Fertility Control for Wildlife: A European Perspective
Abstract
Simple Summary
Abstract
1. Introduction
2. Wildlife Contraceptives
2.1. Immunocontraceptive Vaccines
2.2. Oral Contraceptives
3. Delivery Methods
4. Feasibility, Costs and Public Attitudes
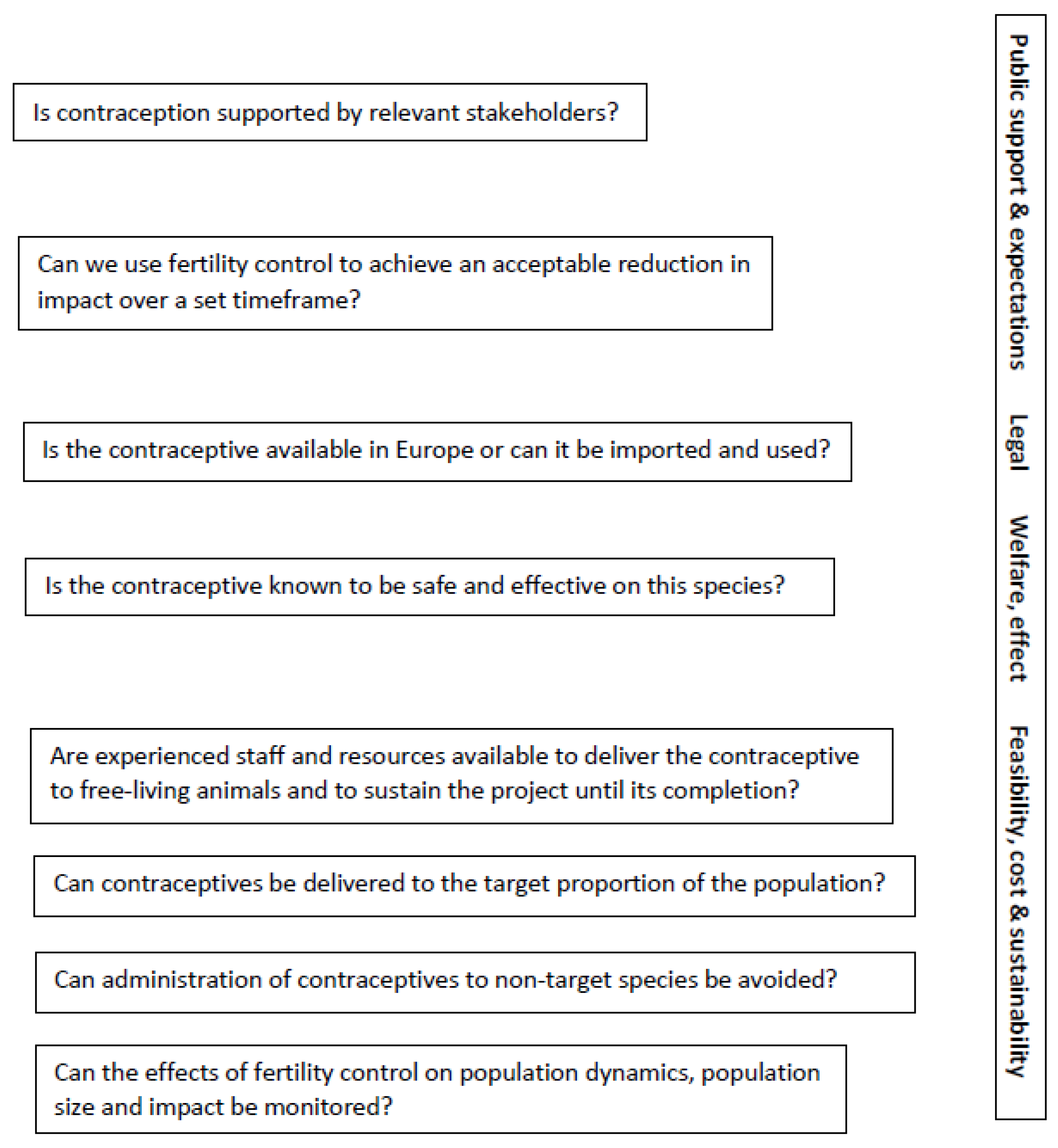
5. When to Use Wildlife Fertility Control? A Framework for Decision Making
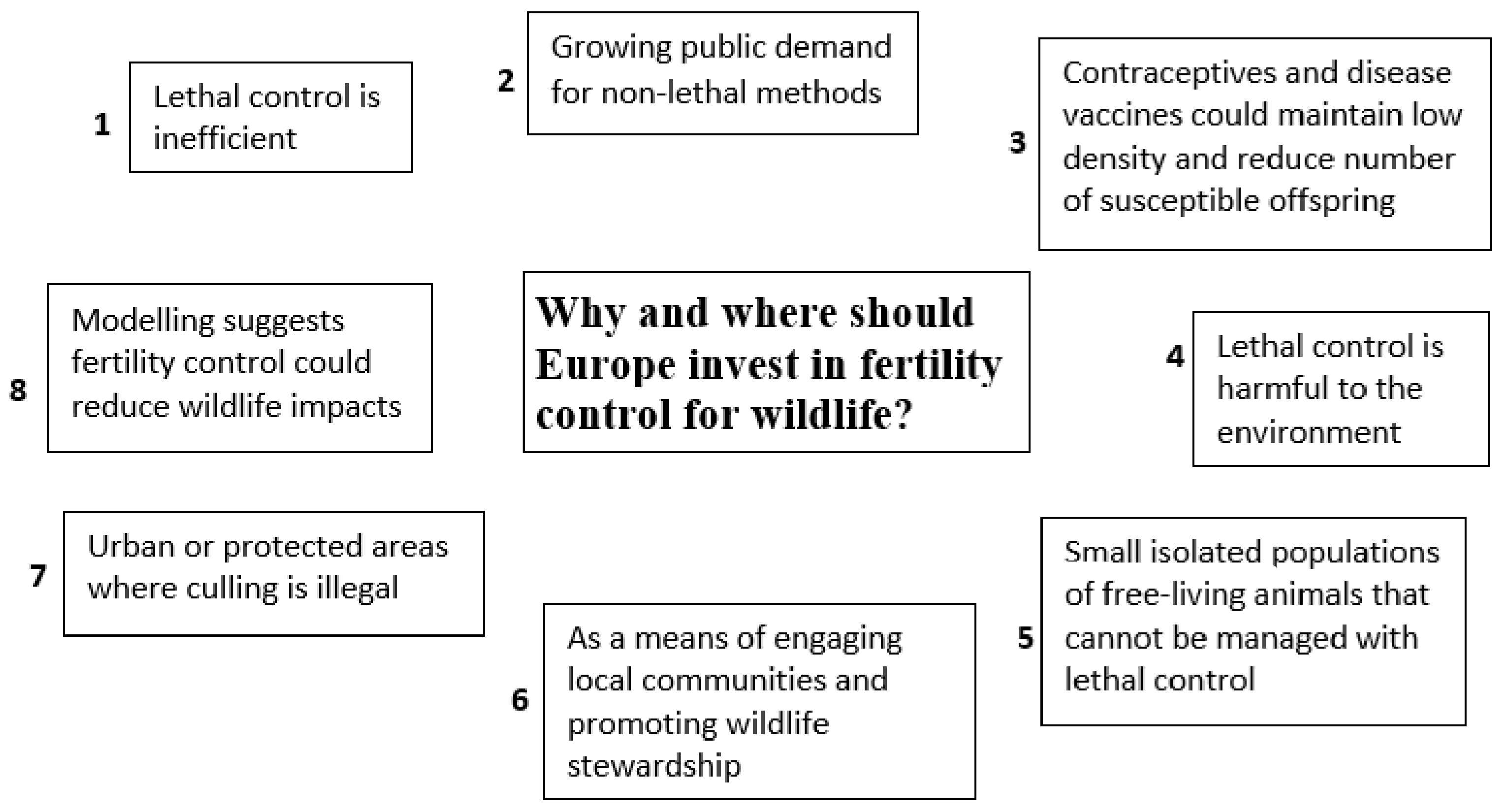
6. The Future: Why Should Europe Invest in Wildlife Fertility Control?
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abrahms, B. Human-wildlife conflict under climate change. Science 2021, 373, 484–485. [Google Scholar] [CrossRef] [PubMed]
- Mesmer, T.A. The emergence of human-wildlife conflict management: Turning challenges into opportunities. Int. Biodeterior. Biodegrad. 2000, 45, 97–102. [Google Scholar] [CrossRef]
- Messmer, T.A. Human–wildlife conflicts: Emerging challenges and opportunities. Hum. Wildl. Confl. 2009, 3, 10–17. [Google Scholar]
- Valente, A.M.; Acevedo, P.; Figueiredo, A.M.; Fonseca, C.; Torres, R.T. Overabundant wild ungulate populations in Europe: Management with consideration of socio-ecological consequences. Mamm. Rev. 2020, 50, 353–366. [Google Scholar] [CrossRef]
- Conover, M.R.; Conover, D.O. Human-Wildlife Interactions: From Conflict to Coexistence. CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Putman, R.; Apollonio, M.; Andersen, R. Ungulate Management in Europe: Problems and Practices. Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Carpio, A.J.; Apollonio, M.; Acevedo, P. Wild ungulate overabundance in Europe: Contexts, causes, monitoring and management recommendations. Mamm. Rev. 2021, 51, 95108. [Google Scholar] [CrossRef]
- Rode, J.; Flinzberger, L.; Karutz, R.; Berghoefer, A.; Schroeter-Schlaack, C. Why so negative? Exploring the socio-economic impacts of large carnivores from a European perspective. Biol. Conserv. 2021, 255, 108918. [Google Scholar] [CrossRef]
- Strand, T.M.; Lundkvist, Å. Rat-borne diseases at the horizon. A systematic review on infectious agents carried by rats in Europe 1995–2016. Infect. Ecol. Epidemiol. 2019, 9, 1553461. [Google Scholar] [CrossRef]
- Jacob, J.; Imholt, C.; Caminero-Saldaña, C.; Couval, G.; Giraudoux, P.; Herrero-Cófreces, S.; Horváth, G.; Luque-Larena, J.J.; Tkadlec, E.; Wymenga, E. Europe-wide outbreaks of common voles in 2019. J. Pest Sci. 2020, 93, 703–709. [Google Scholar] [CrossRef]
- Peck, H.L.; Pringle, H.E.; Marshall, H.H.; Owens, I.P.; Lord, A.M. Experimental evidence of impacts of an invasive parakeet on foraging behavior of native birds. Behav. Ecol. 2014, 25, 582–590. [Google Scholar] [CrossRef]
- Fox, A.D. Urban Geese–looking to North America for experiences to guide management in Europe. Wildfowl 2019, 69, 3–27. [Google Scholar]
- Fox, A.D.; Madsen, J. Threatened species to super-abundance: The unexpected international implications of successful goose conservation. Ambio 2017, 46, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Salgado, I. Is the raccoon (Procyon lotor) out of control in Europe? Biodivers. Conserv. 2018, 27, 2243–2256. [Google Scholar] [CrossRef]
- Jacob, J.; Tkadlec, E. Rodent outbreaks in Europe: Dynamics and damage. In Rodent Outbreaks: Ecology and Impacts; Singleton, G.S., Belmain, S.R., Brown, P.R., Hardy, B., Eds.; International Rice Research Institute: Los Baños, Philippines, 2010; pp. 207–224. [Google Scholar]
- Chambers, M.A.; Carter, S.P.; Wilson, G.J.; Jones, G.; Brown, E.; Hewinson, R.G.; Vordermeier, M. Vaccination against tuberculosis in badgers and cattle: An overview of the challenges, developments and current research priorities in Great Britain. Vet. Rec. 2014, 175, 9096. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-de-Santa-María, A.; Tellería, J.L. Wildlife-vehicle collisions in Spain. Eur. J. Wildl. Res. 2015, 61, 399–406. [Google Scholar] [CrossRef]
- van Beeck Calkoen, S.T.; Mühlbauer, L.; Andrén, H.; Apollonio, M.; Balčiauskas, L.; Belotti, E.; Carranza, J.; Cottam, J.; Filli, F.; Gatiso, T.T.; et al. Ungulate management in European national parks: Why a more integrated European policy is needed. J. Environ. Manag. 2020, 260, 110068. [Google Scholar] [CrossRef]
- Sharp, T.; Saunders, G. A Model for Assessing the Relative Humaneness of Pest Animal Control Methods; Australian Government Department of Agriculture Fisheries and Forestry: Canberra, Australia, 2008. [Google Scholar]
- Dubois, S.; Fenwick, N.; Ryan, E.A.; Baker, L.; Baker, S.E.; Beausoleil, N.J.; Carter, S.; Cartwright, B.; Costa, F.; Draper, C.; et al. International consensus principles for ethical wildlife control. Conserv. Biol. 2017, 31, 753–760. [Google Scholar] [CrossRef]
- Jacoblinnert, K.; Jacob, J.; Zhang, Z.; Hinds, L.A. The status of fertility control for rodents—Recent achievements and future directions. Integr. Zool. 2022, 17, 964–980. [Google Scholar] [CrossRef] [PubMed]
- Quinn, N.; Kenmuir, S.; Krueger, L. A California without rodenticides: Challenges for commensal rodent management in the future. Hum. Wildl. Interact. 2019, 13, 212–225. [Google Scholar] [CrossRef]
- Hunold, C.; Mazuchowski, M. Human–Wildlife Coexistence in Urban Wildlife Management: Insights from Nonlethal Predator Management and Rodenticide Bans. Animals 2020, 10, 1983. [Google Scholar] [CrossRef]
- Broughton, R.K.; Searle, K.R.; Walker, L.A.; Potter, E.D.; Pereira, M.G.; Carter, H.; Sleep, D.; Noble, D.G.; Butler, A.; Johnson, A.C. Long-term trends of second generation anticoagulant rodenticides (SGARs) show widespread contamination of a bird-eating predator, the Eurasian Sparrowhawk (Accipiter nisus) in Britain. Environ. Pollut. 2022, 314, 120269. [Google Scholar] [CrossRef] [PubMed]
- Fagerstone, K.A.; Miller, L.A.; Killian, G.J.; Yoder, C.A. Review of issues concerning the use of reproductive inhibitors, with particular emphasis on resolving human wildlife conflicts in North America. Integr. Zool. 2010, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, J.F.; Lyda, R.O.; Frank, K.M. Contraceptive vaccines for wildlife: A review. Am. J. Reprod. Immun. 2011, 66, 40–50. [Google Scholar] [CrossRef]
- Rutberg, A.T. Managing wildlife with contraception: Why is it taking so long? J. Zoo Wildl. Med. 2013, 44, 38–46. [Google Scholar] [CrossRef]
- Massei, G.; Cowan, D. Fertility control to mitigate human–wildlife conflicts: A review. Wildl. Res. 2014, 41, 1–21. [Google Scholar] [CrossRef]
- Manfredo, M.J.; Berl, R.E.; Teel, T.L.; Bruskotter, J.T. Bringing social values to wildlife conservation decisions. Front. Ecol. Envir. 2021, 19, 355–362. [Google Scholar] [CrossRef]
- Manfredo, M.J.; Teel, T.L.; Dietsch, A.M. Implications of human value shift and persistence for biodiversity conservation. Conserv. Biol. 2016, 30, 287–296. [Google Scholar] [CrossRef]
- Genovesi, P. Guidelines for Eradication of Terrestrial Vertebrates: A European Contribution to the Invasive Alien Species Issue. Other Publications in Wildlife Management. 24. 2001. Available online: https://digitalcommons.unl.edu/icwdmother/24 (accessed on 15 December 2022).
- IUCN. Information on Non-Lethal Measures to Eradicate or Manage Vertebrates Included on the Union List. Technical Note Prepared by IUCN for the European Commission. 2017. Available online: https://circabc.europa.eu/sd/a/518231a9-abdd-47b1-b455-9d78a7e98f0e/Non-lethal%20measures.pdf (accessed on 15 December 2022).
- Scapin, P.; Ulbano, M.; Ruggiero, C.A.; Marsan, A.; Ferrari, N.; Bertolino, S. Surgical sterilization of male and female grey squirrels (Sciurus carolinensis) of an urban population introduced in Italy. J. Vet. Med. Sci. 2019, 81, 18–0319. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.A.; Crane, K.; Gaddis, S.; Killian, G.J. Porcine zona pellucida immuno-contraception: Long-term health effects on white-tailed deer. J. Wildl. Manage. 2001, 65, 941–945. [Google Scholar] [CrossRef]
- Bechert, U.S.; Fraker, M.A. Twenty years of SpayVac® research: Potential implications for regulating feral horse and burro populations in the United States. Hum. Wildl. Interact. 2018, 12, 117–130. [Google Scholar]
- Killian, G.; Thain, D.; Diehl, N.K.; Rhyan, J.; Miller, L. Four-year contraception rates of mares treated with single-injection porcine zona pellucida and GnRH vaccines and intrauterine devices. Wildl. Res. 2008, 35, 531–539. [Google Scholar] [CrossRef]
- Gray, M.E.; Cameron, E.Z. Does contraceptive treatment in wildlife result in side effects? A review of quantitative and anecdotal evidence. Reproduction 2010, 139, 45–55. [Google Scholar] [CrossRef]
- Miller, L.A.; Fagerstone, K.A.; Wagner, D.C.; Killian, G.J. Factors contributing to the success of a single-shot, multiyear PZP immunocontraceptive vaccine for white-tailed deer. Hum. Wildl. Confl. 2009, 3, 103–115. [Google Scholar]
- Rutberg, A.T.; Naugle, R.E.; Turner, J.W.; Fraker, M.A.; Flanagan, D.R. Field testing of single-administration porcine zona pellucida contraceptive vaccines in white-tailed deer (Odocoileus virginianus). Wildl. Res. 2013, 40, 281–288. [Google Scholar] [CrossRef]
- Bechert, U.; Bartell, J.; Kutzler, M.; Menino, A., Jr.; Bildfell, R.; Anderson, M.; Fraker, M. Effects of two porcine zona pellucida immunocontraceptive vaccines on ovarian activity in horses. J. Wildl. Manag. 2013, 77, 13861400. [Google Scholar] [CrossRef]
- Roelle, J.E.; Germaine, S.S.; Kane, A.J.; Cade, B.S. Efficacy of SpayVac® as a contraceptive in feral horses. Wildl. Soc. Bull. 2017, 41, 107–115. [Google Scholar] [CrossRef]
- Turner, J.W.; Flanagan, D.R.; Rutberg, A.T.; Kirkpatrick, J.F. Immunocontraception in wild horses: One inoculation provides two years of infertility. J. Wildl. Manag. 2007, 71, 662–667. [Google Scholar] [CrossRef]
- Ransom, J.I.; Roelle, J.E.; Cade, B.S.; Coates-Markle, L.; Kane, A.J. Foaling rates in feral horses treated with the immunocontraceptive porcine zona pellucida. Wildl. Soc. Bull. 2011, 35, 343–352. [Google Scholar] [CrossRef]
- Rutberg, A.; Grams, K.; Turner, J.W.; Hopkins, H. Contraceptive efficacy of priming and boosting doses of controlled-release PZP in wild horses. Wildl. Res. 2017, 44, 174–181. [Google Scholar] [CrossRef]
- Rosu, O.; Birtoiu, A.I.; Franz, S. Porcine zona pellucida (PZP) vaccination in a free-roaming feral horse population following individual chemical immobilisation and remote booster: Is it feasible? In Joint AAZV/EAZWV/IZW Conference Proceedings; Leibniz Institute for Zoo and Wildlife Research: Altanta, GA, USA, 2016; pp. 317–325. [Google Scholar]
- Curtis, P.D.; Richmond, M.E.; Miller, L.A.; Quimby, F.W. Pathophysiology of white-tailed deer vaccinated with porcine zona pellucida immunocontraceptive. Vaccine 2007, 25, 46234630. [Google Scholar] [CrossRef]
- Kirkpatrick, J.F.; Rowan, A.; Lamberski, N.; Wallace, R.; Frank, K.; Lyda, R. The practical side of immunocontraception: Zona proteins and wildlife. J. Reprod. Immun. 2009, 83, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, C.M.; Adelman, J.S.; Rubenstein, D.I. Immunocontraception in wild horses (Equus caballus) extends reproductive cycling beyond the normal breeding season. PLoS ONE 2010, 5, e13635. [Google Scholar] [CrossRef] [PubMed]
- Nunez, C.M.; Adelman, J.S.; Mason, C.; Rubenstein, D.I. Immunocontraception decreases group fidelity in a feral horse population during the non-breeding season. Appl. Anim. Behav. Sci. 2009, 117, 7483. [Google Scholar] [CrossRef]
- Hernandez, S.; Locke, S.L.; Cook, M.W.; Harveson, L.A.; Davis, D.S.; Lopez, R.R.; Silvy, N.J.; Fraker, M.A. Effects of SpayVac® on Urban Female White-Tailed Deer Movements. Wildl. Soc. Bull. 2006, 34, 1430–1434. [Google Scholar] [CrossRef]
- Ransom, J.I.; Cade, B.S.; Hobbs, N.T. Influences of immunocontraception on time budgets, social behavior, and body condition in feral horses. Appl. Anim. Behav. Sci. 2010, 124, 51–60. [Google Scholar] [CrossRef]
- Turner, A.; Kirkpatrick, J. Effects of immunocontraception on population, longevity and body condition in wild mares (Equus caballus). Reproduction 2002, 60, 187–195. [Google Scholar]
- Kirkpatrick, J.; Turner, A. Reversibility of action and safety during pregnancy of immunization against porcine zona pellucida in wild mares (Equus caballus). Reproduction 2001, 60, 197–202. [Google Scholar]
- Perdok, A.A.; De Boer, W.F.; Stout, T.A.E. Prospects for managing African elephant population growth by immunocontraception: A review. Pachyderm 2007, 42, 95–105. [Google Scholar]
- Miller, L.A.; Gionfriddo, J.P.; Fagerstone, K.A.; Rhyan, J.C.; Killian, G.J. The Single-Shot GnRH Immunocontraceptive Vaccine (GonaCon™) in White-Tailed Deer: Comparison of Several GnRH Preparations. Am. J. Reprod. Immun. 2008, 60, 214–223. [Google Scholar] [CrossRef]
- Miller, L.A.; Rhyan, J.C.; Drew, M. Contraception of bison by GnRH vaccine: A possible means of decreasing transmission of brucellosis in bison. J. Wildl. Dis. 2004, 40, 725–730. [Google Scholar] [CrossRef]
- Massei, G.; Cowan, D.P.; Coats, J.; Bellamy, F.; Quy, R.; Pietravalle, S.; Miller, L.A. Long term effects of immunocontraception on wild boar fertility, physiology and behaviour. Wildl. Res. 2012, 39, 378–385. [Google Scholar] [CrossRef]
- Pinkham, R.; Koon, K.K.; To, J.; Chan, J.; Vial, F.; Gomm, M.; Eckery, D.C.; Massei, G. Long-term effect of a GnRH-based immunocontraceptive on feral cattle in Hong Kong. PLoS ONE 2022, 17, e0272604. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.L.; Powers, J.G.; Ransom, J.I.; McCann, B.E.; Oehler, M.W.; Bruemmer, J.E.; Galloway, N.L.; Eckery, D.C.; Nett, T.M. Reimmunization increases contraceptive effectiveness of gonadotropin-releasing hormone vaccine (GonaCon-Equine) in free-ranging horses (Equus caballus): Limitations and side effects. PLoS ONE 2018, 13, e0201570. [Google Scholar] [CrossRef]
- Massei, G.; Cowan, D.; Coats, J.; Gladwell, F.; Lane, J.; Miller, L. Effect of the GnRH vaccine GonaCon on the fertility, physiology and behaviour of wild boar. Wildl. Res. 2008, 35, 540–547. [Google Scholar] [CrossRef]
- Massei, G.; Koon, K.K.; Benton, S.; Brown, R.; Gomm, M.; Orahood, D.S.; Eckery, D.C. Immunocontraception for managing feral cattle in Hong Kong. PLoS ONE 2015, 10, e0121598. [Google Scholar] [CrossRef] [PubMed]
- Quy, R.J.; Massei, G.; Lambert, M.S.; Coats, J.; Miller, L.A.; Cowan, D.P. Effects of a GnRH vaccine on the movement and activity of free-living wild boar (Sus scrofa). Wildl. Res. 2014, 41, 185–193. [Google Scholar] [CrossRef]
- Cowan, D.; Smith, G.C.; Brash, M.; Bellamy, F.; Massei, G.; Conwell, R.; Vial, F. Evaluation of a single-shot gonadotropin-releasing hormone (GnRH) immunocontraceptive vaccine in captive badgers. Eur. J. Wildl. Res. 2019, 65, 118. [Google Scholar] [CrossRef]
- Cowan, D.P.; Van der Waal, Z.; Pidcock, S.; Gomm, M.; Stephens, N.; Brash, M.; White, P.C.; Mair, L.; Mill, A.C. Adaptive management of an iconic invasive goat Capra hircus population. Mamm. Rev. 2020, 50, 180186. [Google Scholar] [CrossRef]
- Gionfriddo, J.P.; Denicola, A.J.; Miller, L.A.; Fagerstone, K.A. Health effects of GnRH immunocontraception of wild white-tailed deer in New Jersey. Wildl. Soc. Bull. 2011, 35, 149–160. [Google Scholar] [CrossRef]
- Krause, S.K.; Kelt, D.A.; Gionfriddo, J.P.; Van Vuren, D.H. Efficacy and health effects of a wildlife immunocontraceptive vaccine on fox squirrels. J. Wildl. Manag. 2014, 78, 12–23. [Google Scholar] [CrossRef]
- Krause, S.K.; Van Vuren, D.H.; Laursen, C.; Kelt, D.A. Behavioral effects of an immuno-contraceptive vaccine on eastern fox squirrels. J. Wildl. Manag. 2015, 79, 1255–1263. [Google Scholar] [CrossRef]
- Pai, M.; Bruner, R.; Schlafer, D.H.; Yarrow, G.K.; Yoder, C.A.; Miller, L.A. Immuno-contraception in eastern gray squirrels (Sciurus carolinensis): Morphologic changes in reproductive organs. J. Zoo Wildl. Med. 2011, 42, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Fagerstone, K.A.; Miller, L.A.; Eisemann, J.D.; O’Hare, J.R.; Gionfriddo, J.P. Registration of wildlife contraceptives in the United States of America, with OvoControl and GonaCon immunocontraceptive vaccines as examples. Wildl. Res. 2008, 35, 586–592. [Google Scholar] [CrossRef]
- Yoder, C.A.; Miller, L.A. Effect of GonaCon™ vaccine on black-tailed prairie dogs: Immune response and health effects. Vaccine 2010, 29, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Pyzyna, B.; Whish, S.; Dyer, C.A.; Mayer, L.P.; Witmer, G.; Moulton, R. Free ranging wild-caught Norway rats have reduced fecundity after consuming liquid oral fertility bait containing 4-vinylcyclohexene diepoxide and triptolide. In Proceedings of the 27th Vertebrate Pest Conference; Timm, R.M., Baldwin, R.A., Eds.; University of California: Davis, CA, USA, 2016; pp. 314–316. [Google Scholar]
- Siers, S.; Sugihara, R.T.; Leinbach, I.; Pyzyna, B.R.; Witmer, G. Laboratory evaluation of the effectiveness of the fertility control bait ContraPest® on wild-captured black rats (Rattus rattus). In Proceedings of the 29th Vertebrate Pest Conference; Woods, D.M., Ed.; University of California: Davis, CA, USA, 2020; pp. 1–7. [Google Scholar]
- Zhao, M.; Liu, M.; Li, D.; Wan, X.; Hinds, L.A.; Wang, Y.; Zhang, Z. Anti-fertility effect of levonorgestrel and quinestrol in Brandt’s voles (Lasiopodomys brandtii). Integr. Zool. 2007, 2, 260–268. [Google Scholar] [CrossRef]
- Liu, M.; Qu, J.; Yang, M.; Wang, Z.; Wang, Y.; Zhang, Y.; Zhang, Z. Effects of quinestrol and levonorgestrel on populations of plateau pikas, Ochotona curzoniae, in the Qinghai-Tibetan Plateau. Pest Manag. Sci. 2012, 68, 592–601. [Google Scholar] [CrossRef]
- Shi, L.; Li, X.; Ji, Z.; Wang, Z.; Shi, Y.; Tian, X.; Wang, Z. The reproductive inhibitory effects of levonorgestrel, quinestrol, and EP-1 in Brandt’s vole (Lasiopodomys brandtii). PeerJ 2020, 8, e9140. [Google Scholar] [CrossRef]
- Pellizzari, M. Control of pigeon numbers through contraception. Internat. Pest Control 2017, 59, 20–22. [Google Scholar]
- Pyzyna, B.R.; Trulove, N.F.; Mansfield, C.H.; McMillan, R.A.; Ray, C.N.; Mayer, L.P. ContraPest®, A new tool for rodent control. In Proceedings of the 28th Vertebrate Pest Conference; Woods, D.M., Ed.; University of California: Davis, CA, USA, 2018; pp. 284–286. [Google Scholar]
- Mayer, L.P.; Pearsall, N.A.; Christian, P.J.; Devine, P.J.; Payne, C.M.; McCuskey, M.K.; Hoyer, P.B. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod. Toxicol. 2002, 16, 775–781. [Google Scholar] [CrossRef]
- Mayer, L.P.; Devine, P.J.; Dyer, C.A.; Hoyer, P.B. The follicle-deplete mouse ovary produces androgen. Biol. Reprod. 2004, 71, 130–138. [Google Scholar] [CrossRef]
- Witmer, G.W.; Raymond-Whish, S.; Moulton, R.S.; Pyzyna, B.R.; Calloway, E.M.; Dyer, C.A.; Mayer, L.P.; Hoyer, P.B. Compromised fertility in free feeding of wild-caught Norway rats (Rattus norvegicus) with a liquid bait containing 4-vinylcyclohexene diepoxide and triptolide. J. Zoo Wildl. Med. 2017, 48, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Witmer, G.W.; Raymond-Whish, S. Reduced fecundity in free-ranging Norway rats after baiting with a liquid fertility control bait. Hum. Wildl. Interact. 2021, 15, 111–123. [Google Scholar]
- Imakando, C.; Fernández-Grandon, M.; Singleton, G.R.; Belmain, S.R. Impact of fertility vs. mortality control on the demographics of Mastomys natalensis in maize fields. Integr. Zool. 2021, 17, 1028–1040. [Google Scholar]
- Selemani, M.; Makundi, R.; Massawe, A.W.; Mhamphi, G.; Mulungu, L.S.; Belmain, S.R. Impact of contraceptive hormones on the reproductive potential of male and female commensal black rats (Rattus rattus). Integr. Zool. 2021, 17, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.M.; Herawati, N.A.; LIU, M.; Zhang, Z.; Singleton, G.R.; Hinds, L.A. Reproductive responses of rice field rats (Rattus argentiventer) following treatment with the contraceptive hormones, quinestrol and levonorgestrol. Integr. Zool. 2022, 17, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Massawe, A.W.; Makundi, R.H.; Zhang, Z.; Mhamphi, G.; Liu, M.; Li, H.J.; Belmain, S.R. Effect of synthetic hormones on reproduction in Mastomys natalensis. J. Pest Sci. 2018, 91, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wan, X.; Yin, Y.; Li, Y.X.; Sun, F.; Zhang, Z.; Wang, Y.L. Subfertile effects of quinestrol and levonorgestrel in male rats. Reprod. Fertil. Develop. 2012, 24, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hou, X.; Feng, T.; Han, N.; Wang, J.; Chang, G. Anti-fertility effect of levonorgestrel and/or quinestrol on striped field mouse (Apodemus agrarius), evidence from both laboratory and field experiments. Integr. Zool 2021, 17, 1041–1052. [Google Scholar] [CrossRef]
- Jones, J.E.; Hughes, B.L.; Solis, J.; Castaldo, D.J.; Toler, J.E. Effect of nicarbazin on brown-egg layer-breeders. Appl. Agric. Res. 1990, 5, 149–152. [Google Scholar]
- Turner, J.W.; Rutberg, A.T. From the pens to the field: Real-world wildlife contraception. J. Zoo Wildl. Med. 2013, 44, 102–110. [Google Scholar] [CrossRef]
- Turner, J.W.; Liu, I.K.M.; Kirkpatrick, J.F. Remotely delivered immunocontraception in free-roaming feral burros (Equus asinus). J. Reprod. Fertil. 1996, 107, 31–35. [Google Scholar] [CrossRef]
- Carey, K.A.; Ortiz, A.; Grams, K.; Elkins, D.; Turner, J.W.; Rutberg, A.T. Efficacy of dart-delivered PZP-22 immunocontraceptive vaccine in wild horses (Equus caballus) in baited traps in New Mexico, USA. Wildl. Res. 2019, 46, 713718. [Google Scholar] [CrossRef]
- DeNicola, A.J.; Kesler, D.J.; Swihart, R.K. Dose determination and efficacy of remotely delivered norgestomet implants on contraception of white-tailed deer. Zoo Biol. 1997, 16, 3137. [Google Scholar] [CrossRef]
- DeNicola, A.J.; VerCauteren, K.C.; Curtis, P.D.; Hygnstrom, S.E. Managing White-Tailed Deer in Suburban Environments: A Technical Guide; Cornell Cooperative Extension: Ithaca, NY, USA, 2000. [Google Scholar]
- Aune, K.; Terry, J.; Kreeger, T.J.; Roffe, T.J. Overview of delivery systems for the administration of vaccines to elk and bison of the Greater Yellowstone Area. In Proceedings of Brucellosis in Elk and Bison in the Greater Yellowstone Area; Kreeger, T.J., Ed.; Wyoming Game and Fish Department: Cheyenne, WY, USA, 2002; pp. 66–79. [Google Scholar]
- Evans, C.S.; DeNicola, A.J.; Eisemann, J.D.; Eckery, D.C.; Warren, R.J. Administering GonaCon to white-tailed deer via hand-injection versus syringe-dart. Hum. Wildl. Interact. 2015, 9, 265. [Google Scholar]
- Massei, G.; Koon, K.K.; Law, S.; Gomm, M.; Mora, D.S.O.; Callaby, R.; Palphramand, K.; Eckery, D.C. Fertility control for managing free-living feral cattle in Hong Kong. Vaccine 2018, 36, 7393–7398. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Zhang, J.; Shi, D.; Wu, X. Effects of levonorgestrel-quinestrol (EP-1) treatment on Mongolian gerbil wild populations: A case study. Integr. Zool. 2013, 8, 277–284. [Google Scholar] [CrossRef] [PubMed]
- González-Crespo, C.; Lavín, S. Use of Fertility Control (Nicarbazin) in Barcelona: An Effective yet Respectful Method towards Animal Welfare for the Management of Conflictive Feral Pigeon Colonies. Animals 2022, 12, 856. [Google Scholar] [CrossRef]
- Massei, G.; Coats, J.; Quy, R.; Storer, K.; Cowan, D.P. The boar-operated-system: A novel method to deliver baits to wild pigs. J. Wildl. Manag. 2010, 74, 333–336. [Google Scholar] [CrossRef]
- Campbell, T.A.; Long, D.B.; Massei, G. Efficacy of the Boar-Operated-System to deliver baits to feral swine. Prev. Vet. Med. 2011, 98, 243249. [Google Scholar] [CrossRef]
- Ferretti, F.; Sforzi, A.; Coats, J.; Massei, G. The BOS™ as a species-specific method to deliver baits to wild boar in a Mediterranean area. Eur. J. Wildl. Res. 2014, 60, 555–558. [Google Scholar] [CrossRef]
- Beatham, S.E.; Goodwin, D.; Coats, J.; Stephens, P.A.; Massei, G. A PIT-tag–based method for measuring individual bait uptake in small mammals. Ecol. Solut. Evid. 2021, 2, e12081. [Google Scholar] [CrossRef]
- Twigg, L.E.; Williams, C.K. Fertility control of overabundant species; can it work for feral rabbits? Ecol. Lett. 1999, 2, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Twigg, L.E.; Lowe, T.J.; Martin, G.R.; Wheeler, A.G.; Gray, G.S.; Griffin, S.L.; O’Reilly, C.M.; Robinson, D.J.; Hubach, P.H. Effects of surgically imposed sterility on free-ranging rabbit populations. J. Appl. Ecol. 2000, 37, 16–39. [Google Scholar] [CrossRef]
- Hobbs, N.T.; Bowden, D.C.; Baker, D.L. Effects of fertility control on populations of ungulates: General, stage-structured models. J. Wildl. Manag. 2000, 64, 473–491. [Google Scholar] [CrossRef]
- Zhang, Z.B. Mathematical models of wildlife management by contraception. Ecol. Model. 2000, 132, 105–113. [Google Scholar] [CrossRef]
- White, P.C.; Lewis, A.J.; Harris, S. Fertility control as a means of controlling bovine tuberculosis in badger (Meles meles) populations in south–west England: Predictions from a spatial stochastic simulation model. Proc. Royal Soc. B 1997, 264, 1737–1747. [Google Scholar] [CrossRef]
- Merrill, J.A.; Cooch, E.G.; Curtis, P.D. Managing an overabundant deer population by sterilization: Effects of immigration, stochasticity and the capture process. J. Wildl. Manag. 2006, 70, 268–277. [Google Scholar] [CrossRef]
- Croft, S.; Franzetti, B.; Gill, R.; Massei, G. Too many wild boar? Modelling fertility control and culling to reduce wild boar numbers in isolated populations. PLoS ONE 2020, 15, e0238429. [Google Scholar] [CrossRef]
- Croft, S.; Aegerter, J.N.; Beatham, S.; Coats, J.; Massei, G. A spatially explicit population model to compare management using culling and fertility control to reduce numbers of grey squirrels. Ecol. Model. 2021, 440, 109386. [Google Scholar] [CrossRef]
- Pepin, K.M.; Davis, A.J.; Cunningham, F.L.; VerCauteren, K.C.; Eckery, D.C. Potential effects of incorporating fertility control into typical culling regimes in wild pig populations. PLoS ONE 2017, 12, e0183441. [Google Scholar] [CrossRef]
- Druce, H.C.; Mackey, R.L.; Slowtow, R. How immunocontraception can contribute to elephant management in small, enclosed reserves: Munyawana population. PLoS ONE 2011, 6, e27952. [Google Scholar] [CrossRef] [PubMed]
- Locke, C.M.; Anhalt-Depies, C.M.; Frett, S.; Stenglein, J.L.; Cameron, S.; Malleshappa, V.; Peltier, T.; Zuckerberg, B.; Townsend, P.A. Managing a large citizen science project to monitor wildlife. Wildl. Soc. Bull. 2019, 43, 4–10. [Google Scholar] [CrossRef]
- Shuttleworth, C.M.; Robinson, N.; Halliwell, E.C.; Clews-Roberts, R.; Peek, H.; Podgornik, G.; Stinson, M.; Rice, S.; Finlay, C.; McKinney, C.; et al. Evolving grey squirrel management techniques in Europe. Manag. Biol. Invasions 2020, 11, 747–761. [Google Scholar] [CrossRef]
- Crowley, S.L.; Hinchliffe, S.; McDonald, R.A. Killing squirrels: Exploring motivations and practices of lethal wildlife management. Environ. Plan. E Nat. Space 2018, 1, 120143. [Google Scholar] [CrossRef]
- Dunn, M.; Marzano, M.; Forster, J.; Gill, R.M. Public attitudes towards “pest” management: Perceptions on squirrel management strategies in the UK. Biol. Conserv. 2018, 222, 52–63. [Google Scholar] [CrossRef]
- Carter, S.P.; Roy, S.S.; Cowan, D.P.; Massei, G.; Smith, G.C.; Ji, W.; Rossi, S.; Woodroffe, R.; Wilson, G.J.; Delahay, R.J. Options for the control of disease 2: Targeting hosts. In Management of Disease in Wild Mammals; Delahay, R.J., Smith, G.C., Hutchings, M.R., Eds.; Springer: Tokyo, Japan, 2009; p. 121146. [Google Scholar]
- Rutberg, A.T.; Naugle, R.E.; Thiele, L.A.; Liu, I.K. Effects of immunocontraception on a suburban population of white-tailed deer Odocoileus virginianus. Biol. Conserv. 2004, 116, 243–250. [Google Scholar] [CrossRef]
- Hone, J. Wildlife Damage Control; CSIRO Publishing: Collingwood, Australia, 2007. [Google Scholar]
- Krull, C.R.; Stanley, M.C.; Burns, B.R.; Choquenot, D.; Etherington, T.R. Reducing wildlife damage with cost-effective management programmes. PLoS ONE 2016, 11, e0146765. [Google Scholar] [CrossRef]
- Massei, G.; Boyles-Griffin, S.L. Stakeholder acceptance of wild equid fertility control mirrors global shifts in attitudes to wildlife management. Hum. Wildl. Interact. in press. 2023. [Google Scholar]
- Merrill, J.A.; Cooch, E.G.; Curtis, P.D. Time to reduction: Factors influencing management efficacy in sterilizing overabundant white-tailed deer. J. Wildl. Manag. 2003, 67, 267–279. [Google Scholar] [CrossRef]
- Huig, N.; Buijs, R.J.; Kleyheeg, E. Summer in the city: Behaviour of large gulls visiting an urban area during the breeding season. Bird Study 2016, 63, 214–222. [Google Scholar] [CrossRef]
- Colomer, J.; Rosell, C.; Rodriguez-Teijeiro, J.D.; Massei, G. Reserve effect’: An opportunity to mitigate human-wild boar conflicts. Sci. Total Environ. 2021, 795, 148721. [Google Scholar] [CrossRef]
- Carroll, M.J.; Singer, A.; Smith, G.C.; Cowan, D.P.; Massei, G. The use of immuno-contraception to improve rabies eradication in urban dog populations. Wildl. Res. 2010, 37, 676–687. [Google Scholar] [CrossRef]
- Smith, G.C.; Wilkinson, D. Modeling control of rabies outbreaks in red fox populations to evaluate culling, vaccination, and vaccination combined with fertility control. J. Wildl. Dis. 2003, 39, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Meerburg, B.G.; van Gent-Pelzer, M.P.; Schoelitsz, B.; van der Lee, T.A. Distribution of anticoagulant rodenticide resistance in Rattus norvegicus in the Netherlands according to Vkorc1 mutations. Pest Manag. Sci. 2014, 70, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Castaño, P.A.; Campbell, K.J.; Baxter, G.S.; Carrión, V.; Cunninghame, F.; Fisher, P.; Griffiths, R.; Hanson, C.C.; Howald, G.R.; Jolley, W.J.; et al. Managing non-target wildlife mortality whilst using rodenticides to eradicate invasive rodents on islands. Biol. Invasions 2022, 24, 3423–3440. [Google Scholar] [CrossRef]
- Luque-Larena, J.J.; Mougeot, F.; Vinuela, J.; Jareno, D.; Arroyo, L.; Lambin, X.; Arroyo, B. Recent large-scale range expansion and outbreaks of the common vole (Microtus arvalis) in NW Spain. Basic Appl. Ecol. 2013, 14, 432–441. [Google Scholar] [CrossRef]
- Pereira, H.M.; Navarro, L.M. Rewilding European Landscapes; Springer Nature: Heidelberg, Germany, 2015. [Google Scholar]
- Lorimer, J.; Driessen, C. Wild experiments at the Oostvaardersplassen: Rethinking environmentalism in the Anthropocene. Trans. Inst. Brit. Geogr. 2014, 39, 169–181. [Google Scholar] [CrossRef]
- Theunissen, B. The Oostvaardersplassen Fiasco. Isis 2019, 110, 341–345. [Google Scholar] [CrossRef]
- Gordon, I.J.; Manning, A.D.; Navarro, L.M.; Rouet-Leduc, J. Domestic livstock and rewilding: Are they mutually exclusive? Front. Sustain. Food Syst. 2021, 5, 550410. [Google Scholar] [CrossRef]
- Massei, G.; Quy, R.J.; Gurney, J.; Cowan, D.P. Can translocations be used to mitigate human–wildlife conflicts? Wildl. Res. 2010, 37, 428–439. [Google Scholar] [CrossRef]
- Bradley, H.S.; Tomlinson, S.; Craig, M.D.; Cross, A.T.; Bateman, P.W. Mitigation translocation as a management tool. Conserv. Bio. 2022, 36, e13667. [Google Scholar] [CrossRef]
- Bauder, J.M.; Ruid, D.; Roberts, N.M.; Kohn, B.; Allen, M.L. Effects of translocation on survival of nuisance bears. Anim. Conserv. 2021, 24, 820–831. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massei, G. Fertility Control for Wildlife: A European Perspective. Animals 2023, 13, 428. https://doi.org/10.3390/ani13030428
Massei G. Fertility Control for Wildlife: A European Perspective. Animals. 2023; 13(3):428. https://doi.org/10.3390/ani13030428
Chicago/Turabian StyleMassei, Giovanna. 2023. "Fertility Control for Wildlife: A European Perspective" Animals 13, no. 3: 428. https://doi.org/10.3390/ani13030428
APA StyleMassei, G. (2023). Fertility Control for Wildlife: A European Perspective. Animals, 13(3), 428. https://doi.org/10.3390/ani13030428





