Histology and Ultrastructure of the Esophagus in European Beaver (Castor fiber) Displays Features Adapted to Seasonal Changes in Diet
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Legal Statement
2.2. Animals
2.3. Histology and Immunohistochemistry
2.4. Morphometric Evaluation
2.5. Degree of Cellular Proliferation
2.6. Ultrastructural Studies
2.7. Statistical Analysis
3. Results
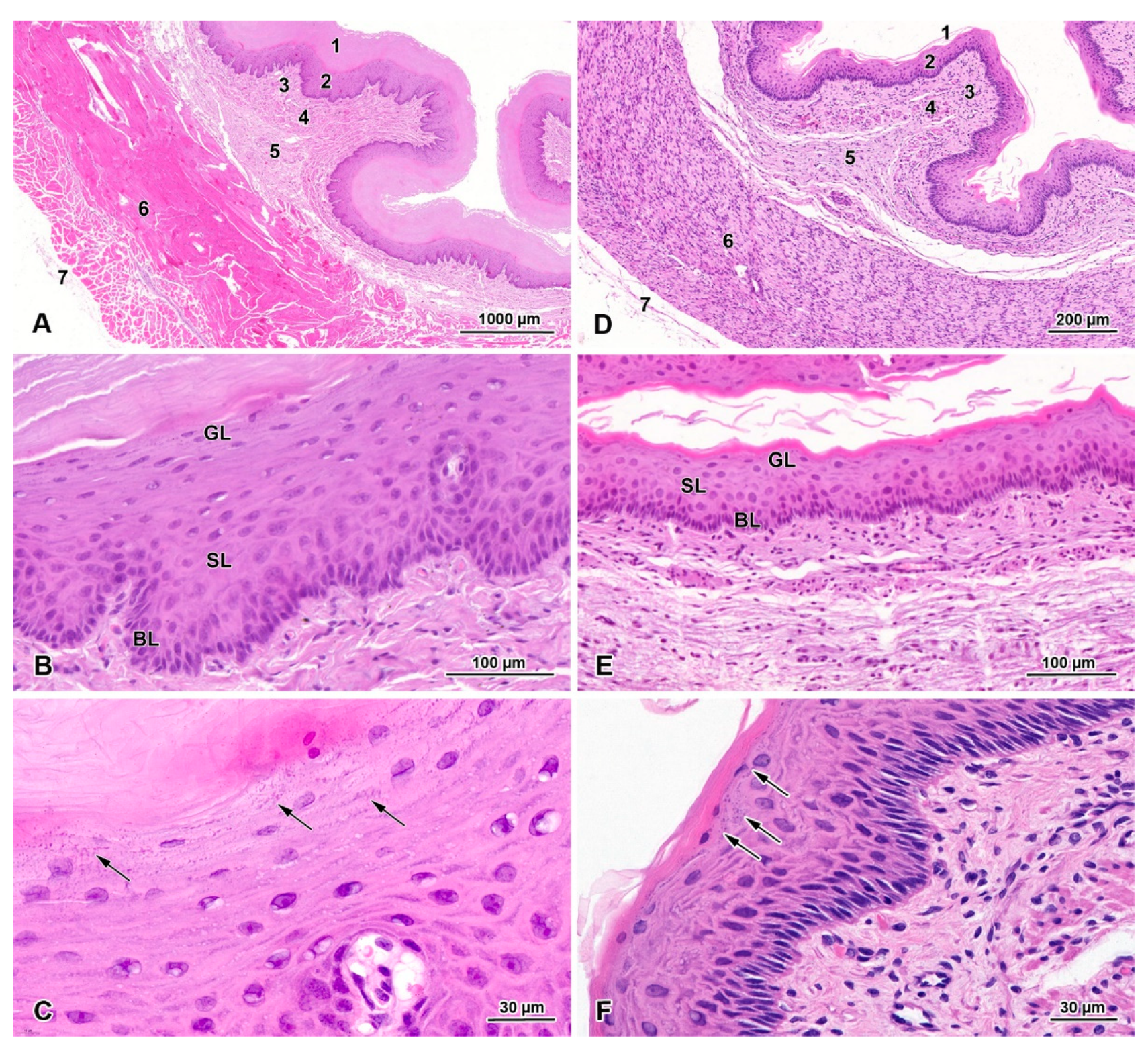
3.1. Histology
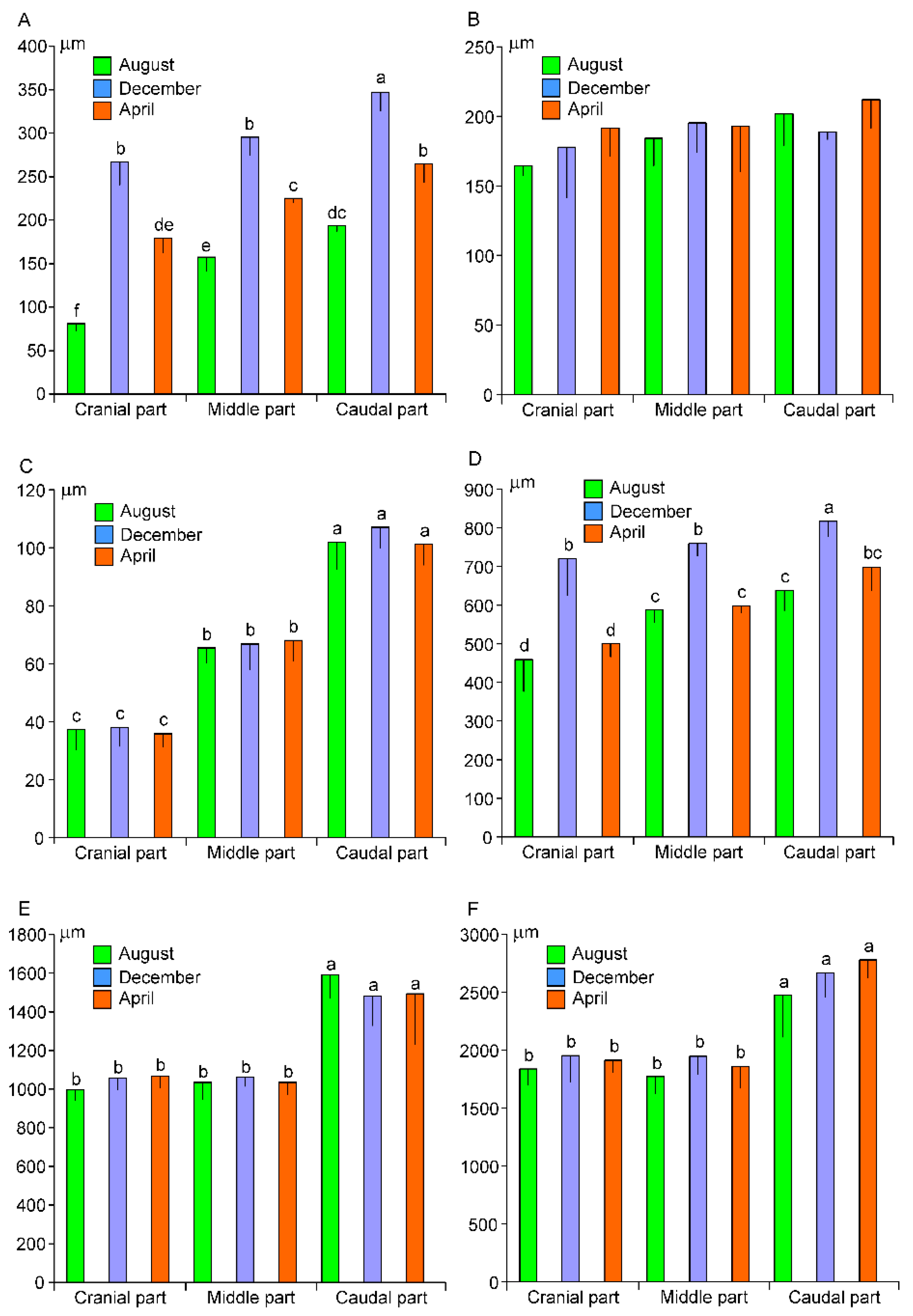
3.2. Morphometry
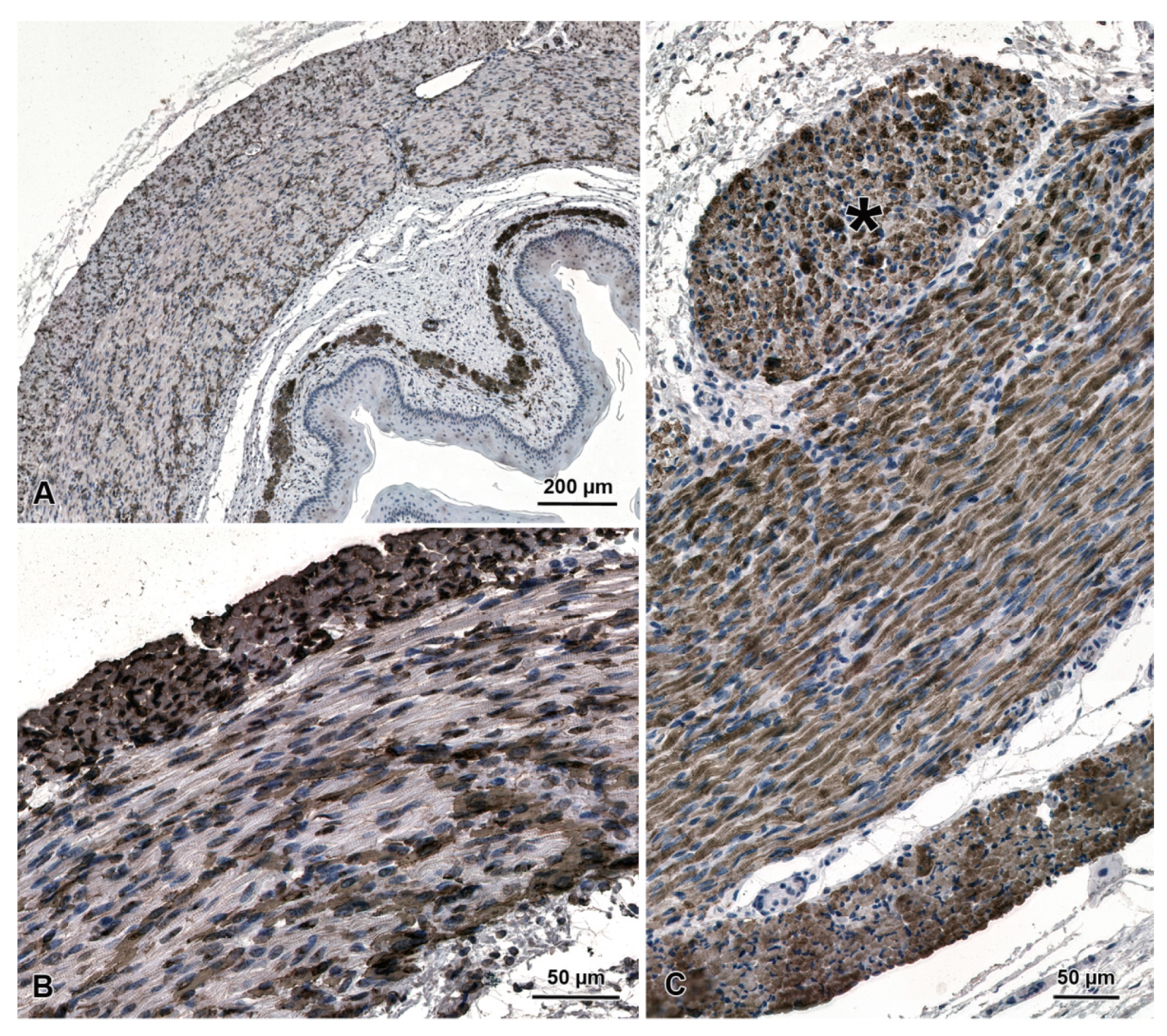
3.3. PCNA Immunohistochemistry
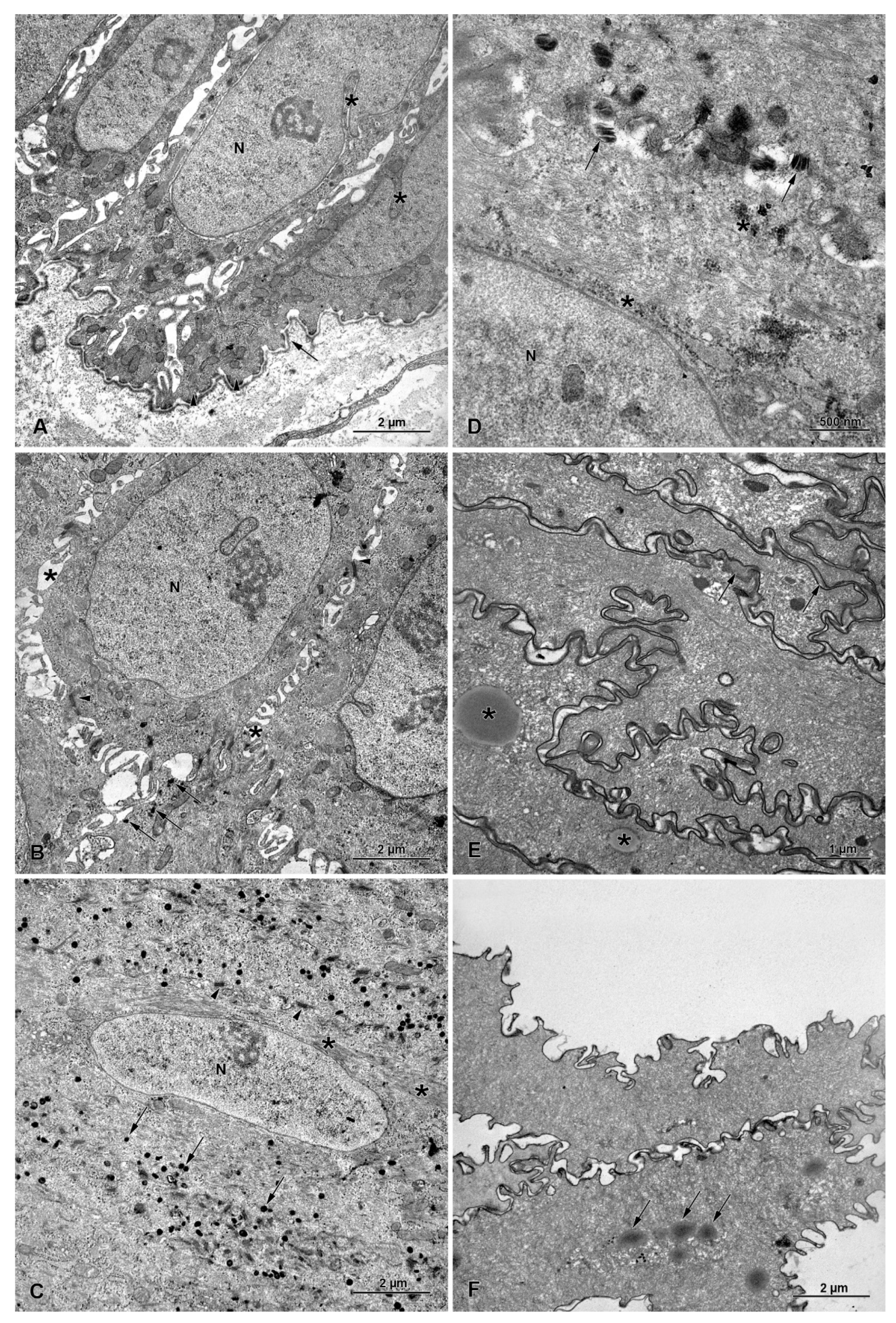
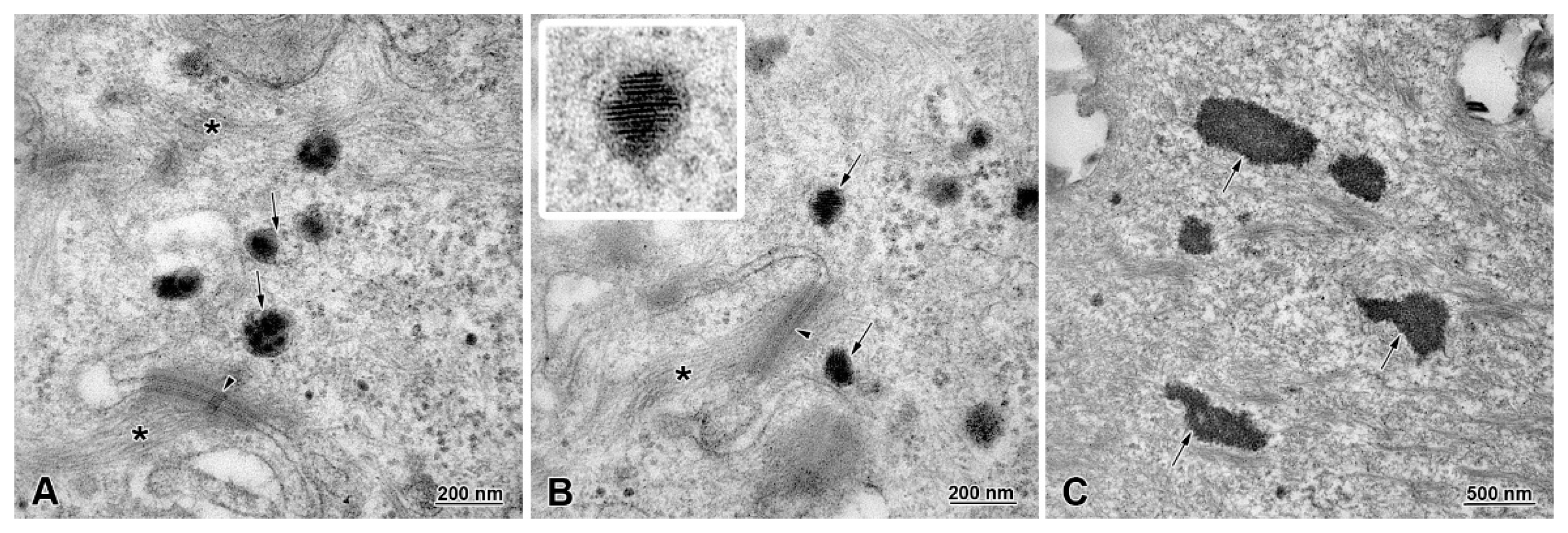
3.4. Ultrastructure of Stratified Squamous Epithelium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamdar, M.N.; Ema, A.N. The submucosal glands and the orientation of the musculature in the oesophagus of the camel. J. Anat. 1982, 135, 165–171. [Google Scholar]
- Windoffer, R.; Beil, M.; Magin, T.M.; Leube, R.E. Cytoskeleton in motion: The dynamics of keratin intermediate filaments in epithelia. J. Cell. Biol. 2011, 194, 669–678. [Google Scholar] [CrossRef]
- Berghes, C.; Tanase, P.; Parvu, M.; Dinu, C.; Cuca, D. Contributions to the study of the esophagus and stomach morphology in guinea pig. Sci. Papers Anim. Sci. Biotech. 2011, 44, 150–154. [Google Scholar]
- Meyer, W.; Schoennagel, B.; Kacza, J.; Busche, R.; Hornickel, I.N.; Hewicker-Trautwein, M.; Schnapper, A. Keratinization of the esophageal epithelium of domesticated mammals. Acta Histochem. 2014, 116, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Shiina, T.; Shimizu, Y.; Izumi, N.; Suzuki, Y.; Asano, M.; Atoji, Y.; Nikami, H.; Takewaki, T. A comperative histological study of the distribution of striated and smooth muscles and glands in the esophagus of wild birds and mammals. J. Vet. Med. Sci. 2005, 67, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Patil, D.; Odze, R.D.; Zhao, L.; Lisovsky, M.; Guindi, M.; Riddel, R.; Bellizzi, A.; Yantiss, R.K.; Nalbantoglu, I.; et al. The microscopic anatomy of the esophagus including the individual layers, specialized tissues, and unique components and their responses to injury. Ann. N.Y. Acad. Sci. 2018, 1434, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Doucet, C.M.; Fryxell, J.M. The effect of nutritional quality on forage preference by beavers. OIKOS 1993, 67, 201–208. [Google Scholar] [CrossRef]
- Nolet, B.A.; Rosell, F. Comeback of the beaver Castor fiber: An overview of old and new conservation problems. Biol. Conserv. 1998, 83, 165–173. [Google Scholar] [CrossRef]
- Haarberg, O.; Rosell, F. Selective foraging on woody plant species by the Eurasian beaver (Castor fiber) in Telemark, Norway. J. Zool. 2006, 270, 201–208. [Google Scholar] [CrossRef]
- Ziółkowska, N.; Lewczuk, B.; Petryński, W.; Palkowska, K.; Prusik, M.; Targońska, K.; Giżejewski, Z.; Przybylska-Gornowicz, B. Light and electron microscopy of the European beaver (Castor fiber) stomach reveal unique morphological features with possible general biological significance. PLoS ONE 2014, 9, e945902014. [Google Scholar] [CrossRef]
- Squier, C.A.; Kremer, M.J. Biology of oral mucosa and esophagus. J. Natl. Cancer Inst. Monogr. 2001, 29, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Pratama, R.; Schneider, D.; Böer, T.; Daniel, R. First Insights Into Bacterial Gastrointestinal Tract Communities of the Eurasian Beaver (Castor fiber). Front. Microbiol. 2019, 10, 1646. [Google Scholar] [CrossRef]
- Alibardi, L.; Maderson, P.F.A. Observations on the histochemistry and ultrastructure of the epidermis of the tuatara, Sphenodon punctatus (Sphenodontida, Lepidosauria, Reptilia): A contribution to an understanding of the Lepidosaurian epidermal generation and the evolutionary origin of the squamate shedding complex. J. Morphol. 2003, 256, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Krmpotic, C.M.; Carlini, A.A.; Galliari, F.C.; Favaronc, P.; Miglinoc, M.A.; Scarano, A.C.; Barbeitoa, C.G. Ontogenetic variation in the stratum granulosum of the epidermis of Chaetophractus vellerosus (Xenarthra, Dasypodidae) in relation to the development of cornified scales. Zoology 2014, 117, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Parakkal, P.F. An electron microscopic study of esophageal epithelium in the newborn and adult mouse. Am. J. Anat. 1967, 121, 175–195. [Google Scholar] [CrossRef]
- Marques-Pererira, J.P.; Leblond, C.P. Mitosis and differentiation in the stratified squamous epithelium of the rat esophagus. Am. J. Anat. 1965, 117, 73–90. [Google Scholar] [CrossRef]
- Borghesi, J.; Mario, L.C.; Carvalho, R.C.; Rodrigues, M.N.; Favaron, P.O.; Miglino, M.A. Morphology of the digestive apparatus in Oligoryzomys nigripes (Rodentia, Sigmodontinae). Open J. Anim. Sci. 2015, 5, 132–141. [Google Scholar] [CrossRef]
- Carrascal Velásquez, J.C.; Ortiz Bedoya, S.A.; Petro Hernández, V.G. Microscopic characterization of esophageal regions of a group of Capybara (Hydrochoerus hydrochaeris) free in Brazil. Rev. CES Med. Zootec. 2016, 11, 73–81. [Google Scholar] [CrossRef]
- Philipsen, H.P.; Fejerskov, O. Normal histology and the effect of acute mechanical stress on the esophagus epithelium in the guinea pig. Acta Odontol. Scand. 1973, 31, 201–210. [Google Scholar] [CrossRef]
- Chu, E.W.; Malmgren, R.A. An inhibitory effect of vitamin A on the induction of tumors of forestomach and cervix in the Syrian hamster by carcinogenic polycyclic hydrocarbons. AACR 1965, 25, 884–895. [Google Scholar]
- Alogninouwa, T.; Agba, K.C.; Agossou, E.; Kpodekon, M. Anatomical, histological and functional specificities of the digestive tract in the male grasscutter (Thryonomys swinderianus, Temminck 1827). Anat. Histol. Embryol. 1996, 25, 15–21. [Google Scholar] [CrossRef]
- Garcia, G.W.; Baptiste, Q.S.; Adogwa, A.O.; Kakuni, M.; Arishima, K.; Makita, T. The digestive system of the agouti (Dasyprocta leporina)-gross anatomy and histology. Japanese J. Zoo Wildl. Med. 2000, 5, 55–66. [Google Scholar] [CrossRef]
- Goetsch, E. The structure of mammalian oesophagus. Am. J. Anat. 1910, 10, 1–40. [Google Scholar] [CrossRef]
- Islam, M.S.; Awal, M.A.; Quasem, M.; Asaduzzaman, M.; Das, S.K. Morphology of esophagus of Black Bengal goat. Bangl. J. Vet. Med. 2008, 6, 223–225. [Google Scholar] [CrossRef]
- Islam, M.S.; Quasem, M.A.; Awal, M.A.; Das, S.K. Histology of esophagus of Black Bengal goat. Bangl. J. Vet. Med. 2005, 3, 152–154. [Google Scholar] [CrossRef]
- Kumar, P.; Mahesh, R.; Kumar, P. Histological architecture of esophagus of goat (Capra hircus). Haryana Vet. 2009, 48, 29–32. [Google Scholar]
- Sokołowska, J.; Urbańska, K.; Matusiak, J.; Wiśniewski, J. New aspects of the esophageal histology of the domestic goat (Capra hircus) and European roe deer (Capreolus capreolus). Vet. Med. Sci. 2021, 7, 1743–1756. [Google Scholar] [CrossRef]
- Hameed, B.K.; Ebraheem, A.H.; Hussein, F.A. Histological structure of the cervical segment oesophagus in goats and sheep (Comparison study). Tikrit J. Pure Sci. 2018, 23, 55–60. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sharma, D.N. Regional histology of the oesophagus of buffalo calves. Indian J. Anim. Sci. 1991, 61, 722–724. [Google Scholar]
- Tseng, S.C.; Hatchell, D.; Tierney, N.; Huang, A.J.; Sun, T.T. Expression of specific keratin markers by rabbit corneal, conjunctival, and esophageal epithelia during vitamin A deficiency. J. Cell. Biol. 1984, 99, 2279–2286. [Google Scholar] [CrossRef]
- Rao, R.S.; Patil, S.; Ganavi, B.S. Oral cytokeratins in health and disease. J. Contemp. Dent. Pract. 2014, 15, 127–136. [Google Scholar] [CrossRef]
- Raymond, C.; Anne, V.; Millane, G. Development of esophageal epithelium in the fetal and neonatal mouse. Anat. Rec. 1991, 230, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.Y.; Slack, J.M.W.; Tosh, D. Conversion of columnar to stratified squamous epithelium in the developing mouse oesophagus. Dev. Biol. 2005, 284, 157–170. [Google Scholar] [CrossRef]
- Meyer, W.; Kacza, J.; Schnapper, A.; Verspohl, J.; Hornickel, I.N.; Seeger, J. A first report on the microbial colonisation of the equine oesophagus. Ann. Anat. 2010, 192, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.; Kacza, J.; Hornickel, I.; Schoennagel, B. Immunolocalization of succinate dehydrogenase in the esophagus epithelium of domesticated mammals. Eur. J. Histochem. 2013, 57, e182013. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, J.; Bowden, P.E.; Coulombe, P.A.; Langbein, L.; Lane, E.B.; Magin, T.M.; Maltais, L.; Omary, M.B.; Parry, D.A.D.; Rogers, M.A.; et al. New consensus nomenclature for mammalian keratins. J. Cell. Biol. 2006, 174, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell. Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef]
- Bragulla, H.H.; Homberger, D.G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 2009, 214, 516–559. [Google Scholar] [CrossRef]
- Grayson, S.; Johnson-Winegar, A.D.; Elias, P.M. Isolation of lamellar bodies from neonatal mouse epidermis by selective sequential filtration. Science 1983, 221, 962–964. [Google Scholar] [CrossRef]
- Freinkel, R.K.; Traczyk, T.N. Lipid composition and acid hydrolase content of lamellar granules of fetal rat epidermis. J. Investig. Dermatol. 1985, 85, 295–298. [Google Scholar] [CrossRef]
- Rassner, U.A.; Crumrine, D.A.; Nau, P.; Elias, P.M. Microwave incubation improves lipolytic enzyme preservation for ultrastructural cytochemistry. Histochem. J. 1997, 29, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Ishida-Yamamoto, A.; Deraison, C.; Bonnart, C.; Bitoun, E.; Robinson, R.; O'Brien, T.J.; Wakamatsu, K.; Ohtsubo, S.; Takahashi, H.; Hashimoto, Y.; et al. LEKTI is localized in lamellar granules separated from KLK5 and KLK7, and is secreted in the extracellular spaces of the superficial stratum granulosum. J. Investig. Dermatol. 2005, 124, 360–366. [Google Scholar] [CrossRef]
- Ishida-Yamamoto, A.; Simon, M.; Kishibe, M.; Miyauchi, Y.; Takahashi, H.; Yoshida, S.; O’Brien, T.J.; Serre, G.; Iizuka, H. Epidermal lamellar granules transport different cargoes as distinct aggregates. J. Investig. Dermatol. 2004, 122, 1137–1144. [Google Scholar] [CrossRef]
- Elias, P.M.; Feingold, K.R. Skin Barrier; CRC Press: New York, NY, USA, 2005. [Google Scholar]
- Dale, B.A.; Holbrook, K.A.; Fleckman, P.; Kimball, J.R.; Brumbaugh, S.; Sybert, V.P. Heterogeneity in harlequin ichthyosis an inborn error of epidermal keratinization: Variable morphology and structural protein expression and a defect in lamellar granules. J. Investig. Dermatol. 1990, 94, 6–18. [Google Scholar] [CrossRef]
- Milner, M.E.; O’Guin, W.M.; Holbrook, K.A.; Dale, B.A. Abnormal lamellar granules in harlequin ichthyosis. J. Investig. Dermatol. 1992, 99, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.J.; Myers, H.M.; Watkins, S.M.; Brown, B.E.; Feingold, K.R.; Elias, P.M.; Farese, R.V., Jr. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J. Biol. Chem. 2004, 279, 11767–11776. [Google Scholar] [CrossRef] [PubMed]
- Matoltsy, A.G.; Parakkal, P.F. Membrane-coating granules of keratinizing epithelia. J. Cell. Biol. 1965, 24, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Sawamura, D.; Nomura, Y.; Sugawara, M.; Shimizu, H. Truncation of CGI-58 protein causes malformation of lamellar granules resulting in ichthyosis in Dorfman-Chanarin syndrome. J. Investig. Dermatol. 2003, 121, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Demerjian, M.; Crumrine, D.A.; Milstone, L.M.; Williams, M.L.; Elias, P.M. Barrier dysfunction and pathogenesis of neutral lipid storage disease with ichthyosis (Chanarin-Dorfman syndrome). J. Investig. Dermatol. 2006, 126, 2032–2038. [Google Scholar] [CrossRef]
- Houben, E.; Hachem, J.P.; De Paepe, K.; Rogiers, V. Epidermal ceramidase activity regulates epidermal desquamation via stratum corneum acidification. Skin Pharmacol. Physiol. 2008, 21, 111–118. [Google Scholar] [CrossRef]
- Menon, G.K.; Orso, E.; Aslanidis, C.; Crumrine, D.; Schmitz, G.; Elias, P.M. Ultrastructure of skin from Refsum disease with emphasis on epidermal lamellar bodies and stratum corneum barrier lipid organization. Arch. Dermatol. Res. 2014, 306, 731–737. [Google Scholar] [CrossRef]
- DeNardi, F.G.; Riddell, R.H. The normal esophagus. Am. J. Surg. Pathol. 1991, 15, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Noguchi, T.; Hashimoto, T.; Uchida, Y.; Shimada, T. The organization of the lamina muscularis mucosae in the human esophagus. Arch. Histol. Cytol. 2003, 66, 281–288. [Google Scholar] [CrossRef]
- Christensen, J.; Rick, G.A.; Soll, D.J. Intramural nerves and interstitial cells revealed by the Champy Maillet stain in the opossum esophagus. J. Auton. Nerv. Syst. 1987, 19, 137–151. [Google Scholar] [CrossRef]
- Uchida, K.; Kamikawa, Y. Muscularis mucosae—The forgotten sibling. J. Smooth Muscle Res. 2007, 43, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Sarosiek, J. Does the healing of the esophageal mucosa improve the function of the esophageal submucosal and salivary glands? Ann. N. Y. Acad. Sci. 2016, 1380, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Su, P.H.; Wang, T.C.; Wong, Z.R.; Huang, B.M.; Yang, H.M. The expression of nestin delineates skeletal muscle differentiation in the developing rat esophagus. J. Anat. 2011, 218, 311–323. [Google Scholar] [CrossRef]
- Fullington, N.M.; Potanos, K.M.; Cauley, R.P.; Purcell, P.; Zurakowski, D.; Fishman, S.J.; Vakili, K.; Kim, H.B. Strain induced esophageal growth in a novel rodent model. J. Pediatr. Surg. 2016, 51, 1273–1278. [Google Scholar] [CrossRef]
- Krauss, R.S.; Chihara, D.; Romer, A.I. Embracing change: Striated-for-smooth muscle replacement in esophagus development. Skelet. Muscle 2016, 6, 27. [Google Scholar] [CrossRef]
- Wörl, J.; Neuhuber, W.L. Ultrastructural analysis of the smooth-to-striated transition zone in the developing mouse esophagus: Emphasis on apoptosis of smooth and origin and differentiation of striated muscle cells. Dev. Dyn. 2005, 233, 964–982. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martyniuk, K.; Ziółkowska, N.; Hanuszewska-Dominiak, M.; Szyryńska, N.; Lewczuk, B. Histology and Ultrastructure of the Esophagus in European Beaver (Castor fiber) Displays Features Adapted to Seasonal Changes in Diet. Animals 2023, 13, 635. https://doi.org/10.3390/ani13040635
Martyniuk K, Ziółkowska N, Hanuszewska-Dominiak M, Szyryńska N, Lewczuk B. Histology and Ultrastructure of the Esophagus in European Beaver (Castor fiber) Displays Features Adapted to Seasonal Changes in Diet. Animals. 2023; 13(4):635. https://doi.org/10.3390/ani13040635
Chicago/Turabian StyleMartyniuk, Kamila, Natalia Ziółkowska, Maria Hanuszewska-Dominiak, Natalia Szyryńska, and Bogdan Lewczuk. 2023. "Histology and Ultrastructure of the Esophagus in European Beaver (Castor fiber) Displays Features Adapted to Seasonal Changes in Diet" Animals 13, no. 4: 635. https://doi.org/10.3390/ani13040635
APA StyleMartyniuk, K., Ziółkowska, N., Hanuszewska-Dominiak, M., Szyryńska, N., & Lewczuk, B. (2023). Histology and Ultrastructure of the Esophagus in European Beaver (Castor fiber) Displays Features Adapted to Seasonal Changes in Diet. Animals, 13(4), 635. https://doi.org/10.3390/ani13040635






