Simple Summary
African elephants commonly enter farmlands across their ranges and trample or consume crops, creating conflicts with farmers and challenges for conservation priorities. Farmers may retaliate and injure elephants, but the extent to which this occurs is relatively unknown. However, scars found on elephants may be evidence of past negative interactions. The objective of our study was to describe the presence of scars in an elephant population in the Greater Tsavo Ecosystem, a region of high human–elephant conflict in Kenya. We monitored and catalogued a population in the Kasigau Wildlife Corridor from 2017 to 2021, noting the number of scars and their placement on individuals. Presumably, evidence of conflict between elephants is more likely to be found on the head or rump areas of elephants, whereas humans are most likely to injure the body of elephants. We found that adult males were much more likely to have scars than adult females, older males were more likely to have scars than younger males, and more scars were located on the body as opposed to the rump and head. Understanding how many elephants in a population have scars and the location of scars can describe the potential level of human–elephant conflict in a population. This valuable information can be used to better understand the level of conflict in a community and aid in management recommendations.
Abstract
Human–elephant conflict (HEC) compromises crop security and threatens elephant conservation. Most commonly, HEC manifests as crop-foraging as elephants modify natural foraging strategies to incorporate crops. Farmers may retaliate by frightening or harming elephants, leaving scars from inflicted wounds. We assessed the prevalence and distribution of scars on the bodies of African savanna elephants (Loxodonta africana) observed in the Kasigau Wildlife Corridor (KWC), part of the Greater Tsavo Ecosystem of Kenya, where conflict is prevalent. We surmised that scars on the body are largely a result of HEC as opposed to scars on the rump or head, which we attributed primarily to elephant–elephant conflict. We hypothesized that: (1) male elephants would have more scars than females; (2) older males would be more likely to have scars than younger males; and (3) most scars would be located on the bodies of elephants. We assessed scars from a photographic catalogue of elephants from the KWC. In line with our hypotheses, male elephants were more likely to have scars than females (32% of males compared to 6% of females); older males had significantly more scars than younger males (61% compared to 24%); and the majority of scars (89%) were located on the body. Scar presence may be useful as an animal-centered indicator to estimate the prevalence and demographic patterns of HEC.
1. Introduction
As wild spaces continue to become reduced or fragmented, wildlife and human populations increasingly come into contact [1,2,3]. Negative interactions occur when humans and wildlife compete over resources such as food, water, and space, creating human–wildlife conflict (HWC) [4,5]. Conflict can result in injury or death for people, wildlife, or both, as evidenced in vehicle collisions [6,7], wildlife attacks on people [8,9], or the intentional killing or injury of wildlife deemed a “nuisance” by stakeholders [10,11]. More common than physical harm are the impacts to rural livelihoods in farming communities, as wildlife and farmers clash over food and water resources. HWC is especially impactful in the form of crop-foraging (also known as crop-raiding, when animals leave protected areas to consume crops belonging to local subsistence farmers) in resource-poor communities [12,13,14]. A variety of mitigation methods exist across species and geographies in which crop-foraging occurs [15,16], but evidence of retaliation continues to be prevalent [17,18]. As climate change exacerbates food insecurity [19], instances of injury to wildlife are likely to increase as farmers attempt to protect their livelihoods and families.
African savanna elephants (Loxodonta africana) are endangered across their range, with primary threats from illegal hunting for ivory, habitat loss, and negative human–elephant interactions or human–elephant conflict (HEC) [20,21,22,23]. The most common form of HEC is crop-foraging [24,25]; crop-foraging events that compromise an entire season’s harvest in one evening occur, but are rare. However, even infrequent partial losses can impact farmers’ harvests, threatening food security [26,27]. In addition, crop-foraging can create tension between stakeholders, wildlife agencies, and conservation practitioners [28,29]. Given this tension, interventions to mitigate these interactions are crucial. However, the widespread occurrence of such incidents, especially in remote areas, makes mitigation a challenge for management agencies.
Farming communities in and around the Greater Tsavo Ecosystem (GTE) of Kenya experience high levels of HEC. Elephants are frequent crop-foragers in the GTE, and remain a source of conflict between community members and corresponding wildlife officials [30,31,32]. Between 2001 and 2016 in the GTE, a total of 45,151 cases of HWC were reported, of which 64.3% involved elephants [33]. Farmers often attempt to mitigate crop-foraging by defending their farms with stationary deterrents, active patrolling, or physically frightening away elephants [34,35,36,37]. While most farmers do not attempt to harm elephants around the GTE, a small percentage reported doing so. In a survey of 206 farmers, the vast majority reported being fearful of elephants [38], which could lead to adverse actions by farmers, threatening elephant and human safety or health. Managing and mitigating HEC can also take time and use valuable conservation resources, as wildlife officers sometimes need to perform costly sedations and treatments for injured elephants [31].
As a potential reflection of HEC, the presence of conspicuous scars on some elephants is evident in the GTE. Firearms are rarely used in this area due to their illegality and lack of availability, and thus most elephant injuries caused by humans are from arrows or occasionally, spears [33], and most wounds likely go untreated. However, in a study on endangered Asian elephants (Elephas maximus) by LaDue et al. [11] in Sri Lanka, gunshots were a common source of injury, especially to male elephants. Obanda et al. [39] used a Kenya Wildlife Service dataset from 1998 to 2007 to evaluate all types of injuries to African elephants in the GTE. Adult males were the most likely to sustain injuries, the legs and body were the most frequently injured body parts, and human-inflicted wounds accounted for two-thirds of injuries. While leg injuries can occur from direct encounters with humans, Obanda et al. surmised that snares and terrain-related causes were the most likely sources of injuries around the legs. Following up on these studies, we sought to determine the relationship between scar presence, age, sex, and body location as a measure of direct negative encounters between people and elephants in the Kasigau Wildlife Corridor (KWC) of Kenya, which is part of the GTE. We focused on scars and not on other forms of injuries such as dislocations, sprains, and swellings [39]. We presumed that scars on the head (or possibly the rump) were more likely to result from interactions between elephants (e.g., mutual sparring, dominance interactions), as elephants mainly spar head-to-head. Conversely, we assumed human-caused scars would be more likely to occur on the body; this area represents a larger target for arrows or spears and other sources of human-inflicted injuries, and humans are unlikely to approach elephants head-on for fear of being detected. Furthermore, we surmised that an elephant would turn away from an imminent anthropogenic threat, leaving very few human-induced scars on the head.
We hypothesized that: (1) adult males were more likely to have scars than adult females; (2) older males were more likely to have scars; and (3) most scars on males would be located on the body as opposed to the head or the rump. Understanding more about how scars are distributed amongst the demographics of an elephant population can give insights into the potential patterns of negative direct human–elephant encounters, which are dangerous to elephants and people. These insights can also assist conservation practitioners in developing management and mitigation strategies.
2. Materials and Methods
2.1. Study Area
The KWC consists of community ranches and lies between Tsavo East and West National Parks in southeastern Kenya (Figure 1) as part of the GTE. The region is home to the country’s largest and growing elephant population of ca. 15,000 individuals [40], and many elephants use the corridor to move between the two parks [41,42]. Rukinga Wildlife Sanctuary, a former cattle ranch and one of the 14 ranches in the corridor that are part of the KWC’s Reducing Emissions from Deforestation and Degradation (REDD+) project, is managed by Wildlife Works. The organization excluded human settlements and livestock grazing to restore the ecosystem function of the ranch, making it a favorable habitat for elephants and other wildlife. In exchange, the organization provides jobs, educational opportunities, and disbursements to local communities, which can be used for numerous improvement projects. The REDD+ project area now contains a subset of the larger elephant population numbering around 2000, with 300 to 500 resident elephants shifting seasonally around the sanctuary [43].
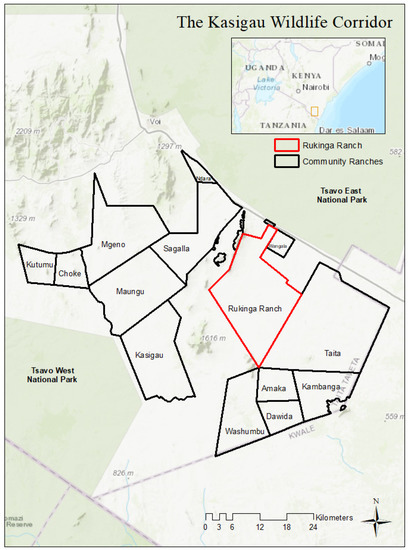
Figure 1.
The Kasigau Wildlife Corridor (KWC) of Kenya, showing the location of Rukinga Ranch Wildlife Sanctuary.
2.2. Elephant Observations and Cataloguing
From 2017 to 2019 and in 2021, we monitored and catalogued elephants passing through the sanctuary from approximately May to late January over the two crop-growing seasons. Elephant observations were collected from six different structured wildlife biodiversity driving transects (which covered portions of the 30,000 ha sanctuary), opportunistic sightings, and images from camera traps placed in Sasenyi village for an elephant crop-foraging study [34]. Transects were originally conducted three times per week approaching dusk and starting around 16:45. During the survey periods, we selected one of the six transects randomly without repeating the same transect, until each of the six transects were performed. In 2019, we shifted to one transect per month until natural forage was present, as wildlife presence greatly diminished during drought periods. Information from all transects informed our study. All elephants were recorded and photographed (if possible) when encountered. Specifically, we noted the number of elephants in a group, type of group (family (matriarchal), bachelor, mixed, or lone individual), sex, estimated age and age class, and any special characteristics (such as presence of scars or injury). Approximate elephant ages were determined using visual indicators such as height, height relative to other elephants, wrinkle prevalence, tusk size and length, and head shape [44,45,46]. We subsequently assessed all photographs to confirm field sightings and/or fill in any gaps in data that could not be ascertained from the original sighting (e.g., sex, age) [45,46,47]. Elephant age classes were assigned for males and females (Table 1) [45,47,48]. Prime males are distinguished from young adult and senescing males because prime males are most likely to enter musth, a heightened period of reproductive activity associated with increased aggression and movement [49]. To ensure that the age estimates of adults were accurate from original sightings, each of the authors listed their age group estimates (disagreements occurred in <15% of cases) and those with any differences were resolved through further examination of photos and 100% consensus. For all elephants, a photograph of each ear, their full front, each side, tusks, and rear were added to assist with identification. Because males (especially those aged ≤ 20 years) without distinguishing markings on their ears or variation in tusks often looked similar, we required a minimum of a clear picture of one ear and both tusks to have enough information to re-identify a bull. Ear markings vary between individual elephants as they receive tears and notches from sparring and moving through the bush, and ear vein patterns are also unique. Tusks also have variation in breaks and wear patterns. Thus, we used a combination of ear and tusk markings to identify individuals and noted each recurrence.

Table 1.
Age classes and respective ages used to categorize elephant groups.
We define “scar” here as any raised area(s) found on an elephant (weeping, scabbed, or healed) that does not appear to be a natural aberration (examples, Figure 2). Whenever scars were noted on an individual, a photograph was taken that included the scar, and its position on the elephant was noted in the catalog. We classified scars into three zones (Figure 3): head, body, and rump. This project operated under the Kenya Wildlife Service’s PIC/MAT agreement with Wildlife Works and with approval from NACOSTI, Kenya’s science agency (License No. NACOSTI/P/20/2292).

Figure 2.
Examples of scars on African elephants. (a) A recent injury that is still healing found on the body of a bull; (b) a bull with multiple scars on its body; (c) a bull with a scar on its rump; (d) a bull with a scar on its head. Photos by R.L. Von Hagen.
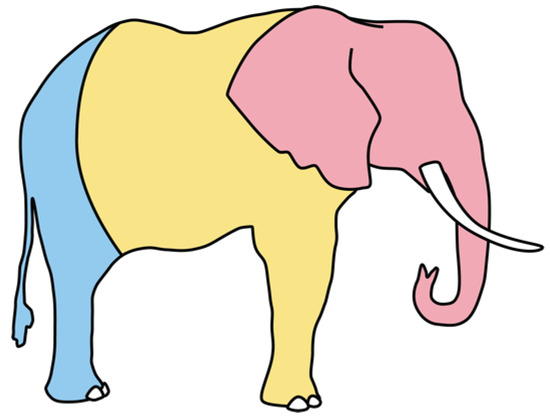
Figure 3.
The three body zones of each elephant used to describe the position(s) of scars: head (shown in pink), body including front legs (yellow), and rump including hind legs (blue).
2.3. Statistical Analysis
For our first hypothesis, we tested for a significant intersexual difference in the presence of scars using Fisher’s exact test (accommodating for small sample sizes) among elephants that we presumed had reached sexual maturity: all males 15 years and older, and adult female elephants 20 years and older [50]. The sample of adult female elephants that had visible scars was very small (n = 3). Therefore, we did not continue with any inter-sex analyses. However, the ages of the male elephants included in this study followed a normal distribution (Kolmogorov–Smirnov test: D = 0.068, p = 0.319). To examine our second hypothesis, we tested for a significant difference between the mean number of scars between adult males and prime adult males using a Welch’s two-sample t-test for unequal variance (we did not have any senescing males in our sample). To examine our third hypothesis, we tested for a significant difference between the presence of scars in the three scar locations (head, body, and rump) amongst all age categories of adult male elephants (age 20 years and older; again, including pubescents did not change the outcome). Because body scars were the most prevalent, we used two separate Fisher’s exact tests: one with head and body and one with rump and body. Alpha values were set at 0.05, and data were analyzed in R [51].
3. Results
Males 20 years of age and older were more likely to have scars (n = 142/161) compared to adult females (n = 3/48) (p < 0.001). Furthermore, prime adult males were significantly more likely to have scars than adult males (p < 0.001). Of all adult males sampled (≥15 years), 32% had one or more scars, including 25% of adult males (n = 24/98) and 61% of prime adults (n = 27/44) (Table 2). Furthermore, prime adult males had significantly more scars (n = 57; almost twice as many) compared to adult males (n = 33; t43.61 = −2.66, p = 0.011). We also found a significantly greater preponderance of scars on the body than either the head or the rump for adult male elephants (both pairwise comparisons, p < 0.001): 89% on the body, 4% on the head, and 7% on the rump.

Table 2.
Scar prevalence among 161 males in the Kasigau Wildlife Corridor of Kenya (see Table 1 for ages).
4. Discussion
We found clear support for our hypotheses predicting demographic patterns of scars observed on African savanna elephants in the KWC. Specifically, (1) adult male elephants were much more likely to have scars than adult females; (2) older, prime adult male elephants (35–54 years) had significantly more scars on average than adult males (20–34 years); and (3) most of the scars we observed were located on the main bodies of elephants compared to the head or rump. In contrast to a prior study on elephant injuries, rarely were any scars or injuries noted on the legs of elephants [39]. We did not detect scars on males or females below the estimated age of 20 years (sexual maturity in males). While we make reasonable assumptions about the cause of the scars we observed, the use of scar patterns is still informative for wildlife managers as they seek to assess populations’ overall wellbeing and aid in the conservation of this endangered species [39,52,53]. We assume many of the scars that we observed, especially those on the body, were acquired from direct encounters with humans [39,53,54]. Increased tension from ongoing HEC is leading many community members to engage in direct conflict with elephants in the GTE, as has occurred in other elephant ranges where HEC is prevalent [55,56,57,58,59,60]. As such, the prevalence of human-inflicted wounds (and scars resulting from those wounds) may increase until effective HEC mitigation strategies can be more widely implemented.
From other studies in areas in which African savanna elephants are likely to engage in crop-foraging, males are more likely to be involved in HEC compared to females [61,62,63,64]. Males may be more willing to exhibit risk-prone behaviors (e.g., crop-foraging) to maintain high body conditions while simultaneously traveling between female groups to locate receptive mates [65,66]. Because males had more scars than females in our study, the difference between the sexes also suggests that many of these scars were incurred during crop-foraging, as the sex bias in crop-foraging occurs in the KWC as well [30,67]. Furthermore, scars were most prevalent on the body, compared to the head or the rump, where scars from sparring among conspecifics presumably would be more common [68,69].
Given that 32% of male elephants in our study had scars, our study demonstrates that scars may be efficacious as a low-cost method to monitor the prevalence and demographic patterns of HEC involving resident elephant populations in this area, even if a proportion of the scars are from sources other than HEC. This type of monitoring could be in addition to farmer reports, which can sometimes be understandably impacted by bias. A similar method was used by LaDue et al. [53] in Sri Lanka to monitor HEC around a protected area for Asian elephants. In that study, males were also observed to have more scars than females, and males appeared to acquire more scars as they aged. Thus, the authors of that study concluded that gunfire was not an effective deterrent for crop-foraging elephants at their study site, with many older elephants observed with dozens of scars incurred from gunfire. In other words, if scars are reflective of a male elephant’s propensity to engage in conflict with humans, then a direct relationship between scar count and age may indicate that certain deterrents (e.g., gunfire, arrows) are not effective in curbing HEC. As in the Sri Lankan study, we found that, in the KWC, male elephants in the older age class (prime adult, 35–54 years) had more scars than individuals in the younger adult male age class (20–34 years). This indicates that older males may be more likely to have HEC encounters, accumulating scars over time. However, a lack of scars in older males could also indicate that certain individuals are more “successful” crop-foragers, or adept at avoiding farmers, further complicating our knowledge on the source of scars. Furthermore, if the presence of scars is unequally distributed among males within an age group, such a pattern would indicate certain males are more likely to crop-forage. This information is important for developing animal-centered approaches to mitigating HEC.
Guns are not the primary means of injuring elephants in the KWC, but arrows (especially those that are poisoned) can be lethal to elephants; spears, snares, and poisoned pumpkins are all tactics that have also been used in response to crop-foraging [70,71]. As we were unable to ascertain the exact causes of the scars we observed, and because there is a limited understanding of wound healing in elephants [72], future work should describe how often scars are obtained from various sources (i.e., from conspecifics, the physical environment, and negative interactions with humans) and the progression of scarring in wild elephants. This is impetus for further research to obtain a more complete profile of the prevalence of injuries. Such research would help to inform conservation and management strategies for free-ranging elephants and determine the consequences for elephant health.
While the issue of HEC is complex and should be informed by research from a range of disciplines, there is particular value in adopting an animal-centered approach to understanding some of the drivers of this conflict [73]. We have justified our presumptions on the source of scars in this population with knowledge of elephant behavior. Thus, our study is distinctly useful in this regard, as scars may act as a real-time, adaptive method with which we can monitor HEC with an understanding of elephant behavior and ecology. For example, male elephants regularly undergo a heightened sexual state of “musth”, which makes males more attractive as mates. However, musth requires males to maintain a high enough body condition to sustain this state [74,75]. Thus, males must balance an inherent trade-off: a male elephant may opt to forage so that it can prolong musth, but at the cost of acquiring mating opportunities [49]. Human crops are calorically dense and may act as a readily available, nutritionally rich food source for these males, who in turn may be prone to take risks to acquire these resources. The results of this study seem to support this paradigm, as male elephants were more likely to have scars than females, and older males (who appear to be preferred as mates over younger males [76,77]) had more scars than younger males. However, if scars were more prevalent among females in a population than what we found, then it is possible that management approaches would need to be adapted accordingly. For example, females travel in larger groups than males, and these groups include offspring [78,79]. Additionally, family groups tend to move less per day and stay in one region longer than adult males [80,81,82,83]. Thus, crop-foraging by the same family groups could be an ongoing issue compared to males that may come and go from particular farms. Indeed, two groups of females with some of their calves have already been observed crop-foraging near Rukinga Ranch [84], demonstrating the potential for variation in local populations. While the majority of the communities around the KWC desire to coexist with elephants, many members feel as if local wildlife officials are not able to adequately control crop-foraging [85]. Observing scars can help guide management strategies to conserve endangered African savanna elephants, but ultimately management practices will also need to be informed by a sound understanding of how individual and family groups of elephants behave and navigate human-dominated landscapes.
5. Conclusions
Our study described the demographic patterns of scars observed on African savanna elephants in the KWC in southeastern Kenya. Most of the scars we observed were located on the body, and we surmise that these resulted from injuries during negative interactions with humans. Thus, we suggest that these scars reflect trends in HEC. Our study makes reasonable assumptions as to the origins of scars, yet points to the gap in knowledge of how elephants acquire injuries from various sources in the wild. However, our results indicate, as expected, that adult male elephants had more scars than adult females, demonstrating that males are more likely to engage in crop-foraging. During these interactions, humans may directly retaliate with activities such as shooting elephants with arrows or spears in the KWC, and with bullets in other areas. Furthermore, prime males (35–54 years) had more scars than younger adult males (20–34 years), indicating that these retaliatory activities were not necessarily effective in curbing crop-foraging among the male elephants in this area. Interestingly, we found no evidence of scars on males or females in the 10–19 year age class. To validate the methods proposed in this study, there are opportunities for further research to describe how scars occur in elephants, how they heal and persist among elephants, and the threat posed from these injuries to elephant conservation priorities.
Author Contributions
Conceptualization, L.V.H., C.A.L. and B.A.S.; methodology, L.V.H. and B.A.S.; formal analysis, L.V.H.; investigation, L.V.H. and B.A.S.; resources, L.V.H. and B.A.S.; data curation, L.V.H.; writing—original draft preparation, L.V.H. and C.A.L.; writing—review and editing, L.V.H., C.A.L. and B.A.S.; visualization, L.V.H. and C.A.L.; supervision, B.A.S.; project administration, L.V.H. and B.A.S.; funding acquisition, L.V.H. and B.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
Major funding for this research was provided by Auburn University, The Earthwatch Institute, The Elephant Managers Association, The International Elephant Foundation, The Richard Lounsbery Foundation, and Western Kentucky University.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Western Kentucky University (protocol designation 16-02 and 3/31/16).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding authors.
Acknowledgments
We would like to thank Mwangi Githiru, Simon Kasaine, Bernard Amakobe, and the staff and rangers of our partners at Wildlife Works, without whom this work would not be possible. We also give special thanks to the Earthwatch volunteers teams, Sophia Corde, and Keith Hellyer for his insights from the air over Tsavo.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Manfredo, M.J. Essays on human–wildlife conflict 10 years after the Durban World Parks Congress: An introduction. Hum. Dimens. Wildl. 2015, 20, 285–288. [Google Scholar] [CrossRef]
- Soulsbury, C.D.; White, P.C.L. Human-wildlife interactions in urban Areas: A review of conflicts, benefits and opportunities. Wildl. Res. 2015, 42, 541–553. [Google Scholar] [CrossRef]
- Pooley, S.; Bhatia, S.; Vasava, A. Rethinking the study of human-wildlife coexistence. Conserv. Biol. 2020, 35, 784–793. [Google Scholar] [CrossRef]
- Hill, C.M. Perspectives of “conflict” at the wildlife–agriculture boundary: 10 years on. Hum. Dimens. Wildl. 2015, 20, 296–301. [Google Scholar] [CrossRef]
- IUCN SSC HWCTF. What Is Human-Wildlife Conflict? Briefing Paper by the IUCN SSC Human-Wildlife Conflict Task Force; IUCN SSC HWCTF: Gland, Swtizerland, 2020; Available online: www.hwctf.org.2020 (accessed on 15 December 2022).
- Blackwell, B.F.; DeVault, T.L.; Fernández-Juricic, E.; Gese, E.M.; Gilbert-Norton, L.; Breck, S.W. No single solution: Application of behavioural principles in mitigating human–wildlife conflict. Anim. Behav. 2016, 120, 245–254. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Fantle-Lepczyk, J.E.; Misajon, K.; Hu, D.; Duffy, D.C. Long-term history of vehicle collisions on the endangered nēnē (Branta sandvicensis). PLoS ONE 2019, 14, e0210180. [Google Scholar] [CrossRef]
- Dunham, K.M.; Ghiurghi, A.; Cumbi, R.; Urbano, F. Human-wildlife conflict in Mozambique: A national perspective, with emphasis on wildlife attacks on humans. Oryx 2010, 44, 185–193. [Google Scholar] [CrossRef]
- Acharya, K.P.; Paudel, P.K.; Neupane, P.R.; Köhl, M. Human-wildlife conflicts in Nepal: Patterns of human fatalities and injuries caused by large mammals. PLoS ONE 2016, 11, e0161717. [Google Scholar] [CrossRef]
- Treves, A.; Wallace, R.B.; Naughton-Treves, L.; Morales, A. Co-managing human–wildlife conflicts: A review. Hum. Dimens. Wildl. 2006, 11, 383–396. [Google Scholar] [CrossRef]
- LaDue, C.A.; Eranda, I.; Jayasinghe, C.; Vandercone, R.P.G. Mortality patterns of Asian elephants in a region of human–elephant conflict. J. Wildl. Manag. 2021, 85, 794–802. [Google Scholar] [CrossRef]
- Hill, C.M. Crop-raiding by wild vertebrates: The farmer’s perspective in an agricultural community in western Uganda. Int. J. Pest. Manag. 1997, 43, 77–84. [Google Scholar] [CrossRef]
- Sukumar, R. Ecology of the Asian elephant in southern India. II. Feeding habits and crop raiding patterns. J. Trop. Ecol. 1990, 6, 33–53. [Google Scholar] [CrossRef]
- Warren, Y. Crop-raiding baboons (Papio anubis) and defensive farmers: A West African perspective. West Afr. J. Appl. Ecol. 2008, 14, 1–11. [Google Scholar] [CrossRef]
- Distefano, E. Human-Wildlife Conflict Worldwide: Collection of Case Studies, Analysis of Management Strategies and Good Practices; FAO: Rome, Italy, 2005. [Google Scholar]
- Gilsdorf, J.M.; Hygnstrom, S.E.; VerCauteren, K.C. Use of frightening devices in wildlife damage management. Integ. Pest. Manag. Rev. 2002, 7, 29–45. [Google Scholar] [CrossRef]
- Nyhus, P.J. Human–wildlife conflict and coexistence. Annu. Rev. Environ. Resour. 2016, 41, 143–171. [Google Scholar] [CrossRef]
- Dickman, A.J.; Hazzah, L. Money, myths and man-eaters: Complexities of human-wildlife conflict. In Problematic Wildlife: A Cross-Disciplinary Approach; Angelici, F.M., Ed.; Springer Nature: London, UK, 2015; pp. 1–603. [Google Scholar]
- IPCC. Climate Change 2022-Impacts Adaptation and Vulnerability, Summary for Policymakers; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2022. [Google Scholar]
- Wittemyer, G.; Northrup, J.M.; Blanc, J.; Douglas-Hamilton, I.; Omondi, P.; Burnham, K.P. Illegal killing for ivory drives global decline in African elephants. Proc. Natl. Acad. Sci. USA 2014, 111, 13117–13121. [Google Scholar] [CrossRef]
- Douglas-Hamilton, I. African elephants: Population trends and their causes. Oryx 1987, 21, 11–24. [Google Scholar] [CrossRef]
- Hoare, R. African elephants and humans in conflict: The outlook for co-existence. Oryx 2000, 34, 34–38. [Google Scholar] [CrossRef]
- Di Minin, E.; Slotow, R.; Fink, C.; Bauer, H.; Packer, C.A. Pan-African spatial assessment of human conflicts with lions and elephants. Nat. Commun. 2021, 12, 2978. [Google Scholar] [CrossRef]
- Chiyo, P.I.; Cochrane, E.P.; Naughton, L.; Basuta, G.I. Temporal patterns of crop raiding by elephants: A response to changes in forage quality or crop availability? Afr. J. Ecol. 2005, 43, 48–55. [Google Scholar] [CrossRef]
- Tiller, L.N.; Humle, T.; Amin, R.; Deere, N.J.; Lago, B.O.; Leader-Williams, N.; Sinoni, F.K.; Sitati, N.; Walpole, M.; Smith, R.J. Changing seasonal, temporal and spatial crop-raiding trends over 15 years in a human-elephant conflict hotspot. Biol. Conserv. 2021, 254, 108941. [Google Scholar] [CrossRef]
- Raphela, T.D.; Pillay, N. Explaining the effect of crop-raiding on food security of subsistence farmers of KwaZulu Natal, South Africa. Front. Sustain. Food. Syst. 2021, 5, 687177. [Google Scholar] [CrossRef]
- Salerno, J.; Stevens, F.R.; Gaughan, A.E.; Hilton, T.; Bailey, K.; Bowles, T.; Cassidy, L.; Mupeta-Muyamwa, P.; Biggs, D.; Pricope, N.; et al. Wildlife impacts and changing climate pose compounding threats to human food security. Curr. Biol. 2021, 31, 5077–5085.e6. [Google Scholar] [CrossRef]
- Marshall, K.; White, R.; Fischer, A. Conflicts between humans over wildlife management: On the diversity of stakeholder attitudes and implications for conflict management. Biodivers. Conserv. 2007, 16, 3129–3146. [Google Scholar] [CrossRef]
- Naughton-Treves, L.; Treves, A. Socio-ecological factors shaping local support for wildlife: Crop-raiding by elephants and other wildlife in Africa. In People and Wildlife: Conflict or Coexistence? Woodroffe, R., Thirgood, S., Rabinowitz, A., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 252–277. [Google Scholar]
- Kagwa, S. Spatial Distribution of Human Elephant Conflict (HEC) and Characterization of Crop-Raiding Elephants in Kasigau Region, Kenya. Master’s Thesis, Western Kentucky University, Bowling Green, KY, USA, 2011. [Google Scholar]
- Litoroh, M.; Omondi, P.; Kock, R.; Amin, R. Conservation and Management Strategy for the Elephant in Kenya, 2012–2021; Kenya Wildlife Service: Nairoboi, Kenya, 2012.
- Githiru, M.; Mutwiwa, U.; Kasaine, S.; Schulte, B. A spanner in the works: Human–elephant conflict complicates the food—water—energy nexus in drylands of Africa. Front. Environ. Sci. 2017, 5, 69. [Google Scholar] [CrossRef]
- Mukeka, J.M.; Ogutu, J.O.; Kanga, E.; Roskaft, E. Characteristics of human-wildlife conflicts in Kenya: Examples of Tsavo and Maasai Mara regions. Environ. Nat. Res. Res. 2018, 8, 148. [Google Scholar] [CrossRef]
- Von Hagen, R.L.; Kasaine, S.; Githiru, M.; Amakobe, B.; Mutwiwa, U.N.; Schulte, B.A. Metal strip fences for preventing African elephant (Loxodonta africana) crop foraging in the Kasigau Wildlife Corridor, Kenya. Afr. J. Ecol. 2020, 59, 293–298. [Google Scholar] [CrossRef]
- King, L.E.; Douglas-Hamilton, I.; Vollrath, F. Beehive fences as effective deterrents for crop-raiding elephants: Field trials in northern Kenya. Afr. J. Ecol. 2011, 49, 431–439. [Google Scholar] [CrossRef]
- Karidozo, M.; Osborn, F. Community based conflict mitigation trials: Results of field tests of chilli as an elephant deterrent. J. Biodivers. Endanger. Species 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Sitati, N.W.; Walpole, M.J. Assessing farm-based measures for mitigating human-elephant conflict in Transmara District, Kenya. Oryx 2006, 40, 279. [Google Scholar] [CrossRef]
- Von Hagen, R.L.; Schulte, B.A.; Steury, T.D.; Dunning, K.; Githiru, M.; Zohdy, S.; Lepczyk, C.A. The need to identify and reach stakeholders trying to reduce African elephant crop raiding. Conserv. Sci. Pract. 2023. In review. [Google Scholar]
- Obanda, V.; Ndeereh, D.; Mijele, D.; Lekolool, I.; Chege, S.; Gakuya, F.; Omondi, P. Injuries of free ranging African elephants (Loxodonta africana africana) in various ranges of Kenya. Pachyderm 2008, 44, 54–58. [Google Scholar]
- Waweru, J.; Omondi, P.; Ngene, S.; Mukeka, J.; Wanyonyi, E.; Ngoru, B.; Mwiu, S.; Muteti, D.; Lala, F.; Kariuki, L.; et al. National Wildlife Census 2021 Report; Kenya Wildlife Service: Nairoboi, Kenya, 2021.
- Ngene, S.; Lala, F.; Nzisa, M.; Kimitei, K.; Mukeka, J.; Kiambi, S.; Davidson, Z.; Bakari, S.; Lyimo, E.; Khayale, C.; et al. Aerial Total Count of Elephants, Buffalo and Giraffe in the Tsavo-Mkomazi Ecosystem; Kenya Wildlife Service: Nairobi, Kenya, 2017.
- Omondi, P.; Bitok, E.K.; Mukeka, J.; Mayienda, R.M.; Litoroh, M. Total Aerial Count of Elephants and Other Large Mammal Species of Tsavo/Mkomazi Ecosystem; Kenya Wildlife Service: Nairobi, Kenya, 2008.
- Githiru, M. Personal Communication; Wildlife Works: Nairobi, Kenya, 2022. [Google Scholar]
- Henley, M.D. Illustration and Guide to Aging Animals within a Family Unit; Unpublished Guide to the Associated Private Nature Reserves; Save the Elephants: Nairobi, Kenya, 2008. [Google Scholar]
- Moss, C.J. Getting to know a population. In Studying Elephants; Kangwana, K., Ed.; African Wildlife Foundation: Nairobi, Kenya, 1996; pp. 58–74. [Google Scholar]
- Bedetti, A.; Greyling, C.; Paul, B.; Blondeau, J.; Clark, A.; Malin, H.; Horne, J.; Makukule, R.; Wilmot, J.; Eggeling, T.; et al. System for elephant ear-pattern knowledge (SEEK) to identify individual African elephants. Pachyderm 2020, 61, 63–77. [Google Scholar]
- Henley, M.D.; Henley, S.R. Population Dynamics and Elephant Movements within the Associated Private Nature Reserves (APNR) Adjoining the Kruger National Park; Unpublished December Progress Report to the Associated Private Nature Reserves. Appendix 3 (53pp); Save the Elephants: Nairobi, Kenya, 2005; Available online: http://umbabat.com/wp-content/uploads/2018/08/Elephant-Aging-Criteria.pdf (accessed on 10 October 2022).
- Loizi, H.; Goodwin, T.E.; Rasmussen, L.E.L.; Whitehouse, A.M.; Schulte, B.A. Sexual dimorphism in the performance of chemosensory investigatory behaviours by African elephants (Loxodonta africana). Behaviour 2009, 146, 373–392. [Google Scholar] [CrossRef]
- LaDue, C.A.; Schulte, B.A.; Kiso, W.K.; Freeman, E.W. Musth and sexual selection in elephants: A review of signaling properties and potential fitness consequences. Behaviour 2021, 159, 207–242. [Google Scholar] [CrossRef]
- Suissa, S.; Shuster, J.J. Exact unconditional sample sizes for the 2 X 2 binomial trial. J. R. Stat. Soc. 1985, 148, 317–327. [Google Scholar] [CrossRef]
- R Core Team. Version 4.0.2. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 15 December 2022).
- Wittemyer, G. Effects of economic downturns on mortality of wild African elephants. Conserv. Biol. 2011, 25, 1002–1009. [Google Scholar] [CrossRef]
- LaDue, C.A.; Vandercone, R.P.G.; Kiso, W.K.; Freeman, E.W. Scars of human–elephant conflict: Patterns inferred from field observations of Asian elephants in Sri Lanka. Wildl. Res. 2021, 48, 540–553. [Google Scholar] [CrossRef]
- Hall-Martin, A.J.; de Boom, H.P.A. Dislocation of the elbow and its social consequences for an African elephant. J. S. Afr. Vet. Assoc. 1979, 50, 19–22. [Google Scholar]
- Givá, N.; Raitio, K. ‘Parks with people’ in Mozambique: Community dynamic responses to human-elephant conflict at Limpopo National Park. J. S. Afr. Stud. 2017, 43, 1199–1214. [Google Scholar] [CrossRef]
- Rohini, C.K.; Aravindan, T.; Das, K.S.A.; Vinayan, P.A. People’s attitude towards wild elephants, forest conservation and human-elephant conflict in Nilambur, Southern Western Ghats of Kerala, India. J. Threat. Taxa 2018, 10, 11710–11716. [Google Scholar] [CrossRef]
- van de Water, A.; Matteson, K. Human-elephant conflict in western Thailand: Socio-economic drivers and potential mitigation strategies. PLoS ONE 2018, 13, e0194736. [Google Scholar] [CrossRef]
- Compaore, A.; Sirima, D.; Hema, E.M.; Doamba, B.; Ajong, S.N.; di Vittorio, M.; Luiselli, L. Correlation between increased human-elephant conflict and poaching of elephants in Burkina Faso (West Africa). Eur. J. Wildl. Res. 2020, 66, 24. [Google Scholar] [CrossRef]
- Kolinski, L.; Milich, K.M. Human-wildlife conflict mitigation impacts community perceptions around Kibale National Park, Uganda. Diversity 2021, 13, 145. [Google Scholar] [CrossRef]
- Thant, Z.M.; May, R.; Røskaft, E. Effect of human–elephant conflict on local attitudes toward the conservation of wild Asian elephants in Myanmar. Hum. Dimens. Wildl. 2022. [Google Scholar] [CrossRef]
- Hoare, R.E. Determinants of human-elephant conflict in a land-use mosaic. J. App. Ecol. 1999, 36, 689–700. [Google Scholar] [CrossRef]
- Osborn, F.V. Seasonal variation of feeding patterns and food selection by crop-raiding elephants in Zimbabwe. Afr. J. Ecol. 2004, 42, 322–327. [Google Scholar] [CrossRef]
- Chiyo, P.I.; Cochrane, E.P. Population structure and behaviour of crop-raiding elephants in Kibale National Park, Uganda. Afr. J. Ecol. 2005, 43, 233–241. [Google Scholar] [CrossRef]
- Ahlering, M.A.; Millspaugh, J.J.; Woods, R.J.; Western, D.; Eggert, L.S. Elevated levels of stress hormones in crop-raiding male elephants. Anim. Conserv. 2010, 14, 124–130. [Google Scholar] [CrossRef]
- Chiyo, P.I.; Moss, C.J.; Alberts, S.C. The influence of life history milestones and association networks on crop-raiding behavior in male African elephants. PLoS ONE 2012, 7, e31382. [Google Scholar] [CrossRef]
- Chiyo, P.I.; Wilson, J.W.; Archie, E.A.; Lee, P.C.; Moss, C.J.; Alberts, S.C. The influence of forage, protected areas, and mating prospects on grouping patterns of male elephants. Behav. Ecol. 2014, 25, 1494–1504. [Google Scholar] [CrossRef]
- Von Hagen, R.L. An Evaluation of Deterrent Methods Utilized to Prevent Crop Raiding by African Elephants (Loxodonta africana) in the Kasigau Wildlife Corridor, Kenya. Master’s Thesis, Western Kentucky University, Bowling Green, KY, USA, 2018. [Google Scholar]
- Poole, J.; Granli, P. The elephant ethogram: A library of African elephant behaviour. Pachyderm 2020, 62, 105–111. [Google Scholar]
- Olson, D. Elephant Husbandry Resource Guide; Allen Press: Lawrence, KS, USA, 2004. [Google Scholar]
- Kamau, P. Elephants, Local Livelihoods, and Landscape Change in Tsavo, Kenya. Doctoral Dissertation, Louisiana State University, Baton Rouge, LA, USA, 2017. [Google Scholar]
- Smith, R.J.; Kasiki, S.M. A Spatial Analysis of Human-Elephant Conflict in the Tsavo Ecosystem, Kenya; A Report to the African Elephant Specialist Group, Human -Elephant Conflict Task Force of IUCN; IUCN: Gland, Switzerland, 2000.
- Mikota, S.K. Integument system. In Biology, Medicine, and Surgery of Elephants; Fowler, M.E., Mikota, S.K., Eds.; Blackwell Publishing: Ames, IA, USA, 2006; pp. 253–261. [Google Scholar]
- Mumby, H.S.; Plotnik, J.M. Taking the elephants’ perspective: Remembering elephant behavior, cognition and ecology in human-elephant conflict mitigation. Front. Ecol. Evol. 2018, 6, 20. [Google Scholar] [CrossRef]
- Silva, I.D.; Kuruwita, W.Y. Hematology, plasma, and serum biochemistry values in free-ranging elephants (Elephas maximus ceylonicus) in Sri Lanka. J. Zoo Wild. Med. 1993, 24, 434–439. [Google Scholar]
- Dickerman, R.D.; Pernikoff, D.; Zachariah, N.Y.; McConathy, W.J.; Gracy, R.W.; Raven, P.V. Creatinine kinase and lactic dehydrogenase isozyme measurements in male Asian elephants (Elephas maximus) during musth and nonmusth. Clin. Chem. 1994, 40, 989. [Google Scholar]
- Poole, J.H. Signals and assessment in African elephants: Evidence from playback experiments. Anim. Behav. 1999, 58, 185–193. [Google Scholar] [CrossRef]
- Poole, J.H. Announcing intent: The aggressive state of musth in African elephants. Anim. Behav. 1989, 37, 140–152. [Google Scholar] [CrossRef]
- Wittemyer, G.; Getz, W.M. Hierarchical dominance structure and social organization in African elephants, Loxodonta africana. Anim. Behav. 2007, 73, 671–681. [Google Scholar] [CrossRef]
- Wittemyer, G.; Douglas-Hamilton, I.; Getz, W. The socioecology of elephants: Analysis of the processes creating multitiered social structures. Anim. Behav. 2005, 69, 1357–1371. [Google Scholar] [CrossRef]
- Wyatt, J.R.; Eltringham, S.K. The daily activity of the elephant in the Rwenzori National Park, Uganda. Afr. J. Ecol. 1974, 12, 273–289. [Google Scholar] [CrossRef]
- Shannon, G.; Page, B.R.; Mackey, R.L.; Duffy, K.J. Activity budgets and sexual segregation in African elephants (Loxodonta africana). J. Mammal. 2008, 89, 467–476. [Google Scholar] [CrossRef]
- Ngene, S.M.; Skidmore, A.K.; Gils, H.V.; Douglas-Hamilton, I.; Omondi, P. Elephant distribution around a volcanic shield dominated by a mosaic of forest and savanna (Marsabit, Kenya). Afr. J. Ecol. 2009, 47, 234–245. [Google Scholar] [CrossRef]
- Stokke, S.; du Toit, J.T. Sex and size related differences in the dry season feeding patterns of elephants in Chobe National Park, Botswana. Ecography 2000, 23, 70–80. [Google Scholar] [CrossRef]
- Von Hagen, R.L. Personal Observations; Auburn University: Auburn, AL, USA, 2021. [Google Scholar]
- Von Hagen, R.L. Drivers and Consequences of Human-Elephant Interactions in an Agricultural Landscape of Rural Kenya. Doctoral Dissertation, Auburn University, Auburn, AL, USA, 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).