Histopathological Changes and Inflammatory Response in Specific Pathogen-Free (SPF) with Porcine Circovirus Type 3 Infection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and Animals
2.2. Animal Treatment
2.3. Clinical Evaluation
2.4. Sample Collection
2.5. Evaluation of Viremia, Viral Shedding and Detection of PCV3 in Tissues by qRT-PCR Assay
2.6. Histopathology Evaluation
2.7. ELISA of Cytokine
2.8. Statistical Analysis
3. Results
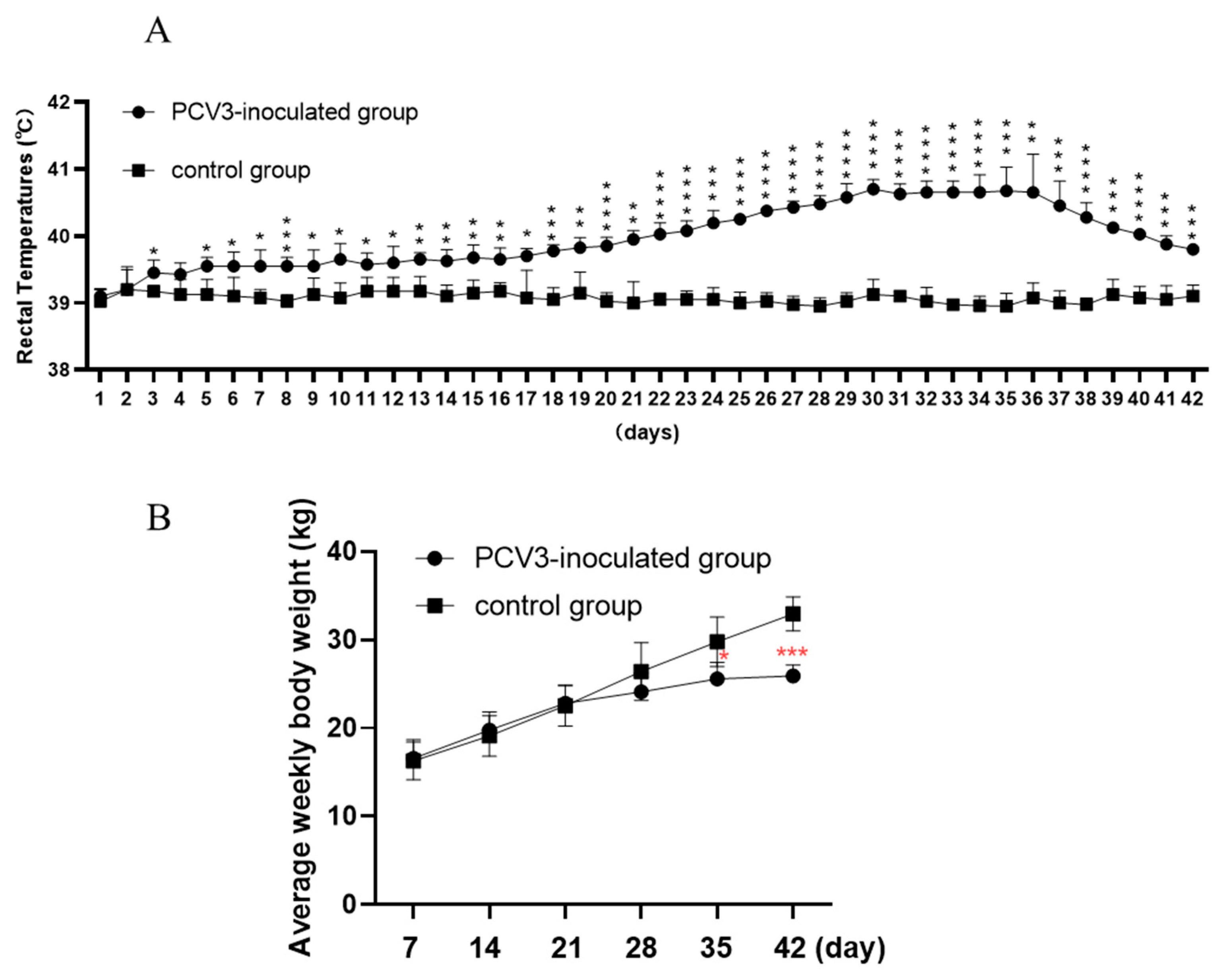
3.1. Clinical Signs Induced by PCV3 Infection
3.2. PCV3 Produces Persistent Viremia
3.3. PCV3 Produces Shedding in Nasal and Faecal Secretions
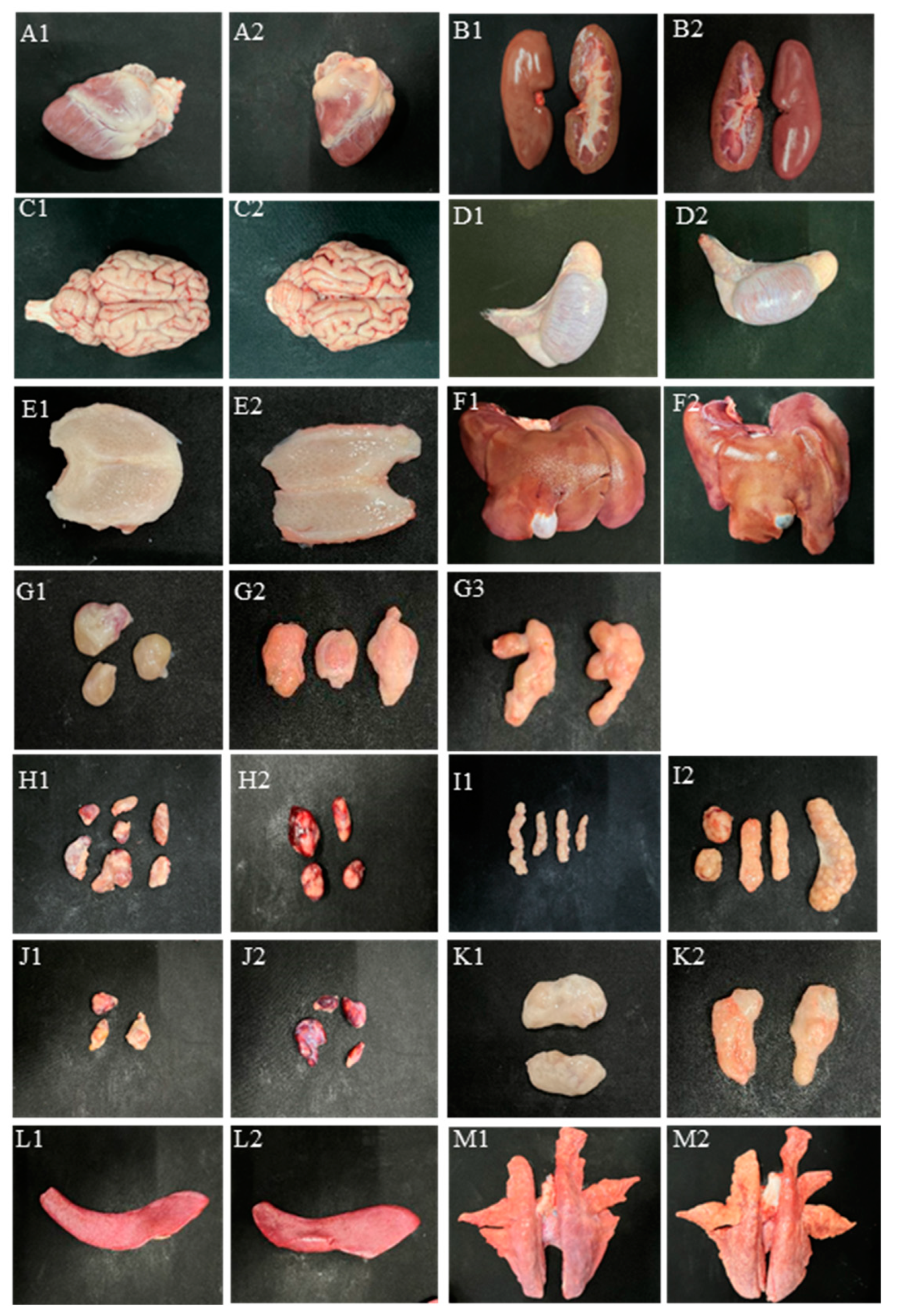
3.4. Gross Lesions Caused by PCV3 Infection
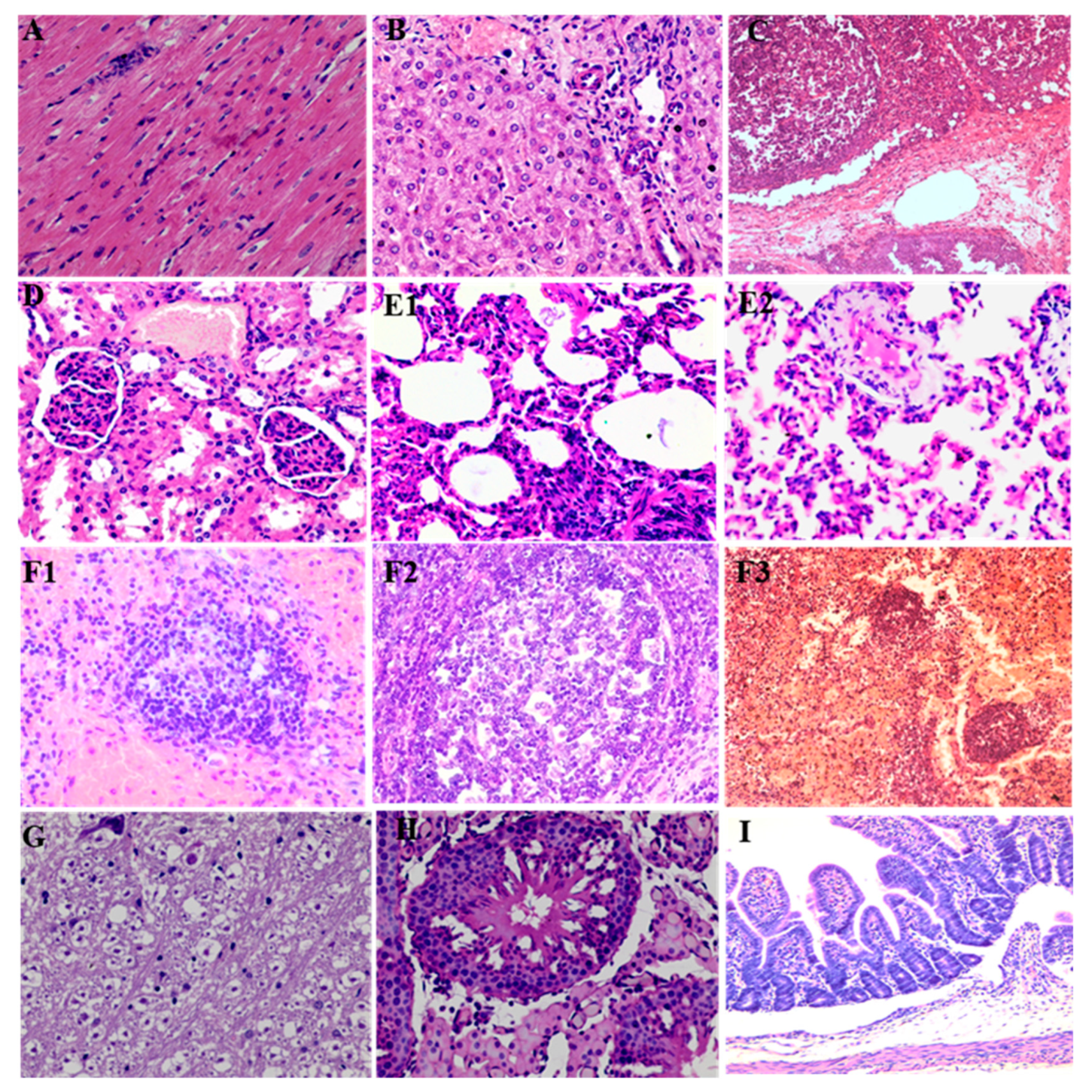
3.5. Histopathology Change Caused by PCV3 Infection
3.6. The PCV3 in Various Tissues and Organs
3.7. The Effect of PCV3 Infection on Cytokine Expression Levels in Serum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, L.; Sun, W.; Lu, H.; Tian, M.; Xie, C.; Zhao, G.; Han, J.; Wang, W.; Zheng, M.; Du, R.; et al. Genetic variation analysis of PCV1 strains isolated from Guangxi Province of China in 2015. BMC Vet. Res. 2018, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.J. Porcine circovirus type 2 (PCV2): Pathogenesis and interaction with the immune system. Annu. Rev. Anim. Biosci. 2013, 1, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Langohr, I. Current state of knowledge on porcine circovirus type 2-associated lesions. Vet. Pathol. 2013, 50, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Palinski, R.; Piñeyro, P.; Shang, P.; Yuan, F.; Guo, R.; Fang, Y.; Byers, E.; Hause, B.M. A Novel Porcine Circovirus Distantly Related to Known Circoviruses Is Associated with Porcine Dermatitis and Nephropathy Syndrome and Reproductive Failure. J. Virol. 2017, 91, e01879-16. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Giannitti, F.; Rossow, S.; Marthaler, D.; Knutson, T.P.; Li, L.; Deng, X.; Resende, T.; Vannucci, F.; Delwart, E. Detection of a novel circovirus PCV3 in pigs with cardiac and multi-systemic inflammation. Virol. J. 2016, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Ku, X.; Chen, F.; Li, P.; Wang, Y.; Yu, X.; Fan, S.; Qian, P.; Wu, M.; He, Q. Identification and genetic characterization of porcine circovirus type 3 in China. Transbound. Emerg. Dis. 2017, 64, 703–708. [Google Scholar] [CrossRef]
- Fu, X.; Fang, B.; Ma, J.; Liu, Y.; Bu, D.; Zhou, P.; Wang, H.; Jia, K.; Zhang, G. Insights into the epidemic characteristics and evolutionary history of the novel porcine circovirus type 3 in southern China. Transbound. Emerg. Dis. 2018, 65, e296–e303. [Google Scholar] [CrossRef]
- Faccini, S.; Barbieri, I.; Gilioli, A.; Sala, G.; Gibelli, L.R.; Moreno, A.; Sacchi, C.; Rosignoli, C.; Franzini, G.; Nigrelli, A. Detection and genetic characterization of Porcine circovirus type 3 in Italy. Transbound. Emerg. Dis. 2017, 64, 1661–1664. [Google Scholar] [CrossRef]
- Sun, J.; Wei, L.; Lu, Z.; Mi, S.; Bao, F.; Guo, H.; Tu, C.; Zhu, Y.; Gong, W. Retrospective study of porcine circovirus 3 infection in China. Transbound. Emerg. Dis. 2018, 65, 607–613. [Google Scholar] [CrossRef]
- Tochetto, C.; Lima, D.A.; Varela, A.P.M.; Loiko, M.R.; Paim, W.P.; Scheffer, C.M.; Herpich, J.I.; Cerva, C.; Schmitd, C.; Cibulski, S.P.; et al. Full-Genome Sequence of Porcine Circovirus type 3 recovered from serum of sows with stillbirths in Brazil. Transbound. Emerg. Dis. 2018, 65, 5–9. [Google Scholar] [CrossRef]
- Yuzhakov, A.G.; Raev, S.A.; Alekseev, K.P.; Grebennikova, T.V.; Verkhovsky, O.A.; Zaberezhny, A.D.; Aliper, T.I. First detection and full genome sequence of porcine circovirus type 3 in Russia. Virus Genes 2018, 54, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Klaumann, F.; Franzo, G.; Sohrmann, M.; Correa-Fiz, F.; Drigo, M.; Núñez, J.I.; Sibila, M.; Segalés, J. Retrospective detection of Porcine circovirus 3 (PCV-3) in pig serum samples from Spain. Transbound. Emerg. Dis. 2018, 65, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Stadejek, T.; Woźniak, A.; Miłek, D.; Biernacka, K. First detection of porcine circovirus type 3 on commercial pig farms in Poland. Transbound. Emerg. Dis. 2017, 64, 1350–1353. [Google Scholar] [CrossRef]
- Kwon, T.; Yoo, S.J.; Park, C.K.; Lyoo, Y.S. Prevalence of novel porcine circovirus 3 in Korean pig populations. Vet. Microbiol. 2017, 207, 178–180. [Google Scholar] [CrossRef]
- Zheng, S.; Wu, X.; Zhang, L.; Xin, C.; Liu, Y.; Shi, J.; Peng, Z.; Xu, S.; Fu, F.; Yu, J.; et al. The occurrence of porcine circovirus 3 without clinical infection signs in Shandong Province. Transbound. Emerg. Dis. 2017, 64, 1337–1341. [Google Scholar] [CrossRef]
- Mora-Díaz, J.; Piñeyro, P.; Shen, H.; Schwartz, K.; Vannucci, F.; Li, G.; Arruda, B.; Giménez-Lirola, L. Isolation of PCV3 from Perinatal and Reproductive Cases of PCV3-Associated Disease and In Vivo Characterization of PCV3 Replication in CD/CD Growing Pigs. Viruses 2020, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Temeeyasen, G.; Lierman, S.; Arruda, B.L.; Main, R.; Vannucci, F.; Gimenez-Lirola, L.G.; Piñeyro, P.E. Pathogenicity and immune response against porcine circovirus type 3 infection in caesarean-derived, colostrum-deprived pigs. J. Gen. Virol. 2021, 102, 001502. [Google Scholar] [CrossRef]
- Wang, M.; Yu, Y.; Wu, J.; Meng, F.; Tang, Y.; Wang, S.; Wang, Y.; Cui, H.; He, X.; Tu, Y.; et al. Pathogenic Characterization of a Porcine Circovirus Type 3 Isolate from Heilongjiang, China. Dis. Mrk. 2021, 2021, 9434944. [Google Scholar] [CrossRef]
- Oh, T.; Chae, C. First isolation and genetic characterization of porcine circovirus type 3 using primary porcine kidney cells. Vet. Microbiol. 2020, 241, 108576. [Google Scholar] [CrossRef]
- Shen, H.; Liu, X.; Zhang, P.; Wang, L.; Liu, Y.; Zhang, L.; Liang, P.; Song, C. Genome characterization of a porcine circovirus type 3 in South China. Transbound. Emerg. Dis. 2018, 65, 264–266. [Google Scholar] [CrossRef]
- Franzo, G.; Tucciarone, C.M.; Drigo, M.; Cecchinato, M.; Martini, M.; Mondin, A.; Menandro, M.L. First report of wild boar susceptibility to Porcine circovirus type 3: High prevalence in the Colli Euganei Regional Park (Italy) in the absence of clinical signs. Transbound. Emerg. Dis. 2018, 65, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.L.; Zhang, Y.; Zhao, Y.; Zheng, H.H.; Han, H.Y.; Zhang, H.X.; Chen, H.Y.; Yang, M.F.; Zheng, L.L. Detection and phylogenetic analysis of porcine circovirus type 3 in central China. Transbound. Emerg. Dis. 2018, 65, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, M.; Temeeyasen, G.; Piñeyro, P.E. Five years of porcine circovirus 3: What have we learned about the clinical disease, immune pathogenesis, and diagnosis. Virus Res. 2022, 314, 198764. [Google Scholar] [CrossRef] [PubMed]
- Klaumann, F.; Correa-Fiz, F.; Sibila, M.; Núñez, J.I.; Segalés, J. Infection dynamics of porcine circovirus type 3 in longitudinally sampled pigs from four Spanish farms. Vet. Rec. 2019, 184, 619. [Google Scholar] [CrossRef]
- Kedkovid, R.; Woonwong, Y.; Arunorat, J.; Sirisereewan, C.; Sangpratum, N.; Lumyai, M.; Kesdangsakonwut, S.; Teankum, K.; Jittimanee, S.; Thanawongnuwech, R. Porcine circovirus type 3 (PCV3) infection in grower pigs from a Thai farm suffering from porcine respiratory disease complex (PRDC). Vet. Microbiol. 2018, 215, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Eddicks, M.; Müller, M.; Fux, R.; Ritzmann, M.; Stadler, J. Detection of porcine circovirus type 3 DNA in serum and semen samples of boars from a German boar stud. Vet. J. 2022, 279, 105784. [Google Scholar] [CrossRef]
- Hou, L.; Wang, J.; Zhang, W.; Quan, R.; Wang, D.; Zhu, S.; Jiang, H.; Wei, L.; Liu, J. Dynamic Alterations of Gut Microbiota in Porcine Circovirus Type 3-Infected Piglets. Front. Microbiol. 2020, 11, 1360. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, D.; Wang, J.; Zhu, S.; She, R.; Ren, X.; Tian, J.; Quan, R.; Hou, L.; Li, Z.; et al. Induction of Porcine Dermatitis and Nephropathy Syndrome in Piglets by Infection with Porcine Circovirus Type 3. J. Virol. 2019, 93, e02045-18. [Google Scholar] [CrossRef]
- Nieto, D.; Aramouni, M.; Sibila, M.; Fraile, L.; Kekarainen, T.; Segalés, J. Lack of effect of piglet vaccination against Porcine circovirus type 2 (PCV2) on serum viral loads of Torque teno sus virus 2 (TTSuV2). Vet. Microbiol. 2012, 157, 8–12. [Google Scholar] [CrossRef]
- Sibila, M.; Martínez-Guinó, L.; Huerta, E.; Mora, M.; Grau-Roma, L.; Kekarainen, T.; Segalés, J. Torque teno virus (TTV) infection in sows and suckling piglets. Vet. Microbiol. 2009, 137, 354–358. [Google Scholar] [CrossRef]

| The PCV3 Genome Copies of Serum in Two Groups (Copies/μL) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days of post inoculation | 0 | 3 | 7 | 10 | 14 | 17 | 21 | 23 | 25 | 27 | 29 | 31 | 33 | 35 | 37 | 39 | 41 |
| control group (All piglets) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| PCV3-inoculated groups No.1 | - | - | - | - | - | 102.05 | 102.24 | 102.48 | 102.81 | 103.13 | 103.55 | 103.47 | 103.85 | 104.17 | 104.16 | 104.17 | 104.12 |
| PCV3-inoculated groups No.2 | - | - | - | - | - | - | 102.03 | 102.22 | 102.42 | 102.59 | 103.02 | 103.25 | 104.17 | 104.25 | 104.08 | 104.09 | 104.16 |
| PCV3-inoculated groups No.3 | - | - | - | - | - | - | 102.26 | 102.49 | 102.69 | 102.83 | 103.26 | 103.40 | 104.09 | 104.46 | 104.21 | 104.02 | 104.12 |
| PCV3-inoculated groups No.4 | - | - | - | - | - | - | - | 102.07 | 102.69 | 103.11 | 103.16 | 103.26 | 104.14 | 104.24 | 104.17 | 104.11 | 103.87 |
| Percentage of positive animals | 0% | 0% | 0% | 0% | 0% | 25% | 75% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| The PCV3 Genome Copies of Nasal Swabs in Two Groups (Copies/μL) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days of post inoculation | 0 | 3 | 7 | 10 | 14 | 17 | 21 | 23 | 25 | 27 | 29 | 31 | 33 | 35 | 37 | 39 | 41 |
| control group (All piglets) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| PCV3-inoculated groups No.1 | - | - | - | - | - | - | 101.87 | 102.08 | 102.22 | 102.47 | 102.68 | 102.99 | 102.90 | 102.35 | - | - | - |
| PCV3-inoculated groups No.2 | - | - | - | - | - | - | 101.76 | - | - | - | 102.30 | 102.73 | 102.59 | - | - | - | - |
| PCV3-inoculated groups No.3 | - | - | - | - | - | - | - | 101.85 | 102.10 | - | 102.56 | 102.81 | 102.86 | 102.45 | - | - | - |
| PCV3-inoculated groups No.4 | - | - | - | - | - | - | - | - | 102.08 | 102.38 | 102.65 | 102.93 | - | 102.39 | - | - | - |
| The PCV3 Genome Copies of Fecal Swabs in Two Groups (Copies/μL) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days of post inoculation | 0 | 3 | 7 | 10 | 14 | 17 | 21 | 23 | 25 | 27 | 29 | 31 | 33 | 35 | 37 | 39 | 41 |
| control group (All piglets) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| PCV3-inoculated groups No.1 | - | - | - | - | - | - | 101.96 | 102.13 | 102.07 | 101.86 | - | - | - | 101.96 | - | 101.78 | - |
| PCV3-inoculated groups No.2 | - | - | - | - | - | - | - | 101.88 | - | - | 101.96 | - | 101.84 | - | - | - | - |
| PCV3-inoculated groups No.3 | - | - | - | - | - | - | - | - | 101.90 | 102.00 | - | 101.86 | 101.90 | - | - | - | - |
| PCV3-inoculated groups No.4 | - | - | - | - | - | - | - | 101.94 | - | - | 101.80 | 102.00 | - | - | - | 101.93 | - |
| 42 Days Infection of PCV3 | ||||
|---|---|---|---|---|
| Tissues | The Genome Copies in PCV3-Inoculated Groups (Copies/μL) | Percentage of Positive Tissues in 4 Piglets of PCV3-Inoculated Groups | Control Group | Percentage of Positive Tissues in 4 Piglets of Control Group |
| Heart | 102.35 ± 64.19 | 50% | - | 0% |
| Liver | 103.28 ± 145.49 | 50% | - | 0% |
| Spleen | 103.88 ± 609.79 | 100% | - | 0% |
| Lung | 103.38 ± 93.64 | 50% | - | 0% |
| Kidney | 102.35 ± 140.27 | 50% | - | 0% |
| Tonsil | 103.47 ± 1561.79 | 50% | - | 0% |
| Inguinal lymph node | 104.12 ± 898.445 | 50% | - | 0% |
| Submaxillary lymph node | 104.48 ± 7072.02 | 100% | - | 0% |
| Mesenteric lymph node | 104.35 ± 8088.98 | 50% | - | 0% |
| Hilar lymph node | 104.18 ± 7215.42 | 100% | - | 0% |
| Liver portal lymph node | 104.19 ± 4424.75 | 75% | - | 0% |
| Brain | - | 0% | - | 0% |
| small intestine | - | 0% | - | 0% |
| Testis | - | 0% | - | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Zhu, S.; Zhu, L.; Jian, Z.; Zhou, Y.; Li, F.; Deng, L.; Deng, J.; Deng, Y.; Lai, S.; et al. Histopathological Changes and Inflammatory Response in Specific Pathogen-Free (SPF) with Porcine Circovirus Type 3 Infection. Animals 2023, 13, 530. https://doi.org/10.3390/ani13030530
Deng H, Zhu S, Zhu L, Jian Z, Zhou Y, Li F, Deng L, Deng J, Deng Y, Lai S, et al. Histopathological Changes and Inflammatory Response in Specific Pathogen-Free (SPF) with Porcine Circovirus Type 3 Infection. Animals. 2023; 13(3):530. https://doi.org/10.3390/ani13030530
Chicago/Turabian StyleDeng, Huidan, Song Zhu, Ling Zhu, Zhijie Jian, Yuancheng Zhou, Fengqin Li, Lishuang Deng, Junliang Deng, Youtian Deng, Siyuan Lai, and et al. 2023. "Histopathological Changes and Inflammatory Response in Specific Pathogen-Free (SPF) with Porcine Circovirus Type 3 Infection" Animals 13, no. 3: 530. https://doi.org/10.3390/ani13030530
APA StyleDeng, H., Zhu, S., Zhu, L., Jian, Z., Zhou, Y., Li, F., Deng, L., Deng, J., Deng, Y., Lai, S., & Xu, Z. (2023). Histopathological Changes and Inflammatory Response in Specific Pathogen-Free (SPF) with Porcine Circovirus Type 3 Infection. Animals, 13(3), 530. https://doi.org/10.3390/ani13030530








