A Scoping Review of Non-Structural Airway Disease as a Cause of Poor Performance in Racehorses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Horse or race speed/time-based measures.
- Measures based on race earnings.
- Measures based on finishing position within race.
- Categorical (yes/no) measures of starting, winning and/or placing in races.
- Count measures of number of starts, wins and/or places.
- Measures of career duration (in days).
- Time to (return to) racing (in days).
- No objective measure of performance.
3. Results
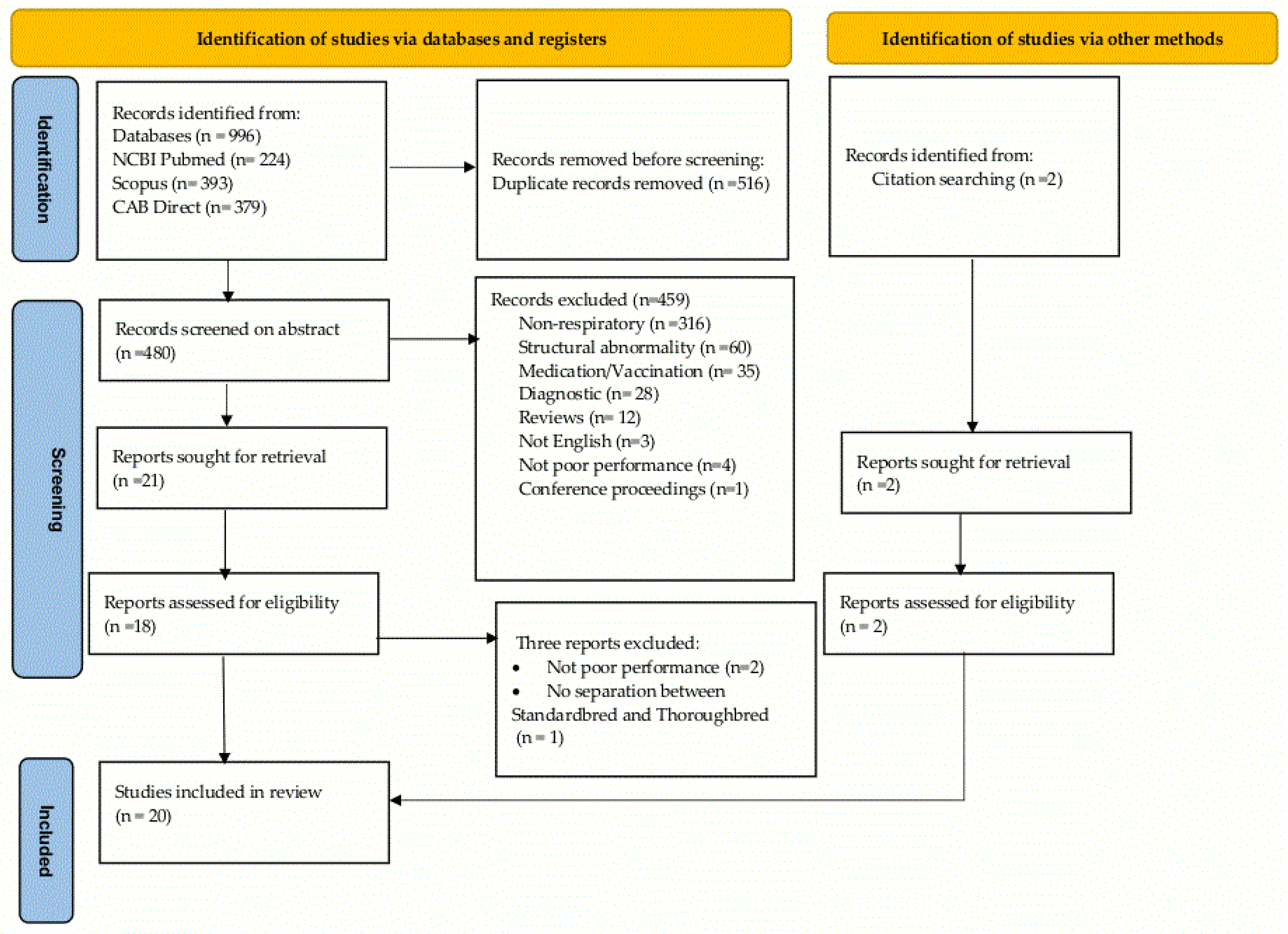
3.1. Search Results
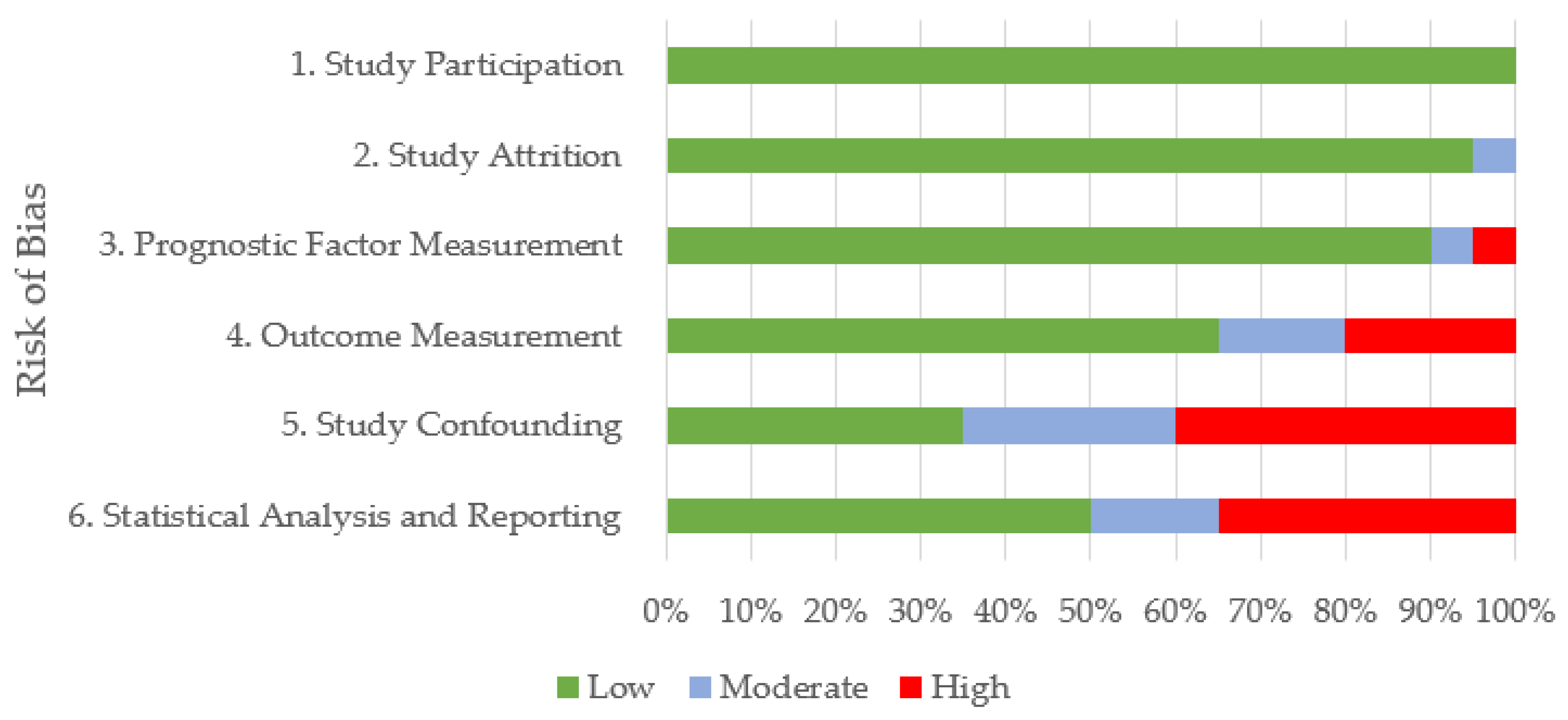
3.2. Quality Appraisal
| Risk of Bias | |||||||
|---|---|---|---|---|---|---|---|
| Focus | Study | 1. Study Participation | 2. Study Attrition | 3. Prognostic Factor Measurement | 4. Outcome Measurement | 5. Study Confounding | 6. Statistical Analysis and Reporting |
| EA | Fogarty and Buckley 1991 [22] | Low | Low | Low | Low | Moderate | Moderate |
| EA | Holcombe et al. 2006 [23] | Low | Low | Moderate | Low | Low | Low |
| EA | Ivester et al. 2018 [24] | Low | Low | Low | Low | Low | Low |
| EA | Salz et al. 2016 [25] | Low | Low | Low | Low | Moderate | Low |
| EA | McKane et al. 1995 [21] | Low | Low | Low | Moderate | High | High |
| EA | Nolen- Walston et al. 2013 [18] | Low | Low | Low | Moderate | High | High |
| EA | Saulez and Gummow 2009 [10] | Low | Low | Low | Low | High | High |
| EA | Allen et al. 2006 [20] | Low | Low | Low | High | High | High |
| EA | Kusano et al. 2008a [19] | Low | Low | Low | High | High | High |
| P | Ainsworth et al. 2000 [34] | Low | Moderate | Low | Low | Low | Low |
| p | Seltzer et al. 1996 [35] | Low | Low | Low | Low | Moderate | Moderate |
| EIPH | Crispe et al. 2017 [26] | Low | Low | Low | Low | Low | Low |
| EIPH | Hinchcliff et al. 2005 [29] | Low | Low | Low | Low | Low | Low |
| EIPH | Morley et al. 2015 [30] | Low | Low | Low | Low | Low | Low |
| EIPH | Sullivan et al. 2015 [28] | Low | Low | Low | Moderate | Moderate | Low |
| EIPH | Preston et al. 2015 [27] | Low | Low | High | High | High | High |
| RA | Couetil et al. 2021 [31] | Low | Low | Low | Low | Low | Low |
| RA | Araneda and Cavada 2022 [33] | Low | Low | Low | Low | Moderate | Moderate |
| RA | Back et al. 2019 [32] | Low | Low | Low | Low | High | Low |
| C | Kusano et al. 2008b [36] | Low | Low | Low | High | High | High |
| Authors | Country | Primary Focus in Relation to Performance | Centre | Study Design | No. of TB Horses | Flat | Jump | Status | Performance |
|---|---|---|---|---|---|---|---|---|---|
| Ainsworth et al. 2000 [34] | USA/ Canada | Pulmonary abscesses: Diagnostic radiographs and ultrasonographic images described and affected regions identified. | Four hospitals. | Retrospective cohort. | 20 | U | U | Medical records of a primary lung abscess. | Y- ii and v (no. of starts and earnings per start preceding and following the horses illness) |
| Allen et al. 2006 [20] | UK | IAD: Three different case definitions of disease are used. Tracheal mucus scored from 0–3. >20% neutrophils in TW. >5% neutrophils in BAL. | One referral clinic. | Retrospective case series. | 91 | Y | Referred for investigation of poor athletic performance. | viii | |
| Araneda and Cavada 2022 [33] | Chile | Atmospheric pollutants (PM10, PM2.5, O3, NO2, NO, SO2, and CO), humidity, and temperature. | One racetrack. | Correlational observational. | 162 | Y | Race winners. | Y- i | |
| Back et al. 2019 [32] | Ireland | Equine rhinitis virus seroconversion detected by complement fixation test. | One yard. | Longitudinal cohort. | 30 | Y | In training. | Y- iv | |
| Couetil et al. 2021 [31] | USA | Equine herpesviruses and equine rhinitis viruses. Virus-specific qPCR. | Five barns. | Prospective observational. | 31 | Y | Racing. | Y- i | |
| Crispe et al. 2017 [26] | Australia | EIPH: tracheobronchoscopic examinations graded 0-4 using a referenced scoring system. | Three racetracks. | Observational cross- sectional. | 1567 | Y | Racing. | Y- i, ii, iii, and iv | |
| Fogarty and Buckley 1991 [22] | Ireland | BAL: neutrophil %, haemosiderophage %, TCFU. Lower airway infection was characterised by a TCFU >40, neutrophil % > 10%, in association with toxic cellular changes, cellular clumping, and increased mucus production. EIPH detection was based on the presence of haemosiderophages with or without intra- or extracellular red blood cells. | One laboratory. | Case control. | 65 + 11 controls | U | U | Total of 65 with severe exercise intolerance during strenuous exercise and 11 having competed successfully. | Y- iv |
| Hinchcliff et al. 2005 [29] | Australia | EIPH: tracheobronchoscopic examinations graded 0–4 using a referenced scoring system. | Four racetracks. | Cross- sectional. | 744 | Y | Racing. | Y- ii and iii | |
| Holcombe et al. 2006 [23] | USA | Tracheal mucus grade (0–4) as described by Gerber et al. 2004 [37], PLH grade (0–4) as described by Raker and Boles 1978 [38] and TW turbidity and differential cytology counts. | One racetrack. | Longitudinal cohort. | 327 | Y | Racing. | Y- iii and iv | |
| Ivester et al. 2018 [24] | USA | BAL differential cytology counts. Mild asthmatics were defined as horses with >5% neutrophils, >2% mast cells, or >1% eosinophils, or any combination thereof. Respirable and inhalable dust, respirable endotoxin, and respirable β-glucan exposure measurements were obtained within one week after racing. | Three race meets. | Prospective cohort. | 64 | Y | Racing. | Y- i, iii, and iv | |
| Kusano et al. 2008a [19] | Japan | Assessment of horses IAD vs. non-IAD. Tracheal aspirates containing >20% neutrophils were considered indicative of IAD. | One training facility. | Cross- sectional. | 76 | Y | Presenting with cough or poor performance. | viii | |
| Kusano et al. 2008b [36] | Japan | Tracheal mucus grades (0–3), tracheal aspirate cytology (> 20% neutrophils diagnostic for IAD) SAA, Fbg and SP-D measurements from coughing vs. non-coughing horses. | One Training facility. | Cross- sectional. | 95 (86 + 9 controls) | Y | Total of 86 presenting with cough or poor performance and 9 controls without respiratory abnormality. | Y- iv, v, and vii | |
| McKane et al. 1995 [21] | Australia | BAL cytology (differential cell counts including neutrophil, erythrocyte, and haemosiderophage percentage and the total nucleated cell concentration) and oxygen saturation of haemoglobin in arterial blood. | One referral clinic. | Case series. | 24 | U | U | Reported poor racing performance. | viii |
| Morley et al. 2015 [30] | South Africa | EIPH: tracheobronchoscopic examinations graded 0-4 using a referenced scoring system. | Five racetracks. | Prospective cross- sectional. | 886 | Y | Racing. | Y- ii, iii, and iv | |
| Nolen- Walston et al 2013 [18] | USA | IAD (now referred to as asthma) subtypes: eosinophilic-mastocytic: ≥0.5% eosinophils, ≥ 2% mast cells or both. Neutrophilic, ≥ 5% neutrophils, and Mixed ≥ 5%.neutrophils as well as ≥ 0.5% eosinophils, ≥ 2% mast cells, or all three. | One referral clinic. | Retrospective case series. | 45 | Y (42) | Y (3) | Examined because of poor performance. | viii |
| Preston et al. 2015 [27] | Hong Kong | EIPH: tracheobronchoscopic examinations. Graded 0–4 using a referenced scoring system. | Three racetracks. | Retrospective cross sectional. | 822 | Y | In training. | Y- v and vi | |
| Salz et al. 2016 [25] | Australia | Tracheal mucus grade (0–4), tracheal blood grade (0–4), and TW differential cytology counts >20% neutrophils was considered to be significant. | One yard. | Cross- sectional. | 155 | Y | In training. | Y- iii | |
| Saulez and Gummow (2009) [10] | South Africa | Laryngeal function grade (1–4), PLH grade (1–4), and tracheal mucus grade (0–5), on the basis of referenced grading systems. | Five racetracks. | Prospective cross- sectional. | 1005 | Y | Racing. | Y- ii and v | |
| Seltzer et al. 1996 [35] | USA | Pleuropneumonia-clinical signs, ultrasonography, and bacteriology. | One hospital. | Retrospective case series. | 70 | U | U | Medical records of pneumonia and pleural effusion | Y- iv Post treatment won≥ 1 race, had earnings, raced without earnings or did not race. |
| Sullivan et al. 2015 [28] | Australia | EIPH: tracheobronchoscopic examinations. Graded 0–4 using a referenced scoring system | Four racetracks. | Prospective longitudinal. | 744 | Y | Racing. | Y- ii, v, and vi |
| Authors | Primary Focus in Relation to Performance | Endoscopy | TW | BAL | Bacterial Culture | Envir. | Blood Gas | Misc. | Statistics |
|---|---|---|---|---|---|---|---|---|---|
| Ainsworth et al. 2000 [34] | Pulmonary abscesses: Diagnostic radiographs and ultrasonographic images described and affected regions identified. | Y | Radiography | Wilcoxon rank sum, Wilcoxon signed ranks, and Fischer exact tests. | |||||
| Allen et al. 2006 [20] | IAD: Three different case definitions of disease are used. Tracheal mucus scored from 0–3. >20% neutrophils in TW. >5% neutrophils in BAL. | Y | Y | Y | Y | Kappa statistics, Spearman rank correlations, and binomial logistic regression analysis. | |||
| Araneda and Cavada 2022 [33] | Atmospheric pollutants (PM10, PM2.5, O3, NO2, NO, SO2, and CO), humidity, and temperature. | Y | Pearson’s correlation co-efficient. | ||||||
| Back et al. 2019 [32] | Equine rhinitis virus seroconversion detected by complement fixation test. | Serology | Chi-squared test. | ||||||
| Couetil et al. 2021 [31] | Equine herpesviruses and equine rhinitis viruses. Virus-specific qPCR. | Y | Y | Y | qPCR | Generalised linear mixed models. | |||
| Crispe et al. 2017 [26] | EIPH: tracheobronchoscopic examinations graded 0-4 using a referenced scoring system. | Y | Linear mixed effects and multiple logistic regression models. Generalised estimating equations were used for analysis of binary responses. | ||||||
| Fogarty and Buckley 1991 [22] | BAL: neutrophil %, haemosiderophage %, TCFU. Lower airway infection was characterised by a TCFU >40, neutrophil % > 10%, in association with toxic cellular changes, cellular clumping, and increased mucus production. EIPH detection was based on the presence of haemosiderophages with or without intra- or extracellular red blood cells. | Y | Y | Y | Y | Student’s t test. | |||
| Hinchcliff et al. 2005 [29] | EIPH: tracheobronchoscopic examinations graded 0–4 using a referenced scoring system. | Y | Multi-variate logistic regression. | ||||||
| Ivester et al. 2018 [24] | BAL differential cytology counts. Mild asthmatics were defined as horses with >5% neutrophils, >2% mast cells, or >1% eosinophils, or any combination thereof. Respirable and inhalable dust, respirable endotoxin, and respirable β-glucan exposure measurements were obtained within one week after racing. | Y | Y | Y | Mixed logistic regression models, Spearman rank correlations, and Tukey’s post hoc analysis. | ||||
| Kusano et al. 2008a [19] | Assessment of horses IAD vs. non-IAD. Tracheal aspirates containing >20% neutrophils were considered indicative of IAD. | Y | Y | Y | Fisher’s exact test. | ||||
| Kusano et al. 2008b [36] | Tracheal mucus grades (0–3), tracheal aspirate cytology (>20% neutrophils diagnostic for IAD) SAA, Fbg and SP-D measurements from coughing vs. non-coughing horses. | Y | Inflammatory markers. | Wilcoxon signed ranks, Kruskal–Wallis, and Scheffe’s F tests. | |||||
| McKane et al. 1995 [21] | BAL cytology (differential cell counts including neutrophil, erythrocyte, and haemosiderophage percentage and the total nucleated cell concentration) and oxygen saturation of haemoglobin in arterial blood. | Y | Y | Mann–Whitney U test and Spearman rank correlations. | |||||
| Morley et al. 2015 [30] | EIPH: tracheobronchoscopic examinations graded 0-4 using a referenced scoring system. | Y | Multivariable logistic and linear regression models. | ||||||
| Nolen- Walston et al 2013 [18] | IAD (now referred to as asthma) subtypes: eosinophilic-mastocytic: ≥0.5% eosinophils, ≥ 2% mast cells or both. Neutrophilic, ≥ 5% neutrophils, and Mixed ≥ 5%.neutrophils as well as ≥ 0.5% eosinophils, ≥ 2% mast cells, or all three. | Y | Y | Y | Kruskal–Wallis, Spearman rank correlations, Fischer exact tests, and simple logistic regression. | ||||
| Preston et al. 2015 [27] | EIPH: tracheobronchoscopic examinations. Graded 0–4 using a referenced scoring system. | Y | Kruskal–Wallis and Cox regression analysis. | ||||||
| Salz et al. 2016 [25] | Tracheal mucus grade (0–4), tracheal blood grade (0–4), and TW differential cytology counts >20% neutrophils was considered to be significant. | Y | Y | Ordinal logistic regression and Somer’s D (non-parametric). | |||||
| Saulez and Gummow (2009) [10] | Laryngeal function grade (1–4), PLH grade (1–4), and tracheal mucus grade (0–5), on the basis of referenced grading systems. | Y | Wilcoxon rank sum, Mann–Whitney U test, Kruskal–Wallis, Chi-squared test, and regression analysis. | ||||||
| Seltzer et al. 1996 [35] | Pleuropneumonia-clinical signs, ultrasonography, and bacteriology. | Y | Ultrasonography. | Two sample z test. | |||||
| Sullivan et al. 2015 [28] | EIPH: tracheobronchoscopic examinations. Graded 0–4 using a referenced scoring system | Y | Linear and negative binomial regression. |
3.3. Equine Asthma Formerly Inflammatory Airway Disease (IAD)
3.4. Infectious and Non-Infectious Respiratory Agents
3.5. Exercise-Induced Pulmonary Haemorrhage (EIPH)
3.6. Pleuropneumonia and Pulmonary Abscesses
3.7. Coughing
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jeffcott, L.B.; Rossdale, P.D.; Freestone, J.; Frank, C.J.; Towers-Clark, P.F. An assessment of wastage in thoroughbred racing from conception to 4 years of age. Equine Vet. J. 1982, 14, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Rossdale, P.D.; Hopes, R.; Digby, N.J.; Offord, K. Epidemiological study of wastage among racehorses 1982 and 1983. Vet. Rec. 1985, 116, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, J.M.; Smith, K.C.; Wood, J.L.; Newton, J.R. Infectious risk factors and clinical indicators for tracheal mucus in British National Hunt racehorses. Equine Vet. J. 2014, 46, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, J.M.; Smith, K.C.; Wood, J.L.; Newton, J.R. A longitudinal study of respiratory infections in British National Hunt racehorses. Vet. Rec. 2013, 172, 637. [Google Scholar] [CrossRef] [PubMed]
- Couëtil, L.L.; Cardwell, J.M.; Gerber, V.; Lavoie, J.-P.; Léguillette, R.; Richard, E.A. Inflammatory Airway Disease of Horses—Revised Consensus Statement. J. Vet. Intern. Med. 2016, 30, 503–515. [Google Scholar] [CrossRef]
- Kinnison, T.; Cardwell, J.M. Conflict between Direct Experience and Research-Based Evidence Is a Key Challenge to Evidence-Based Respiratory Medicine on British Racing Yards. Front. Vet. Sci. 2020, 7, 266. [Google Scholar] [CrossRef]
- Kinnison, T.; McGilvray, T.A.; Couëtil, L.L.; Smith, K.C.; Wylie, C.E.; Bacigalupo, S.A.; Gomez-Grau, E.; Cardwell, J.M. Mild-moderate equine asthma: A scoping review of evidence supporting the consensus definition. Vet. J. 2022, 286, 105865. [Google Scholar] [CrossRef]
- Ivester, K.M.; Couëtil, L.L.; Moore, G.E.; Zimmerman, N.J.; Raskin, R.E. Environmental exposures and airway inflammation in young thoroughbred horses. J. Vet. Intern. Med. 2014, 28, 918–924. [Google Scholar] [CrossRef]
- Mazan, M.R.; Hoffman, A.M. Effects of aerosolized albuterol on physiologic responses to exercise in standardbreds. Am. J. Vet. Res. 2001, 62, 1812–1817. [Google Scholar] [CrossRef]
- Saulez, M.N.; Gummow, B. Prevalence of pharyngeal, laryngeal and tracheal disorders in thoroughbred racehorses, and effect on performance. Vet. Rec. 2009, 165, 431–435. [Google Scholar] [CrossRef]
- Hinchcliff, K.W.; Couetil, L.L.; Knight, P.K.; Morley, P.S.; Robinson, N.E.; Sweeney, C.R.; van Erck, E. Exercise induced pulmonary hemorrhage in horses: American College of Veterinary Internal Medicine consensus statement. J. Vet. Intern. Med. 2015, 29, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Lyle, C.H.; Uzal, F.A.; McGorum, B.C.; Aida, H.; Blissitt, K.J.; Case, J.T.; Charles, J.T.; Gardner, I.; Horadagoda, N.; Kusano, K.; et al. Sudden death in racing Thoroughbred horses: An international multicentre study of post mortem findings. Equine Vet. J. 2011, 43, 324–331. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Cochrane. Quality in Prognosis Studies Tool. Available online: http://methods.cochrane.org/sites/methods.cochrane.org.prognosis/files/uploads/QUIPS%20tool.pdf (accessed on 30 November 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Nolen-Walston, R.D.; Harris, M.; Agnew, M.E.; Martin, B.B.; Reef, V.B.; Boston, R.C.; Davidson, E.J. Clinical and diagnostic features of inflammatory airway disease subtypes in horses examined because of poor performance: 98 cases (2004-2010). J. Am. Vet. Med. Assoc. 2013, 242, 1138–1145. [Google Scholar] [CrossRef]
- Kusano, K.; Ishikawa, Y.; Seki, K.; Kusunose, R. Characteristic of inflammatory airway disease in Japanese thoroughbred racehorses. J. Equine Sci. 2008, 19, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.; Tremaine, W.H.; Franklin, S.H. Prevalence of inflammatory airway disease in National Hunt horses referred for investigation of poor athletic performance. Equine Vet. J. 2006, 38, 529–534. [Google Scholar] [CrossRef]
- McKane, S.A.; Rose, R.J.; Evans, D.L. Comparison of bronchoalveolar lavage findings and measurements of gas exchange during exercise in horses with poor racing performance. N. Z. Vet. J. 1995, 43, 179–182. [Google Scholar] [CrossRef]
- Fogarty, U.; Buckley, T. Bronchoalveolar lavage findings in horses with exercise intolerance. Equine Vet.-J 1991, 23, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Holcombe, S.J.; Robinson, N.E.; Derksen, F.J.; Bertold, B.; Genovese, R.; Miller, R.; De Feiter Rupp, H.; Carr, E.A.; Eberhart, S.W.; Boruta, D.; et al. Effect of tracheal mucus and tracheal cytology on racing performance in Thoroughbred racehorses. Equine Vet. J. 2010, 38, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Ivester, K.M.; Couëtil, L.L.; Moore, G.E. An observational study of environmental exposures, airway cytology, and performance in racing thoroughbreds. J. Vet. Intern. Med. 2018, 32, 1754–1762. [Google Scholar] [CrossRef]
- Salz, R.O.; Ahern, B.J.; Boston, R.; Begg, L.M. Association of tracheal mucus or blood and airway neutrophilia with racing performance in Thoroughbred horses in an Australian racing yard. Aust. Vet. J. 2016, 94, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Crispe, E.J.; Lester, G.D.; Secombe, C.J.; Perera, D.I. The association between exercise-induced pulmonary haemorrhage and race-day performance in Thoroughbred racehorses. Equine Vet. J. 2017, 49, 584–589. [Google Scholar] [CrossRef]
- Preston, S.A.; Riggs, C.M.; Singleton, M.D.; Troedsson, M.H. Descriptive analysis of longitudinal endoscopy for exercise-induced pulmonary haemorrhage in Thoroughbred racehorses training and racing at the Hong Kong Jockey Club. Equine Vet. J. 2015, 47, 366–371. [Google Scholar] [CrossRef]
- Sullivan, S.L.; Anderson, G.A.; Morley, P.S.; Hinchcliff, K.W. Prospective study of the association between exercise-induced pulmonary haemorrhage and long-term performance in Thoroughbred racehorses. Equine Vet. J. 2015, 47, 350–357. [Google Scholar] [CrossRef]
- Hinchcliff, K.W.; Jackson, M.A.; Morley, P.S.; Brown, J.A.; Dredge, A.E.; O’Callaghan, P.A.; McCaffrey, J.P.; Slocombe, R.E.; Clarke, A.E. Association between exercise-induced pulmonary hemorrhage and performance in Thoroughbred racehorses. J. Am. Vet. Med. Assoc. 2005, 227, 768–774. [Google Scholar] [CrossRef]
- Morley, P.S.; Bromberek, J.L.; Saulez, M.N.; Hinchcliff, K.W.; Guthrie, A.J. Exercise-induced pulmonary haemorrhage impairs racing performance in Thoroughbred racehorses. Equine Vet. J. 2015, 47, 358–365. [Google Scholar] [CrossRef]
- Couetil, L.; Ivester, K.; Barnum, S.; Pusterla, N. Equine respiratory viruses, airway inflammation and performance in thoroughbred racehorses. Vet. Microbiol. 2021, 257, 109070. [Google Scholar] [CrossRef]
- Back, H.; Weld, J.; Walsh, C.; Cullinane, A. Equine rhinitis a virus infection in thoroughbred racehorses-a putative role in poor performance? Viruses 2019, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- Araneda, O.F.; Cavada, G. Atmospheric Pollutants Affect Physical Performance: A Natural Experiment in Horse Racing Studied by Principal Component Analysis. Biology 2022, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, D.M.; Erb, H.N.; Eicker, S.W.; Yeagar, A.E.; Viel, L.; Sweeney, C.R.; Lavoie, J.P. Effects of pulmonary abscesses on racing performance of horses treated at referral veterinary medical teaching hospitals: 45 cases (1985-1997). J. Am. Vet. Med. Assoc. 2000, 216, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, K.L.; Byars, T.D. Prognosis for return to racing after recovery from infectious pleuropneumonia in Thoroughbred racehorses: 70 cases (1984-1989). J. Am. Vet. Med. Assoc. 1996, 208, 1300–1301. [Google Scholar] [PubMed]
- Kusano, K.; Hobo, S.; Ode, H.; Ishikawa, Y. Tracheal endoscopic and cytological findings and blood examination results in thoroughbred racehorses suspected to have lower respiratory tract disease. J. Equine Sci. 2008, 19, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Gerber, V.; Straub, R.; Marti, E.; Hauptman, J.; Herholz, C.; King, M.; Imhof, A.; Tahon, L.; Robinson, N.E. Endoscopic scoring of mucus quantity and quality: Observer and horse variance and relationship to inflammation, mucus viscoelasticity and volume. Equine Vet. J. 2004, 36, 576–582. [Google Scholar] [CrossRef]
- Raker, C.; Boles, C.L. Pharyngeal lymphoid hyperplasia in the horse. J. Equine Med. Surg. 1978, 2, 202–207. [Google Scholar]
- McKane, S.A.; Canfield, P.J.; Rose, R.J. Equine bronchoalveolar lavage cytology: Survey of thoroughbred racehorses in training. Aust. Vet. J. 1993, 70, 401–404. [Google Scholar] [CrossRef]
- Bonsignore, M.R.; Morici, G.; Vignola, A.M.; Riccobono, L.; Bonanno, A.; Profita, M.; Abate, P.; Scichilone, N.; Amato, G.; Bellia, V.; et al. Increased airway inflammatory cells in endurance athletes: What do they mean? Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2003, 33, 14–21. [Google Scholar] [CrossRef]
- Wylie, C.E.; Newton, J.R. A systematic literature search to identify performance measure outcomes used in clinical studies of racehorses. Equine Vet. J. 2018, 50, 304–311. [Google Scholar] [CrossRef]
- Depecker, M.; Richard, E.A.; Pitel, P.H.; Fortier, G.; Leleu, C.; Couroucé-Malblanc, A. Bronchoalveolar lavage fluid in Standardbred racehorses: Influence of unilateral/bilateral profiles and cut-off values on lower airway disease diagnosis. Vet. J. 2014, 199, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Christley, R.M.; Hodgson, D.R.; Rose, R.J.; Hodgson, J.L.; Wood, J.L.N.; Reid, S.W.J. Coughing in thoroughbred racehorses: Risk factors and tracheal endoscopic and cytological findings. Vet. Rec. 2001, 148, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Burrell, M.H.; Wood, J.L.; Whitwell, K.E.; Chanter, N.; Mackintosh, M.E.; Mumford, J.A. Respiratory disease in thoroughbred horses in training: The relationships between disease and viruses, bacteria and environment. Vet. Rec. 1996, 139, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.S.; Green, C.; Main, J.P.; Taylor, P.M.; Cunningham, F.M.; Cook, A.J.; Marr, C.M. Retrospective study of the relationships between age, inflammation and the isolation of bacteria from the lower respiratory tract of thoroughbred horses. Vet. Rec. 2000, 146, 91–95. [Google Scholar] [CrossRef]
- Newton, J.R.; Wood, J.L.N.; Chanter, N. A case control study of factors and infections associated with clinically apparent respiratory disease in UK Thoroughbred racehorses. Prev. Vet. Med. 2003, 60, 107–132. [Google Scholar] [CrossRef]
- Couetil, L.; Cardwell, J.M.; Leguillette, R.; Mazan, M.; Richard, E.; Bienzle, D.; Bullone, M.; Gerber, V.; Ivester, K.; Lavoie, J.-P.; et al. Equine Asthma: Current Understanding and Future Directions. Front. Vet. Sci. 2020, 7, 450. [Google Scholar] [CrossRef]
- Hansbro, N.G.; Horvat, J.C.; Wark, P.A.; Hansbro, P.M. Understanding the mechanisms of viral induced asthma: New therapeutic directions. Pharmacol. Ther. 2008, 117, 313–353. [Google Scholar] [CrossRef]
- Houtsma, A.; Bedenice, D.; Pusterla, N.; Pugliese, B.; Mapes, S.; Hoffman, A.M.; Paxson, J.; Rozanski, E.; Mukherjee, J.; Wigley, M.; et al. Association between inflammatory airway disease of horses and exposure to respiratory viruses: A case control study. Multidiscip. Respir. Med. 2015, 10, 33. [Google Scholar] [CrossRef]
- Millerick-May, M.L.; Karmaus, W.; Derksen, F.J.; Berthold, B.; Holcombe, S.J.; Robinson, N.E. Local airborne particulate concentration is associated with visible tracheal mucus in Thoroughbred racehorses. Equine Vet. J. 2013, 45, 85–90. [Google Scholar] [CrossRef]
- Costa, M.F.; Thomassian, A. Evaluation of race distance, track surface and season of the year on exercise-induced pulmonary haemorrhage in flat racing thoroughbreds in Brazil. Equine Vet. J. Suppl 2006, 36, 487–489. [Google Scholar] [CrossRef]
- Dauvillier, J.; ter Woort, F.; van Erck-Westergren, E. Fungi in respiratory samples of horses with inflammatory airway disease. J. Vet. Intern. Med. 2019, 33, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Hough, K.P.; Curtiss, M.L.; Blain, T.J.; Liu, R.M.; Trevor, J.; Deshane, J.S.; Thannickal, V.J. Airway Remodeling in Asthma. Front. Med. 2020, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Bessonnat, A.; Hélie, P.; Grimes, C.; Lavoie, J.P. Airway remodeling in horses with mild and moderate asthma. J. Vet. Intern. Med. 2022, 36, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Wilsher, S.; Allen, W.R.; Wood, J.L. Factors associated with failure of thoroughbred horses to train and race. Equine Vet. J. 2006, 38, 113–118. [Google Scholar] [CrossRef]
- Uprety, T.; Sreenivasan, C.C.; Hause, B.M.; Li, G.; Odemuyiwa, S.O.; Locke, S.; Morgan, J.; Zeng, L.; Gilsenan, W.F.; Slovis, N.; et al. Identification of a Ruminant Origin Group B Rotavirus Associated with Diarrhea Outbreaks in Foals. Viruses 2021, 13, 1330. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cullinane, A.; Garvey, M.; Walsh, C.; Gibbons, J.; Creighton, A. A Scoping Review of Non-Structural Airway Disease as a Cause of Poor Performance in Racehorses. Animals 2023, 13, 429. https://doi.org/10.3390/ani13030429
Cullinane A, Garvey M, Walsh C, Gibbons J, Creighton A. A Scoping Review of Non-Structural Airway Disease as a Cause of Poor Performance in Racehorses. Animals. 2023; 13(3):429. https://doi.org/10.3390/ani13030429
Chicago/Turabian StyleCullinane, Ann, Marie Garvey, Cathal Walsh, James Gibbons, and Alan Creighton. 2023. "A Scoping Review of Non-Structural Airway Disease as a Cause of Poor Performance in Racehorses" Animals 13, no. 3: 429. https://doi.org/10.3390/ani13030429
APA StyleCullinane, A., Garvey, M., Walsh, C., Gibbons, J., & Creighton, A. (2023). A Scoping Review of Non-Structural Airway Disease as a Cause of Poor Performance in Racehorses. Animals, 13(3), 429. https://doi.org/10.3390/ani13030429







