Skin Marks in Critically Endangered Taiwanese Humpback Dolphins (Sousa chinensis taiwanensis)
Abstract
Simple Summary
Abstract
1. Introduction
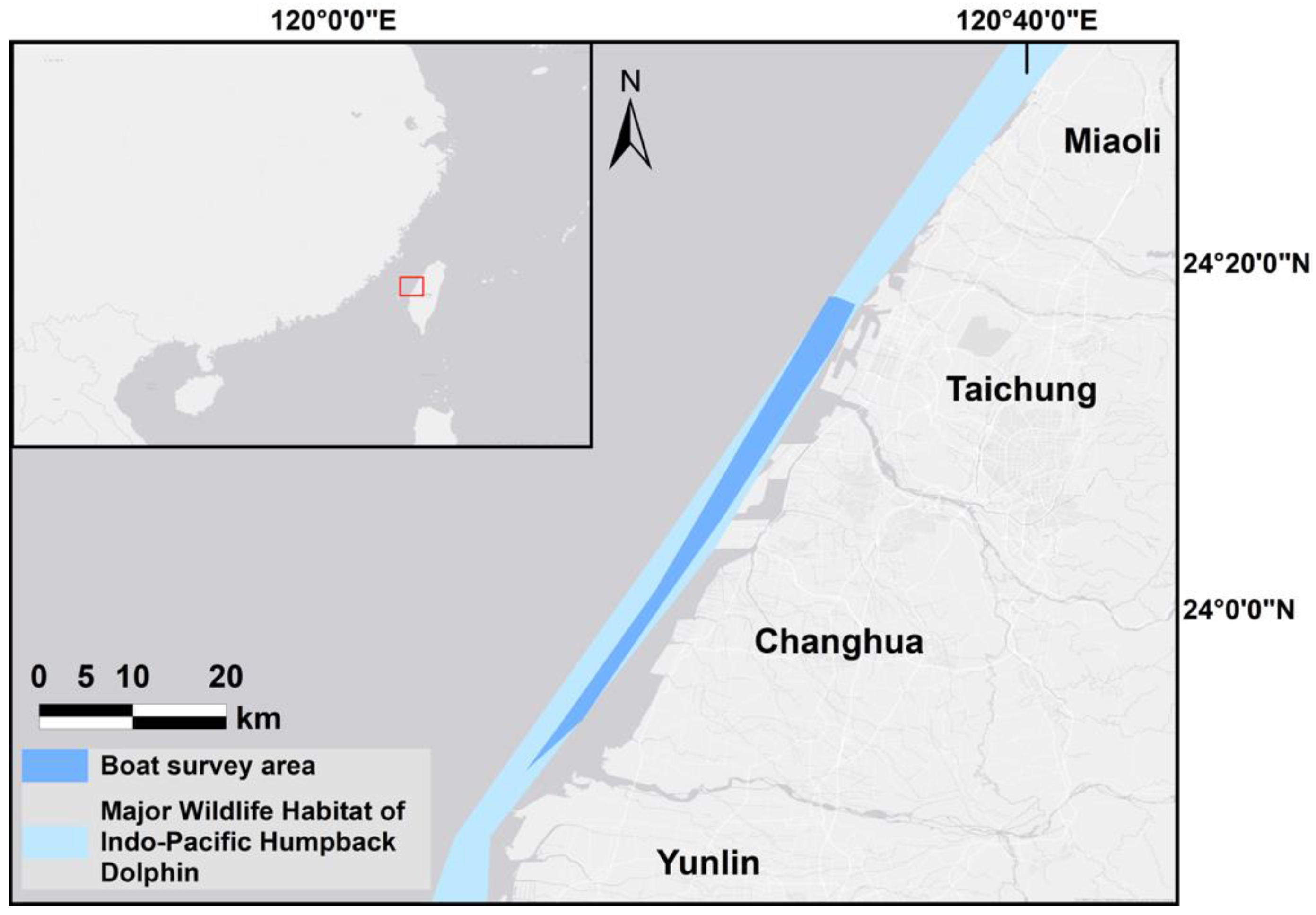
2. Materials and Methods
3. Results
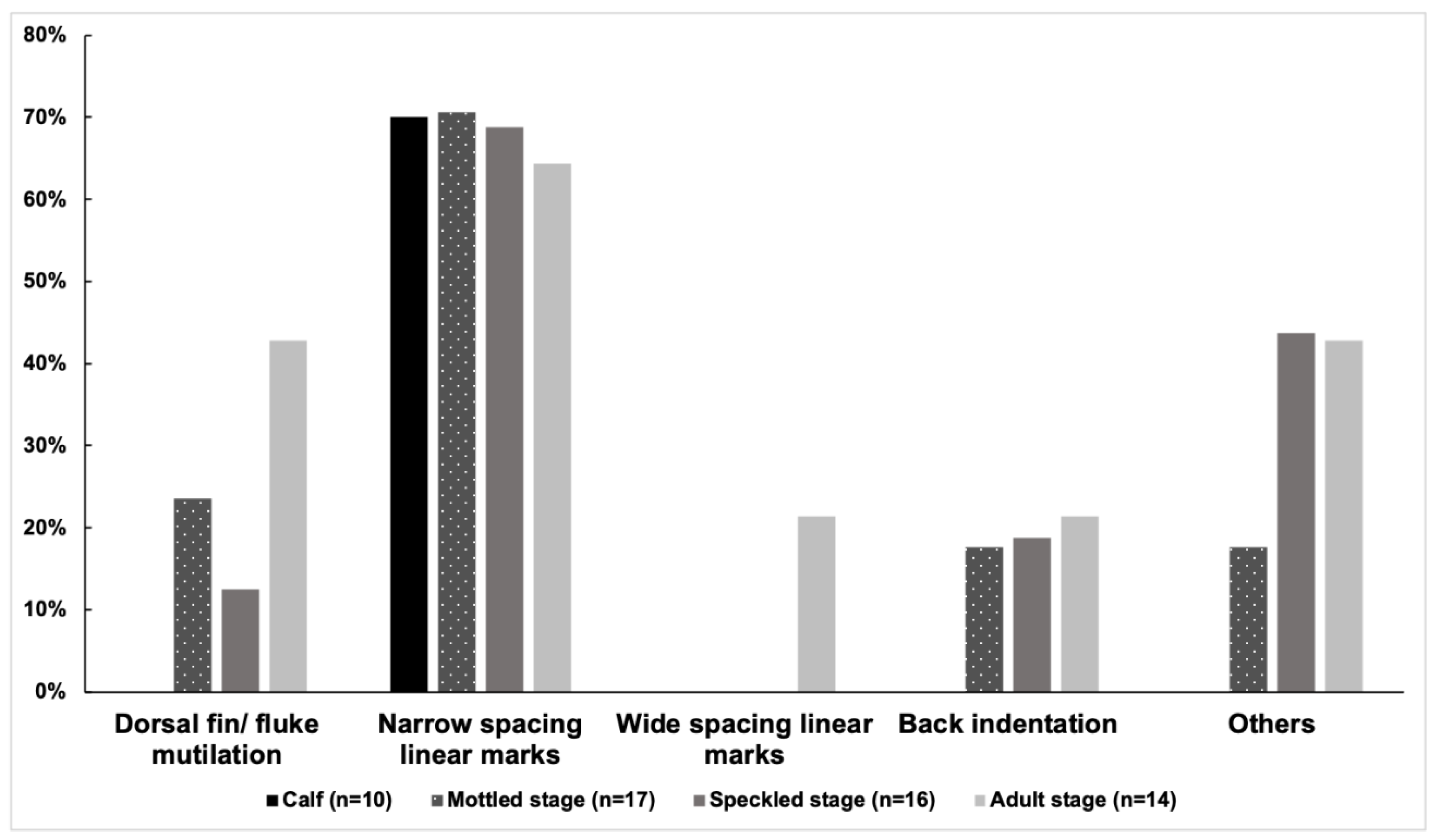
3.1. Prevalence of Injuries
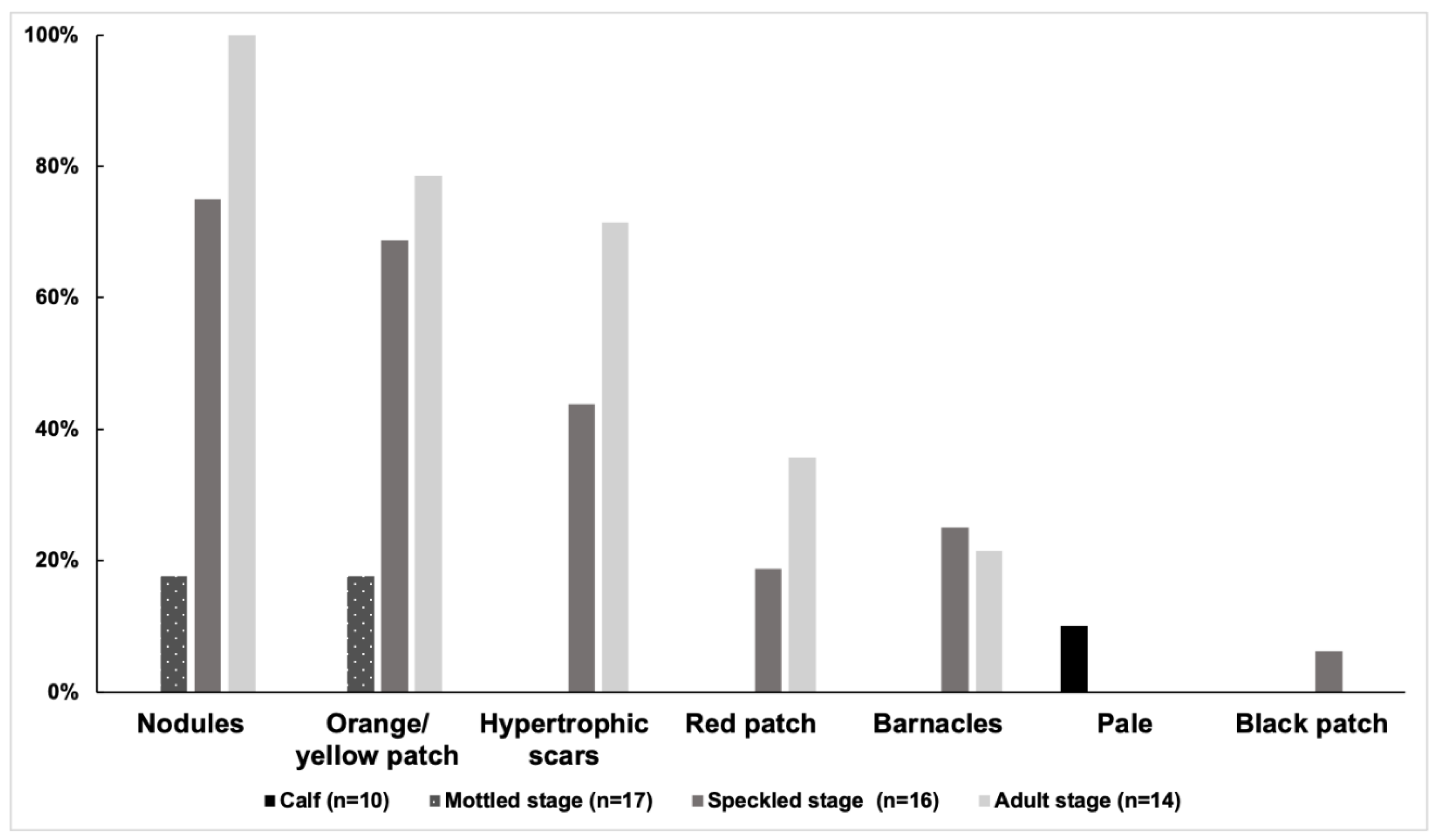
3.2. Prevalence of Skin Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.Y.; Hung, S.K.; Yang, S.-C. Records of Indo-Pacific humpback dolphins, Sousa chinensis (Osbeck, 1765), from the waters of western Taiwan. Aquat. Mamm. 2004, 30, 189–196. [Google Scholar] [CrossRef]
- Ross, P.S.; Dungan, S.Z.; Hung, S.K.; Jefferson, T.A.; Macfarquhar, C.; Perrin, W.F.; Riehl, K.N.; Slooten, E.; Tsai, J.; Wang, J.Y. Averting the baiji syndrome: Conserving habitat for critically endangered dolphins in eastern Taiwan Strait. Aquat. Conserv. 2010, 20, 685–694. [Google Scholar] [CrossRef]
- Wang, J.Y.; Chu Yang, S.; Hung, S.K.; Jefferson, T.A. Distribution, abundance and conservation status of the eastern Taiwan Strait population of Indo-Pacific humpback dolphins, Sousa chinensis. Mammalia 2007, 71, 157–165. [Google Scholar] [CrossRef]
- Wang, J.Y.; Araújo-Wang, C. Sousa chinensis ssp. taiwanensis (amended version of 2017 assessment). IUCN Red List. Threat. Species 2018, e-T133710A122515524. [Google Scholar]
- Chi-Hung, L.I.N.; Lien-Siang, C. Rapid changes in environmental factors could affect the distribution of Taiwanese humpback dolphins (Sousa chinensis taiwanensis) off the coast of Yunlin, Taiwan. Taiwania 2021, 66, 184–192. [Google Scholar]
- Fossi, M.C.; Panti, C. Sentinel Species of Marine Ecosystems. Oxf. Res. Encycl. Environ. Sci. 2017. [Google Scholar] [CrossRef]
- Wells, R.S.; Rhinehart, H.L.; Hansen, L.J.; Sweeney, J.C.; Townsend, F.I.; Stone, R.; Casper, D.R.; Scott, M.D.; Hohn, A.A.; Rowles, T.K. Bottlenose dolphins as marine ecosystem sentinels: Developing a health monitoring system. Ecohealth 2004, 1, 246–254. [Google Scholar] [CrossRef]
- Duignan, P.J.; Stephens, N.S.; Robb, K. Fresh water skin disease in dolphins: A case definition based on pathology and environmental factors in Australia. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Taylor, J.S.; Hart, L.B.; Adams, J. Skin lesion prevalence of estuarine common bottlenose dolphins (Tursiops truncatus) in North Carolina, with comparisons to other east coast study sites. Mar. Mamm. Sci. 2021, 37, 127–141. [Google Scholar] [CrossRef]
- Leone, A.B.; Bonanno Ferraro, G.; Boitani, L.; Blasi, M.F. Skin marks in bottlenose dolphins (Tursiops truncatus) interacting with artisanal fishery in the central Mediterranean Sea. PLoS ONE 2019, 14, e0211767. [Google Scholar] [CrossRef]
- Hart, L.B.; Rotstein, D.S.; Wells, R.S.; Allen, J.; Barleycorn, A.; Balmer, B.C.; Lane, S.M.; Speakman, T.; Zolman, E.S.; Stolen, M. Skin lesions on common bottlenose dolphins (Tursiops truncatus) from three sites in the Northwest Atlantic, USA. PLoS ONE 2012, 7, e33081. [Google Scholar] [CrossRef]
- Reif, J.S.; Peden-Adams, M.M.; Romano, T.A.; Rice, C.D.; Fair, P.A.; Bossart, G.D. Immune dysfunction in Atlantic bottlenose dolphins (Tursiops truncatus) with lobomycosis. Med. Mycol. 2009, 47, 125–135. [Google Scholar] [CrossRef]
- van Bressem, M.-F.; van Waerebeek, K.; Raga, J.A. A review of virus infections of cetaceans and the potential impact of morbilliviruses, poxviruses and papillomaviruses on host population dynamics. Dis. Aquat. Organ. 1999, 38, 53–65. [Google Scholar] [CrossRef]
- van Bressem, M.-F.; van Waerebeek, K.; Raga, J.A.; Gaspar, R.; di Beneditto, A.P.; Ramos, R.; Siebert, U. Tattoo disease of odontocetes as a potential indicator of a degrading or stressful environment: A preliminary report. Sci. Comm. Doc. SC/55/E1 2003. [Google Scholar]
- van Bressem, M.-F.; Raga, J.A.; di Guardo, G.; Jepson, P.D.; Duignan, P.J.; Siebert, U.; Barrett, T.; de Oliveira Santos, M.C.; Moreno, I.B.; Siciliano, S. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis. Aquat. Organ. 2009, 86, 143–157. [Google Scholar] [CrossRef]
- Wilson, B.; Arnold, H.; Bearzi, G.; Fortuna, C.M.; Gaspar, R.; Ingram, S.; Liret, C.; Pribanic, S.; Read, A.J.; Ridoux, V. Epidermal diseases in bottlenose dolphins: Impacts of natural and anthropogenic factors. Proc. R. Soc. Lond. B Biol. Sci. 1999, 266, 1077–1083. [Google Scholar] [CrossRef]
- Read, A.J. The looming crisis: Interactions between marine mammals and fisheries. J. Mammal. 2008, 89, 541–548. [Google Scholar] [CrossRef]
- Yang, W.-C.; Chang, W.-L.; Kwong, K.-H.; Yao, Y.-T.; Chou, L.-S. Prevalence of epidermal conditions in Critically Endangered Indo-Pacific humpback dolphins (Sousa chinensis) from the waters of western Taiwan. Pak. Vet. J. 2013, 33, 505–509. [Google Scholar]
- Slooten, E.; Wang, J.Y.; Dungan, S.Z.; Forney, K.A.; Hung, S.K.; Jefferson, T.A.; Riehl, K.N.; Rojas-Bracho, L.; Ross, P.S.; Wee, A. Impacts of fisheries on the Critically Endangered humpback dolphin Sousa chinensis population in the eastern Taiwan Strait. Endanger. Species. Res. 2013, 22, 99–114. [Google Scholar] [CrossRef]
- Wang, J.Y.; Riehl, K.N.; Yang, S.C.; Araújo-Wang, C. Unsustainable human-induced injuries to the Critically Endangered Taiwanese humpback dolphins (Sousa chinensis taiwanensis). Mar. Pollut. Bull. 2017, 116, 167–174. [Google Scholar] [CrossRef]
- Jefferson, T.A. Population biology of the Indo-Pacific hump-backed dolphin in Hong Kong waters. Wildl. Monogr. 2000, 64, 1–65. [Google Scholar]
- Hupman, K.E.; Pawley, M.D.M.; Lea, C.; Grimes, C.; Voswinkel, S.; Roe, W.D.; Stockin, K.A. Viability of photo-identification as a tool to examine the prevalence of lesions on free-ranging common dolphins (Delphinus sp.). Aquat. Mamm. 2017, 43, 264–278. [Google Scholar] [CrossRef]
- Machernis, A.F.; Stack, S.H.; Olson, G.L.; Sullivan, F.A.; Currie, J.J. External scarring as an indicator of fisheries interactions with bottlenose (Tursiops truncatus) and pantropical spotted (Stenella attenuata) dolphins in Maui Nui, Hawai’i. Aquat. Mamm. 2021, 47. [Google Scholar] [CrossRef]
- Luksenburg, J.A. Prevalence of external injuries in small cetaceans in Aruban waters, Southern Caribbean. PLoS ONE 2014, 9, e88988. [Google Scholar] [CrossRef] [PubMed]
- Feyrer, L.J.; Stewart, M.; Yeung, J.; Soulier, C.; Whitehead, H. Origin and persistence of markings in a long-term photo-identification dataset reveal the threat of entanglement for endangered northern bottlenose whales (Hyperoodon ampullatus). Front. Mar. Sci. 2021, 8, 620804. [Google Scholar] [CrossRef]
- Bradford, A.L.; Weller, D.W.; Ivashchenko, Y.V.; Burdin, A.M.; Brownell Robert, L.J. Anthropogenic scarring of western gray whales (Eschrichtius robustus). Mar. Mamm. Sci. 2009, 25, 161–175. [Google Scholar] [CrossRef]
- Chan, S.C.Y.; Karczmarski, L. Epidermal lesions and injuries of coastal dolphins as indicators of ecological health. Ecohealth 2019, 16, 576–582. [Google Scholar] [CrossRef]
- Flach, L.; van Bressem, M.-F.; Pitombo, F.; Aznar, F.J. Emergence of the epibiotic barnacle Xenobalanus globicipitis in Guiana dolphins after a morbillivirus outbreak in Sepetiba Bay, Brazil. Estuar. Coast. Shelf. Sci. 2021, 263, 107632. [Google Scholar] [CrossRef]
- Toms, C.N.; Stone, T.; Och-Adams, T. Visual-only assessments of skin lesions on free-ranging common bottlenose dolphins (Tursiops truncatus): Reliability and utility of quantitative tools. Mar. Mamm. Sci. 2020, 36, 744–773. [Google Scholar] [CrossRef]
- Li, W.-T.; Chang, H.-W.; Yang, W.-C.; Lo, C.; Wang, L.-Y.; Pang, V.F.; Chen, M.-H.; Jeng, C.-R. Immunotoxicity of silver nanoparticles (AgNPs) on the leukocytes of common bottlenose dolphins (Tursiops truncatus). Sci. Rep. 2018, 8, 5593. [Google Scholar] [CrossRef]
- Li, W.-T.; Wang, L.-Y.; Chang, H.-W.; Yang, W.-C.; Lo, C.; Pang, V.F.; Chen, M.-H.; Jeng, C.-R. Th2 cytokine bias induced by silver nanoparticles in peripheral blood mononuclear cells of common bottlenose dolphins (Tursiops truncatus). PeerJ 2018, 6, e5432. [Google Scholar] [CrossRef]
- Chen, M.-H.; Shih, C.-C.; Chou, C.L.; Chou, L.-S. Mercury, organic-mercury and selenium in small cetaceans in Taiwanese waters. Mar. Pollut. Bull. 2002, 45, 237–245. [Google Scholar] [CrossRef]
- Chou, C.C.; Chen, Y.N.; Li, C.S. Congener-specific polychlorinated biphenyls in cetaceans from Taiwan waters. Arch. Environ. Contam. Toxicol. 2004, 47, 551–560. [Google Scholar] [CrossRef]
- Ko, F.C.; We, N.-Y.; Chou, L.-S. Bioaccumulation of persistent organic pollutants in stranded cetaceans from Taiwan coastal waters. J. Hazard. Mater. 2014, 277, 127–133. [Google Scholar] [CrossRef]
- Chen, M.-H.; Zhuang, M.-F.; Chou, L.-S.; Liu, J.-Y.; Shih, C.-C.; Chen, C.-Y. Tissue concentrations of four Taiwanese toothed cetaceans indicating the silver and cadmium pollution in the western Pacific Ocean. Mar. Pollut. Bull. 2017, 124, 993–1000. [Google Scholar] [CrossRef]
- Li, W.-T.; Chang, H.-W.; Chen, M.-H.; Chiou, H.-Y.; Liou, B.-Y.; Pang, V.F.; Yang, W.-C.; Jeng, C.-R. Investigation of silver (Ag) deposition in tissues from stranded cetaceans by autometallography (AMG). Environ. Pollut. 2018, 235, 534–545. [Google Scholar] [CrossRef]
- Li, W.-T.; Liou, B.-Y.; Yang, W.-C.; Chen, M.-H.; Chang, H.-W.; Chiou, H.-Y.; Pang, V.F.; Jeng, C.-R. Use of autometallography to localize and semi-quantify silver in cetacean tissues. J. Vis. Exp. 2018, 140, e58232. [Google Scholar]
- Hsieh, M.-J.; Yang, W.-C. A field-deployable insulated isothermal PCR (iiPCR) for the global surveillance of Toxoplasma gondii infection in cetaceans. Animals 2022, 12, 506. [Google Scholar] [CrossRef]
- van Bressem, M.-F.; Minton, G.; Sutaria, D.; Kelkar, N.; Peter, C.; Zulkarnaen, M.; Mansur, R.M.; Porter, L.; Vargas, L.H.R.; Rajamani, L. Cutaneous nodules in Irrawaddy dolphins: An emerging disease in vulnerable populations. Dis. Aquat. Organ. 2014, 107, 181–189. [Google Scholar] [CrossRef]
- Maldini, D.; Riggin, J.; Cecchetti, A.; Cotter, M.P. Prevalence of epidermal conditions in California coastal bottlenose dolphins (Tursiops truncatus) in Monterey Bay. Ambio 2010, 39, 455–462. [Google Scholar] [CrossRef]
- Baird, R.W.; Mahaffy, S.D.; Gorgone, A.M.; Cullins, T.; McSweeney, D.J.; Oleson, E.M.; Bradford, A.L.; Barlow, J.; Webster, D.L. False killer whales and fisheries interactions in Hawaiian waters: Evidence for sex bias and variation among populations and social groups. Mar. Mamm. Sci. 2015, 31, 579–590. [Google Scholar] [CrossRef]
- Wells, R.S.; Allen, J.B.; Hofmann, S.; Bassos-Hull, K.; Fauquier, D.A.; Barros, N.B.; DeLynn, R.E.; Sutton, G.; Socha, V.; Scott, M.D. Consequences of injuries on survival and reproduction of common bottlenose dolphins (Tursiops truncatus) along the west coast of Florida. Mar. Mamm. Sci. 2008, 24, 774–794. [Google Scholar] [CrossRef]
- Su, C.-Y.; Hughes, M.W.; Liu, T.-Y.; Chuong, C.-M.; Wang, H.-V.; Yang, W.-C. Defining wound healing progression in cetacean skin: Characteristics of full-thickness wound healing in Fraser’s dolphins (Lagenodelphis hosei). Animals 2022, 12, 537. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C. Studies on the Scars of Humpback Dolphins, Sousa chinensis, in Taiwan. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 2012; pp. 24–30. [Google Scholar]
- Li, W.-T.; Chou, L.-S.; Chiou, H.-Y.; Chen, I.-H.; Yang, W.-C. Analyzing 13 years of cetacean strandings: Multiple stressors to cetaceans in Taiwanese waters and their implications for conservation and future research. Front. Mar. Sci. 2021, 8, 606722. [Google Scholar] [CrossRef]
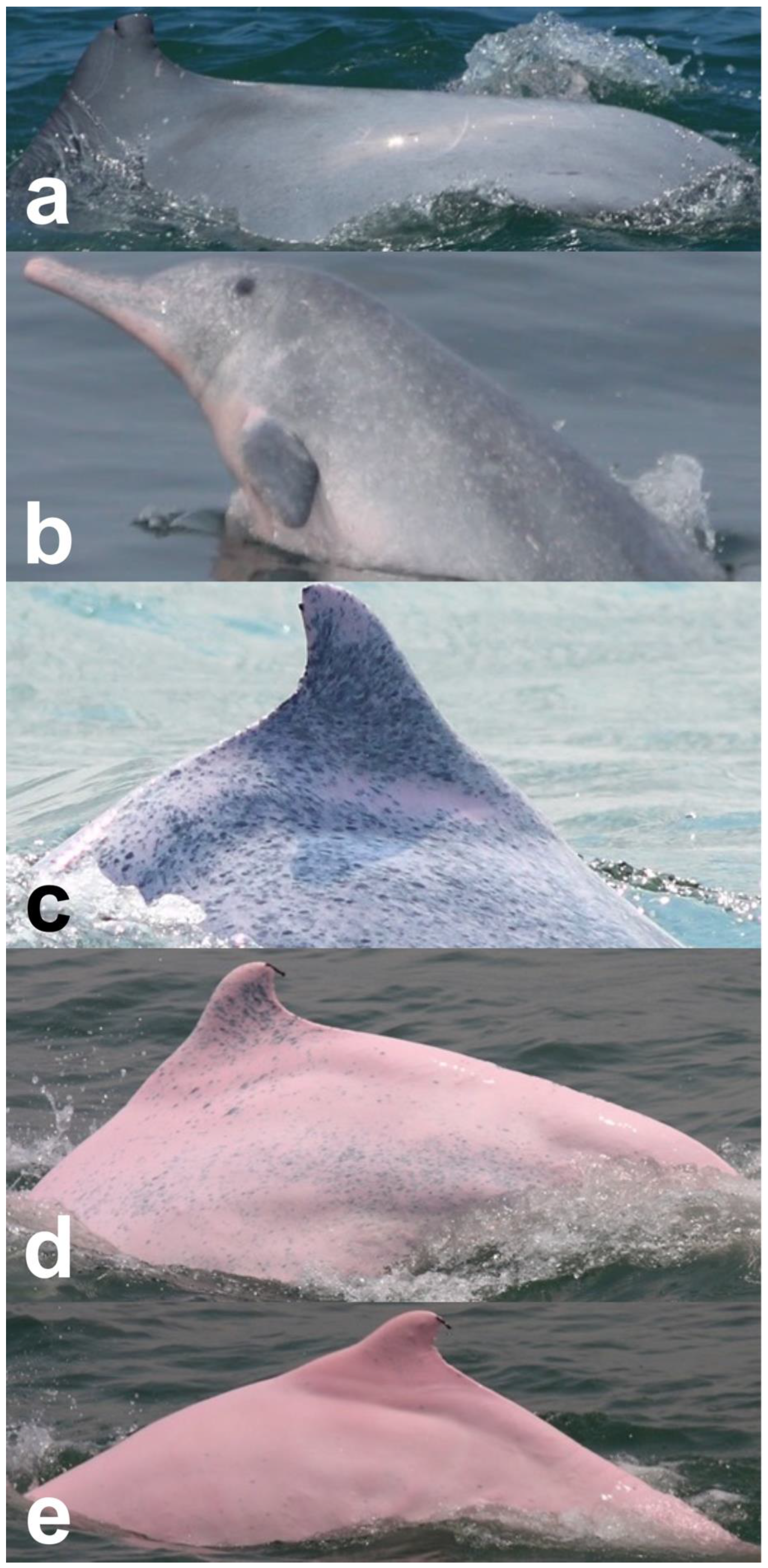



| Injury Categories | Description | Examples | Potential Causes | References |
|---|---|---|---|---|
| Dorsal fin/fluke mutilation | Missing the apex of the dorsal fin/fluke, with a sharp edge or a linear cut down the dorsal fin |  | cut from net entanglement or other object | [10,22,23] |
| Narrow-spaced linear marks | Serial linear marks, including both shallow and deep marks, especially those appearing along the spine of the peduncle |  | net entanglement or inherent deformities | [23,24] |
| Wide-spaced linear marks | Serial linear marks that extend from one side of the peduncle to the other side, with a long and equal interval between each mark | 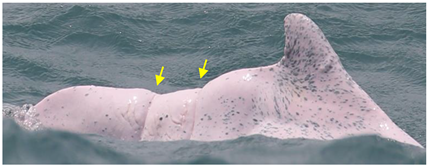 | net entanglement or ship strike | [23,24] |
| Back indentation | V-shaped indentation located along the spine; size varies |  | net entanglement or ship strike | [10,23,25] |
| Others | Marks seemingly unrelated to natural causes and that cannot be assigned to the other scar categories |  | cut from net entanglement or other object | [26] |
| Skin Lesion Categories | Description | Examples | Potential Causes | References |
|---|---|---|---|---|
| Nodules | Oval or circular cutaneous elevations of the skin |  | fungal or bacterial infection | [27] |
| Hypertrophic Scars | Raised lesions with oval shapes | 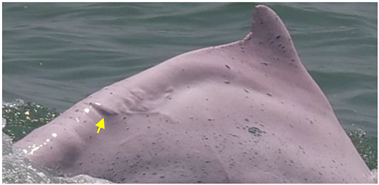 | infection or immune deficiency | [22] |
| Barnacles | One or multiple hanging barnacles, especially seen on dorsal fins and flukes |  | swimming slowly due to health issues | [28] |
| Pale | White in color and irregular in shape; diffuse edges and of any size |  | fungal infection | [9,22] |
| Black patch | Uniform in color and irregular in shape; diffuse edges |  | pox virus infection | [9,22,25] |
| Red patch | Pink patch with multiple small red spots scattered around; irregular in shape | 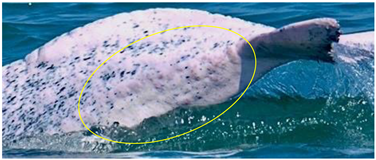 | bacterial or viral infection | [27] |
| Orange/yellow patch | Orange or yellow coloration with diffuse edges | 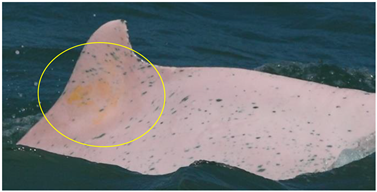 | attached diatom | [22,27,29] |
| Stage (n = 2018, 2019, 2021) | Dorsal Fin/Fluke Mutilation | Narrow-Spaced Linear Marks | Wide-Spaced Linear Marks | Back Indentation | Others |
|---|---|---|---|---|---|
| Calf (n = 5,3,8) | (0,0,0) | (3,2,4) | (0,0,0) | (0,0,0) | (0,0,0) |
| Mottled stage (n = 16,7,11) | (3,1,2) | (10,5,5) | (0,0,0) | (2,0,1) | (1,2,1) |
| Speckled stage (n = 15,10,10) | (2,2,2) | (9,7,6) | (0,0,0) | (3,2,2) | (4,4,3) |
| Spotted adult (n = 12,7,5) | (6,2,2) | (7,4,4) | (2,2,0) | (2,0,1) | (5,2,2) |
| Unspotted adult (n = 2,1,0) | (0,0,0) | (1,0,0) | (1,0,0) | (0,0,0) | (1,0,0) |
| Stage (n = 2018, 2019, 2021) | Nodules | Orange/Yellow Patch | Hypertrophic Scars | Red Patch | Barnacles | Pale | Black Patch |
|---|---|---|---|---|---|---|---|
| Calf (n = 5,3,8) | (0,0,0) | (0,0,0) | (0,0,0) | (0,0,0) | (0,0,0) | (1,0,0) | (0,0,0) |
| Mottled stage (n = 16,7,11) | (1,1,1) | (2,0,1) | (0,0,0) | (0,0,0) | (0,0,0) | (0,0,0) | (0,0,0) |
| Speckled stage (n = 15,10,10) | (11,4,2) | (9,0,3) | (5,4,3) | (6,0,1) | (0,0,4) | (0,0,0) | (1,1,0) |
| Spotted adult (n = 12,7,5) | (11,3,4) | (9,0,1) | (9,2,2) | (5,1,0) | (1,0,1) | (0,0,0) | (0,0,0) |
| Unspotted adult (n = 2,1,0) | (2,0,0) | (2,0,0) | (1,0,0) | (0,0,0) | (1,0,0) | (0,0,0) | (0,0,0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, Y.; Wu, P.-Y.; Chou, L.-S.; Yang, W.-C. Skin Marks in Critically Endangered Taiwanese Humpback Dolphins (Sousa chinensis taiwanensis). Animals 2023, 13, 608. https://doi.org/10.3390/ani13040608
Ho Y, Wu P-Y, Chou L-S, Yang W-C. Skin Marks in Critically Endangered Taiwanese Humpback Dolphins (Sousa chinensis taiwanensis). Animals. 2023; 13(4):608. https://doi.org/10.3390/ani13040608
Chicago/Turabian StyleHo, Yun, Pei-Ying Wu, Lien-Siang Chou, and Wei-Cheng Yang. 2023. "Skin Marks in Critically Endangered Taiwanese Humpback Dolphins (Sousa chinensis taiwanensis)" Animals 13, no. 4: 608. https://doi.org/10.3390/ani13040608
APA StyleHo, Y., Wu, P.-Y., Chou, L.-S., & Yang, W.-C. (2023). Skin Marks in Critically Endangered Taiwanese Humpback Dolphins (Sousa chinensis taiwanensis). Animals, 13(4), 608. https://doi.org/10.3390/ani13040608





