Simple Summary
The Purebred Spanish Horse, according to our clinical experience, is characterized by having a high number of stallions that do not meet the international commercial recommendations for equine-sperm cryopreservation. We investigated if the incorporation of single-layer colloidal centrifugation prior to cryopreservation in clinical conditions could increase the number of ejaculates of Purebred Spanish stallions suitable for this processing. Using colloidal centrifugation, the percentage of ejaculates available to be frozen was increased from 35% to 71%, allowing us to obtain from poor-quality fresh ejaculates thawed sperm doses with similar sperm quality to that of good-quality fresh ejaculates. These results could potentially be of great interest in the equine reproductive industry when dealing with other individuals or breeds in which, initially, low sperm quality prevents or limits their inclusion in sperm-cryopreservation programs.
Abstract
The Purebred Spanish Horse, according to our clinical experience, is characterized by having a high number of stallions that do not meet the international commercial recommendations for equine-sperm cryopreservation. This means that artificial insemination with frozen semen from these stallions is less widespread than in other breeds. In this study, we investigated if the incorporation of single-layer colloidal centrifugation prior to cryopreservation in clinical conditions could increase the number of ejaculates of Purebred Spanish stallions suitable for this processing, observing the influence of centrifugation and freezing extender protocol on post-thawed sperm motility. Using colloidal centrifugation, the percentage of ejaculates available to be frozen was increased from 35% (6/17) to 71% (12/17), doubling the number of samples that could have been subjected to cryopreservation. We only found significant differences in linearity (LIN) and lateral head displacement (ALH) after 5 min of incubation at 37 °C between colloidal and simple centrifugation processing techniques. No significant differences were found between the two different colloidal protocols in any of the variables considered. Colloidal centrifugation allowed us to obtain, from worse fresh-quality ejaculates, thawed sperm doses with similar quality to that of good-quality ejaculates. BotuCrio® produced, in general, higher motility parameters and its characteristics than the other extenders analyzed, with significant differences found in comparison to Inra-Freeze® and Lac-Edta in both total (MOT) and progressive motility (PMOT) when using colloidal centrifugation and only in PMOT when applying simple centrifugation. Colloidal centrifugation optimized the efficiency of cryopreservation, as it allowed us to increase the number of ejaculates of Purebred Spanish Horses suitable to be frozen. Including these semen processing techniques in the freeze test could help to optimize equine-sperm cryopreservation protocols, especially when dealing with individuals or breeds for which initially low sperm quality prevents or limits their inclusion in sperm cryopreservation programs.
1. Introduction
Traditionally, stallions are classified as “Good/Bad Freezers” according to the capacity of their sperm to be cryopreserved [1]. Equine breeder’s selection has caused this individual variability to increase, and, nowadays, it is considered as one of the main factors that has prevented the widespread use of cryopreserved semen [2,3]. In general, 20–30% of stallions are considered to have a good frozen–thawed sperm quality, 40–60% average frozen–thawed quality and the other 20–30% are not suitable for cryopreservation [2], regardless of their fertility in natural mating [2,4,5]. This variability has also been reported between breeds [6,7].
In clinical practice, due to this variability, it is necessary to perform a freeze test in which one ejaculate is divided and processed with different cryopreservation protocols in order to establish which individual procedure better optimizes results [2,8].
There are not many papers presenting the seminal characteristics of the Purebred Spanish (P.R.E.) Horse; however, according to our clinical experience, this breed is characterized by having few stallions with more than 50% progressive-motility fresh ejaculates [9,10], which may explain why many P.R.E. stallions do not meet the international commercial recommendations for equine-sperm cryopreservation, for which percentage of progressive motility is needed to be over 50–70% in the fresh sperm before freezing [2,3,7,11] and 30–35% post-thawing [12,13]. This initial selection regarding sperm motility in raw semen is the most important variable affecting the acceptable post-thaw quality of cryopreserved ejaculates [7].
All these limitations, according to our clinical experience, determine that artificial insemination with frozen semen in P.R.E. horses is less widespread than in other breeds [14] and finding systems and procedures to increase the number of stallions and ejaculates suitable to be cooled and cryopreserved is an important goal. Among these techniques, colloidal purifying systems are proven to improve sperm quality [15,16,17,18,19,20,21] in protocols compatible with equine clinic procedures [22,23] and their use is recommended to improve fertility in sub-fertile stallions [1,24].
In this study, we investigated if the incorporation of two different single-layer colloidal-centrifugation protocols prior to cryopreservation in clinical conditions in the P.R.E. horse could increase the number of stallions or ejaculates suitable for this processing, observing the influence of centrifugation and freezing extender protocol on post-thawed sperm motility.
2. Materials and Methods
2.1. Animals
A total of six P.R.E. horses, located at Centro Militar de Cría Caballar de Ávila (CCFAA) (40.6:N 4.70:W) in Spain, were used. Clinically healthy stallions, with proven fertility, ranging in age from 7 to 19 years, were kept under controlled feeding and housing conditions. Stallions were collected on a regular basis (two collections/week) after the stabilization of the extragonadal sperm reserves, which was performed at the beginning of the reproductive season in March.
Depletion of extragonadal sperm reserves was performed by daily semen collection until the stabilization of the number of sperm in the ejaculates [25]. The number of days required differed between stallions, with a range between 4–5 consecutives days.
All stallions included in the study were subjected to an initial sperm evaluation before the beginning of the reproductive season, where morphological abnormalities could not exceed 30%.
2.2. Semen Collection
Semen was collected by allowing the stallions to mount a phantom and ejaculate into a Missouri-model artificial vagina (Nasco, Fort Atkinson, WL, USA), lubricated with a sterile non-spermicidal gel (IMV technologies, L´Aigle, France) and warmed to 45 to 50 °C prior to collection. A mare in estrous was used as sexual stimulation. Semen was filtered to capture gel. A total of three ejaculates per stallion were used, except from stallion D, for which only two ejaculates were processed (n = 17).
2.3. Experimental Design
Following international commercial recommendation for equine sperm [2,3,7], those ejaculates whose progressive motility in fresh semen was higher than 60% were classified as “Suitable for Cryopreservation” (SC). These ejaculates were subjected to the standard protocol, which includes simple centrifugation.
Ejaculates whose progressive motility in fresh semen was lower than 60% were classified as “Non-Suitable for Cryopreservation” (NSC) and were processed through colloidal centrifugation (Protocols 1 and 2).
After colloidal centrifugation, samples in which progressive motility increased ≥60% were reclassified as “Acceptable After Colloidal Centrifugation” (AACC), distinguishing between “Acceptable After Colloidal Centrifugation with Protocol 1”, (AACC1) and “Acceptable After Colloidal Centrifugation with Protocol 2” (AACC2). Semen samples whose progressive motility did not increase to 60% after colloidal centrifugation were definitely rejected for cryopreservation (non-processed samples, NP).
Samples with ≥60% progressive motility either in fresh semen or after colloidal centrifugation (SC, AACC1 and AACC2) were included in the freeze test (Figure 1).
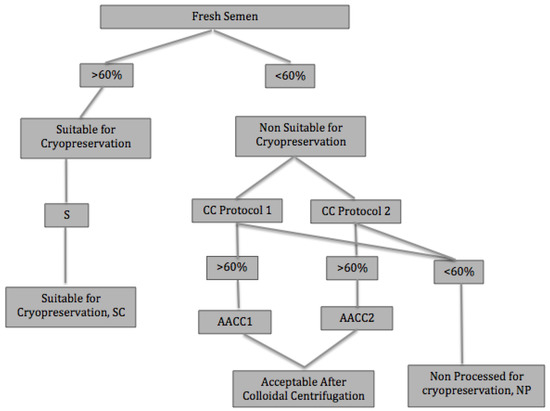
Figure 1.
Semen centrifugation protocols. S: simple centrifugation (10 mL extended semen (1:1, v:v in Inra96®); 450 g for 7 min); CC: colloidal centrifugation protocol 1 (10 mL extended semen (1:1, v:v in Inra96®) over 10 mL of Bottom Layer®; 300 g for 20 min) and Protocol 2 (5 mL raw semen over 5 mL of Bottom Layer®; 300 g for 20 min); SC: suitable for cryopreservation; AACC: acceptable after colloidal centrifugation; AACC1: acceptable after Colloidal centrifugation with Protocol 1; AACC2: acceptable after colloidal centrifugation with Protocol 2.
2.4. Fresh Ejaculate Evaluation
The 17 collected, gel-free ejaculates were immediately transported to the laboratory and maintained at 37 °C for evaluation and processing.
Spermatozoa concentration and motion characteristics were evaluated using a computer-assisted sperm-motion analyzer microscope (Sperm Class Analyzer®, Microptic SL, Barcelona, Spain) equipped with a heated stage and phase contrast optics (X 20 objective, Optiphot-2, Nikon, Japan). Warmed (37 °C) analysis chambers (fixed height of 20 μm) affixed to microscope slides (Leja Standard Count 2 chamber slides; Leja Products, B.V., Nienw-Vennep, The Netherlands) were loaded with 2 μL volume of extended semen (1:40, v:v in Inra96®). The significant settings used for CASA [22] were as follows: STR threshold for progressive motility, 60%; LIN threshold for circular spermatozoa, 50%; 32 frames per sequence; minimum of 15 frames per object; minimum area for objects 25 pix and 10 mm/s as velocity limit for immobile objects. A minimum of 500 spermatozoa were analyzed per sample. Experimental endpoints included: percentage of total motile sperm (%; MOT) and percentage of progressively motile sperm (%; PMOT).
2.5. Semen Centrifugation
Simple centrifugation: 10 mL of extended semen (1:1, v:v in Inra96®) in a 50 mL falcon tube and centrifuged (EBA 21 Centrifuge, Hettich®) at 450 g for 7 min [1].
Colloidal centrifugation: two different colloidal-centrifugation protocols were used. In 50 mL falcon tubes, 10 mL of extended semen (1:1, v:v in Inra96®) were pipetted and carefully layered, to avoid phase mixing, over 10 mL of Equipure Bottom Layer® with a density of 80% (Nidacon, International AB, Mölndal, Sweden) (Protocol 1) or 5 mL of raw semen over 5 mL of Equipure Bottom Layer® (Protocol 2). Both were equilibrated at 22 °C and centrifuged (EBA 21 Centrifuge, Hettich®) at 300 g for 20 min as described by Gutiérrez-Cepeda et al. [22].
2.6. After-Centrifugation Sperm Evaluation
After centrifugation, the supernatant and most of the gradient material was removed by aspiration. An aliquot of the resulting homogenized sperm pellet was added to a tube containing 1 mL of equilibrated Inra96®, to obtain a final concentration of 50 × 106 spermatozoa/mL.
Spermatozoa motion characteristics were evaluated using a computer-assisted sperm-motion analyzer microscope (Sperm Class Analyzer®, Microptic SL, Barcelona, Spain), as described in Section 2.4. Experimental endpoints included: percentage of total motile sperm (%; MOT) and percentage of progressively motile sperm (%; PMOT).
2.7. Freeze-Test
A total of twelve samples were, finally, processed for cryopreservation. Each sample was subjected to three different freezing protocols with three different freezing extenders commonly used in the equine reproductive industry, modified Lac-EDTA [26], Inra-Freeze® (IMV technologies, L´Aigle, France) and BotuCrio® (BioTech, Botucatu, Sao Paulo, Brazil). The required volume of each pellet to obtain a final concentration of 50 × 106 spermatozoa/mL (25 × 106 spermatozoa/per straw) was added to a fixed volume of 5mL of each extender [27]. After resuspension, semen was packed into 0.5 mL polyvinylchloride straws (IMV International, St Paul, MM, USA). Cooling rate used was that described for each extender:
- -
- Lac-EDTA: straws were frozen directly without a cooling phase [28].
- -
- Inra-Freeze®: straws were frozen after being slowly cooled to 4 °C (−0.3 °C/min) over an hour, as recommended by the manufacturer.
- -
- BotuCrio®: straws were frozen after being cooled at 4 to 6 °C for 20 min, as recommended by the manufacturer.
All straws were similarly frozen horizontally in racks placed 4 cm above the surface of liquid nitrogen for 7 min, after which they were directly plunged into liquid nitrogen [29].
2.8. Post-Thaw Sperm Evaluation
After 4 weeks of storage, straws were thawed by immersion in a 37 °C water bath for 1 min. Spermatozoa motion characteristics were evaluated using a computer-assisted sperm-motion analyzer microscope (Sperm Class Analyzer®, Microptic SL, Barcelona, Spain), as described in Section 2.4. Experimental endpoints included: percentage of total motile sperm (%; MOT); percentage of progressively motile sperm (%; PMOT); curvilinear velocity (μm/s; VCL); straight-line velocity (μm/s; VSL); average path velocity (μm/s; VAP); linearity (%; LIN); straightness (%; STR); amplitude of lateral displacement (μm; ALH) and beat cross frequency (Hz; BCF).
A minimum of 500 spermatozoa per sample were counted. The sperm was kept at 37 °C before analysis performed both 5 and 30 min after thawing.
2.9. Statistical Analysis
The effect of centrifugation and freezing extender treatment on the different motility variables were determined by ANOVA and Duncan Tests, both in general and within each group (SC and AACC), independently, for the centrifugation and the freezing extender treatments. The effect of the stallion variability was considered in the analysis. Significance was set at p < 0.05. Data were processed using the SPSS-19 statistical package.
3. Results
3.1. Prior Cryopreservation Evaluation
Progressive motility values for fresh semen and after centrifugation protocols are shown in Table 1.

Table 1.
Progressive motility values for fresh semen and after centrifugation protocols.
From the total of seventeen ejaculates, six presented PMOT > 60% in fresh sperm and were classified as SC. The other eleven ejaculates presented fresh PMOT < 60% and were included as NSC and subjected to colloidal centrifugation (Protocol 1 and 2). From these eleven NSC ejaculates, six obtained enough progressive motility (PMOT > 60%) to be included in the freeze test and were reclassified as AACC.
Five ejaculates did not obtain enough progressive motility (PMOT < 60%) to be frozen and were rejected for cryopreservation, NP.
Therefore, a total of fourteen samples from twelve ejaculates were processed for cryopreservation, three samples (1SC, 2SC, 3SC) from three ejaculates (1, 2 and 3) of stallion A, one sample (1SC) from one ejaculate (1) of stallion B, three samples (1SC, 3AACC1, 3AACC2) from two ejaculates (1 and 3) of stallion C, two samples (1AACC1, 2AACC1) from two ejaculate (1 and 2) of stallion D, one sample (1AACC2) from one ejaculate (1) of stallion and four samples (1SC, 2AACC1, 3AACC1 y 3AACC2) from three ejaculates (1, 2 and 3) of stallion F.
Among the six stallions included in the study, only stallion A presented enough fresh quality to process his three ejaculates. For 64.7% of the ejaculates, single-layer colloidal centrifugation with Equipure® was necessary to try to improve semen quality. Three ejaculates from stallion A and one from stallions B, C and F were cryopreserved following simple centrifugation, while colloidal centrifugation was required to freeze two ejaculates from stallion E, two ejaculates from F and one from stallions C and D.
3.2. Post-Thaw Evaluation
When studying freezing extenders and centrifugation protocols, we observed significant differences between horses (p < 0.05) in all variables considered. The effect of stallion was then considered when analyzing statistically the effect of the different treatments of the study. However, these differences between stallions were reduced within the SC and AACC groups.
Differences in post-thawing values after both 5 and 30 min of incubation between groups are shown in Table 2. There were only significant differences in variables LIN5, where AACC obtained higher values, and in ALH5, where SC presented a significant increased.

Table 2.
Motility parameters values 5 and 30 min after thawing.
Subsequently, differences between freezing extenders were observed in post-thawing motility parameters within the SC group (Table 3).

Table 3.
Motility parameters 5 and 30 min after thawing for freezing extenders in SC group.
In the SC group, the BotuCrio® freezing extender produced significantly higher values than the others in PMOT and BCF both 5 and 30 min and in VSL 5 min after thawing. When considering velocity parameters, both BotuCrio® and Lac-EDTA were higher than Inra-Freeze®, with significant differences found 5 min after thawing in VCL and 30 min after thawing in VCL, VSL and VAP.
Table 4 represents the differences between freeze-test extenders in post-thawing motility parameters within AACC groups.

Table 4.
Motility parameters 5 and 30 min after thawing for freezing extenders in AACC group.
Regarding the AACC group, MOT and PMOT, both 5- and 30-minute post-thawing were significantly higher (p < 0.05) with BotuCrio® than with the other extenders considered. STR and LIN 5 min after thawing were significantly lower with Lac-EDTA than with the other extenders.
Mean post-thawed progressive motility values (%) for each stallion and freezing extender are represented in Table 5.

Table 5.
Mean progressive motility values (%) 5 min after thawing for freezing extenders and stallion.
4. Discussion
International commercial recommendations for equine-sperm cryopreservation stipulates that the percentage of progressive motility must be over 50–70% in the fresh sperm before freezing [2,3,7,11] and 30–35% post-thawing [2,12,13]. These minimal requirements for the raw-semen quality of conventional equine-semen freezing programs are needed to obtain a good post-thawing sperm quality, and, among them, initial sperm motility is proven to be the most important affecting variable [7]. In our study, when seminal characteristics were analyzed in the seventeen fresh ejaculates (Table 1), we observed that only nine of the seventeen ejaculates (52.94%) presented a progressive motility greater than 50% and only six over 60% (35%). Although Miró and Papas found no differences in some sperm-quality parameters of both fresh and frozen/thawed samples between P.R.E. and non-P.R.E. stallions [30], our study showed, as also previously described [9,10], that not many ejaculates from P.R.E. stallions meet these international commercial standards.
Between the eleven ejaculates labelled “NSC” (PMOT<60%), when subjected to colloidal-centrifugation protocols prior to cryopreservation, six obtained sufficient motility to be included in the freeze test (AACC), increasing from 35% (6/17) to 71% (12/17) the percentage of ejaculates available to be frozen. Protocol 1 was more effective clinically, as it improved five out of six of the “NSC”ejaculates, whereas Protocol 2 only optimized three.
Differences between ejaculates of the same stallion were observed. This variability in the sperm motility observed between the ejaculates of the same stallion could be explained by the influence of factors such as seminal plasma composition and volume, which is also affected by sexual stimulation and sperm volume [31,32].
The importance of stallion selection prior to colloidal-centrifugation cryopreservation protocols was described by Mancill et al. [17] and Hoogewijs et al. [33]. In concordance with them, we did not process good-quality fresh ejaculates through colloidal centrifugation. Our study demonstrated that the clinical incorporation of colloidal centrifugation in low-quality ejaculates in cryopreservation protocols is of great interest, as it increases the efficiency of the freezing technique, as the number of ejaculates suitable for cryopreservation in each stallion is increased and it allows obtaining thawed sperm doses from stallions that would otherwise be rejected for cryopreservation according to its fresh sperm quality.
No significant differences were found in post-centrifugation sperm quality between the two different colloidal protocols used. When comparing post-thawed sperm quality between centrifugation protocols, we only found significant differences in LIN and ALH after 5 min of incubation.
What is more, colloidal centrifugation has proved to increase equine [15,16,17,20,34], dog [35,36,37] and buck [38] sperm quality when applied to thawed samples. Various authors [16,26,38,39,40,41,42,43] state that colloidal centrifugation prior to freezing also increases the cryosurvival of spermatozoa and/or post-thawed sperm quality. Macías García et al. [44] stated that colloidal centrifugation selects a sperm subpopulation that responds differently to osmotic shock, helping to withstand the cryopreservation process [43]. This fact could be related to lower reactive oxygen species’ (ROS) production and contamination which, in other studies, have been proven to be achieved after colloidal centrifugation [30,43].
Hoogewijs et al. [33] found an improvement in quality both before and after cryopreservation compared to cushion techniques when applying colloidal centrifugation prior to cryopreservation. This effect was higher in stallions classified as “non-suitable for cryopreservation” (stallions that produced post-thawing progressive motility <30%) [40]. In another study, Hoogewijs et al. [40] also found that when comparing these two selecting techniques prior to cryopreservation, colloidal centrifugation allowed the maintenance of the improvement after 120 min of incubation at 37 °C. Hidalgo et al. [42] also showed that single-layer centrifugation prior to cryopreservation allowed obtaining higher sperm motility and lower DNA fragmentation after thawing than when applying a modified colloid swim up and/or a sperm washing procedure. In addition, El-Essawe et al. [43] described beneficial effects on sperm chromatin integrity, high mitochondrial membrane potential and a reduction in hydrogen-peroxide production after thawing.
Similarly, Mancill et al. [17] found a higher thawing sperm quality in “subfertile stallions” when colloidal centrifugation was performed prior to cryopreservation compared to simple centrifugation, although no differences were found between centrifugation techniques within the fertile group. Mancill et al. [17] determined that the incorporation of colloidal centrifugation prior to cryopreservation in fertile stallions is not effective, as it includes an additional processing step, which causes a significant loss of presumably normal sperm and unnecessarily increases economic and technical costs.
The present colloidal-centrifugation protocols have already been used in previous works without finding a negative impact on outcomes [22,27]. However, as mentioned before, in the present work, Protocol 1 was more effective at improving the quality of NSC ejaculates. This could be related to the higher sperm concentration applied over the colloid in Protocol 2 (raw sperm), as has been previously described [45].
When studying freezing extenders and centrifugation protocols, there were significant differences between horses (p < 0.05) in all variables considered, which reflects the characteristic individual sperm-response variability among the horse population [2].
Regarding our results for freezing extenders, we can determine that BotuCrio® produced, in general, higher values in motility parameters and their characteristics compared to the others both in the SC and AACC groups. It significantly improved progressive motility both immediately and after 30 min of incubation. Lac-EDTA showed higher results for velocity parameters (VCL, VSL and VAP) than Inra-Freeze® but only within the SC group. Studies in dogs [46] and boars [47] have revealed that high sperm velocities are landmarks of fertility both in vivo and in vitro. It has also been reported previously that VCL is of key importance for the formation of the sperm reservoir at the utero-tubal junction in mice [48], that VCL and VAP are linked to the ability of ram spermatozoa to penetrate cervical mucus [49], and that post-thaw VSL is related to the fertility of bull [50] and human [51] spermatozoa. Likewise, some authors [46,52] described a reduction in the sperm’s ability to travel along the uterus and arrive at the fertilization site when velocity variables decreased.
These differences could be related with the cryoprotectants combination presented in the extender. Both Inra-Freeze® and Lac-EDTA extenders include Glycerol as the only cryoprotectant agent, while BotuCrio® includes a lower Glycerol concentration and Metilformamine. It has been published that sperm cryopreservation with amines as cryoprotectants are effective at improving motility, viability in the female genital tract and fertility of frozen–thawed sperm, as well as at reducing individual and breed variability, mainly in horses classified as “bad freezers” [6,53,54,55]. Similarly, lower cryoprotectant concentrations benefits thawed-sperm viability, especially in “bad freezers” stallions [56].
The development of new freezing extenders, as seemed to happen with amides, in combination with sperm selection techniques can be used in equine reproduction clinics to optimize sperm cryopreservation, especially when dealing with low fresh-sperm quality breeds, which limits their inclusion in freezing commercial protocols.
5. Conclusions
The clinical use of colloidal centrifugation on equine sperm freeze test in P.R.E. stallions optimized the efficiency of the cryopreservation technique, as low-quality ejaculates were incorporated into this procedure. Colloidal centrifugation allowed us to obtain from poor-quality fresh ejaculates thawed-sperm doses with similar sperm quality to that of good-quality fresh ejaculates. These results could potentially be of great interest in the equine reproductive industry when dealing with other individuals or breeds in which initially low sperm quality prevents or limits their inclusion in sperm cryopreservation programs. Further studies with a larger sample size are needed to confirm these results.
Author Contributions
The study was conceived by C.S. who, together with L.G.-C. and F.C., participated in its design and coordination; L.G.-C., J.C.B. and F.C. performed the semen collection, processing of samples for centrifugation, cryopreservation, and sperm evaluation; L.G.-C. and C.S. performed the statistical analysis and, with F.C. and J.C.B., interpreted the data; The manuscript was written by L.G.-C., helped by C.S., F.C. and J.C.B., who revised it critically. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
Gutiérrez-Cepeda, L. was supported by MICINN FPU fellowship AP2008-02034.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the fact that samples used in the study were aliquots from ejaculates obtained from the semen extractions that were carried out regularly and routinely on the stallions included in an artificial-insemination program.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are indebted to the FESCCR for their assistance in this work and to Robin Blakey for his assistance in language corrections.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Loomis, P.R. Advanced methods for handling and preparation of stallion semen. Vet. Clin. N. Am. Equine 2006, 22, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Loomis, P.R.; Graham, J.K. Commercial semen freezing: Individual male variation in cryosurvival and the response of stallion sperm to customized freezing protocols. Anim. Reprod. Sci. 2008, 105, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Samper, J.C.; Morris, C.A. Current methods for stallion semen cryopreservation: A survey. Theriogenology 1998, 49, 895–903. [Google Scholar] [CrossRef]
- Brinsko, S.P.; Crockett, E.C.; Squires, E.L. Effect of centrifugation and partial removal of seminal plasma on equine spermatozoal motility after cooling and storage. Theriogenology 2000, 54, 129–136. [Google Scholar] [CrossRef]
- Tischner, M. Evaluation of deep-frozen semen in stallions. J. Reprod. Fertil. 1979, 27, 53–59. [Google Scholar]
- Alvarenga, M.A.; Leao, K.M.; Papa, F.O.; Landim-Alvarenga, F.C.; Medeiros, A.S.L.; Gomes, G.M. The use of alternative cryoprotectors for freezing stallion semen. In Proceedings of a Workshop on Transporting Gametes and Embryos; Squires, E., Wade, J.F., Eds.; Havemeyer Foundation, R&W Publications Limited: Newmarket, UK, 2003; pp. 74–76. [Google Scholar]
- Aurich, J.; Kuhl, J.; Tichy, A.; Aurich, C. Efficiency of Semen Cryopreservation in Stallions. Animals 2020, 10, 1033. [Google Scholar] [CrossRef]
- Amann, R.P.; Pickett, B.W. Principles of cryopreservation and a review of cryopreservation of stallion spermatozoa. J. Equine Vet. Sci. 1987, 7, 145–173. [Google Scholar] [CrossRef]
- Benito, D.; Alvarez, A.; Crespo, F.; Mateos, E.; Gómez-Cuétara, C.; Serres, C. Análisis Computerizado del Semen de Caballo de Pura Raza Española; IV Congreso Ibérico de Reproducción Animal: Las Palmas, España, 2003. [Google Scholar]
- Balmori, A.; Serres, C. Efecto de la vacuna anti-GnRH sobre la calidad espermática en caballos. In Trabajo Fin de Grado; Facultad de Veterinaria, Universidad Complutense d e Madrid: Madrid, España, 2019. [Google Scholar]
- Sieme, H. Freezing semen. In Equine Reproduction, 2nd ed.; McKinnon, A.O., Squires, E.L., Vaala, W.E., Varner, D.D., Eds.; Wiley-Blackwell: Chichester, UK, 2011; Volume 2, pp. 2972–2982. [Google Scholar]
- Vidament, M.; Dupree, A.M.; Julienne, P.; Evian, A.; Noue, P.; Palmer, E. Equine frozen semen: Freezability and fertility field results. Theriogenology 1997, 48, 907–917. [Google Scholar] [CrossRef]
- WBFSH. World Breeding Federation for Sport Horses: Semen Standards. Available online: http://www.wbfsh.org/files/Semen%20standards.pdf (accessed on 19 May 2020).
- Gutiérrez-Cepeda, L. Optimización de Las Técnicas de Acondicionamiento del Semen Equino Para los Procesos de Conservación Seminal. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2013; pp. 47–48. [Google Scholar]
- García, B.M.; Morrell, J.; Ortega-Ferrusola, C.; González-Fernández, L.; Tapia, J.; Rodriguez-Martínez, H.; Peña, F. Centrifugation on a single layer of colloid selects improved quality spermatozoa from frozen-thawed stallion semen. Anim. Reprod. Sci. 2009, 114, 193–202. [Google Scholar] [CrossRef]
- García, B.M.; Fernández, L.G.; Morrell, J.M.; Ferrusola, C.O.; Tapia, J.A.; Martínez, H.R.; Pena, F.J. Single-layer centrifugation through colloid positively modifies the sperm subpopulation structure of frozen-thawed stallion spermatozoa. Reprod. Dome Anim. 2009, 44, 523–526. [Google Scholar] [CrossRef]
- Mancill, S.S.; Love, C.C.; Brinsko, S.P.; Edmond, A.J.; Foster, M.L.; Teague, J.A. Effect of density gradient centrifugation on cryopreservation of equine spermatozoa. Anim. Reprod. Sci. Suppl. 2010, 121, 208–209. [Google Scholar]
- Morrell, J.M.; Johannisson, A.; Dalin, A.M.; Rodriguez-Martínez, H. Morphology and chromatin integrity of stallion spermatozoa prepared by density gradient and single layer centrifugation through silica colloids. Reprod. Domest. Anim. 2009, 44, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Al-Kass, Z.; Brown, A.; Johannisson, A.; Ntallaris, T.; Morrell, J.M. Variation among stallions in sperm quality after single layer centrifugation. Reprod. Domest. Anim. 2021, 56, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Podico, G.; Ellerbrock, R.E.; Curcio, B.R.; Cheong, S.H.; Lima, F.S.; Canisso, I.F. Single-Layer Colloid Centrifugation as a Method to Process Urine-Contaminated Stallion Semen After Freesing-Thawing. J. Equine Vet. Sci. 2020, 87, 102910. [Google Scholar] [CrossRef]
- Morrell, J.M.; Nunes, M.M. Practical guide to single layer centrifugation of stallion semen. Equine Vet. Educ. 2018, 30, 392–398. [Google Scholar] [CrossRef]
- Gutiérrez-Cepeda, L.; Fernández, S.; Crespo, F.; Gósalvez, J.; Serres, C. Simple and economic colloidal centrifugation protocols may be incorporated to the clinical equine sperm processing procedure. Anim. Reprod. Sci. 2011, 124, 85–89. [Google Scholar] [CrossRef]
- Morrell, J.M. Stallion Sperm Selection: Past, Present, and Future Trends. J. Equine Vet. Sci. 2012, 32, 436–440. [Google Scholar] [CrossRef]
- Varner, D.D.; Love, C.C.; Brinsko, S.P.; Blanchard, T.L.; Hartman, D.; Bliss, S.; Carroll, S.; Eslick, M. Semen Processing for the Subfertile Stallion. J. Equine Vet. Sci. 2008, 28, 677–685. [Google Scholar] [CrossRef]
- Stich, K.; Brinsko, S.; Thompson, J.; Love, C.; Miller, C.; Blanchard, T.; Varner, D. Stabilization of extragonadal sperm reserves in stallions: Application for determination of daily sperm output. Theriogenology 2002, 58, 397–400. [Google Scholar]
- Martin, J.C.; Klug, E.; Günzel, A.R. Centrifugation of stallion semen and its storage in large volume straws. J. Reprod. Fertil. Suppl. 1979, 27, 47–51. [Google Scholar]
- Gutiérrez-Cepeda, L.; Fernández, A.; Crespo, F.; Ramírez, M.A.; Gosálvez, J.; Serres, C. The effect of two pre-cryopreservation single layer colloidal centrifugation protocols in combination with different freezing extenders on the fragmentation dynamics of thawed equine sperm DNA. Acta Vet. Scand. 2012, 54, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Henry, M.; Nunes, S.A.; Mello, S.L.V. Effect of packaging on the quality of frozen donkey semen, evaluated in vitro after thawing. Rev. Bras. Reprod. Anim. 1997, 21, 140–146. [Google Scholar]
- Cochran, J.D.; Amann, R.P.; Froman, D.P.; Pickett, B.W. Effects of centrifugation, glicerol level, cooling to 5 °C, freezing rate and thawing rate on the post-thaw motility of equine sperm. Theriogenology 1984, 22, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Miró, J.; Papas, M. Improvement of cryopreservation protocol in both purebred horses including Spanish horses. SPA J. Agric. Res. 2018, 16, e0406. [Google Scholar] [CrossRef]
- Foote, R.H. Factors influencing the quantity and quality of semen harvested from bulls, rams, boars and stallions. J. Anim. Sci. 1978, 47, 1–11. [Google Scholar]
- Ionata, L.M.; Anderson, T.M.; Pickett, B.W.; Heird, J.C.; Squires, E.L. Effect of supplementary sexual preparation on semen characteristics of stallions. Theriogenology 1991, 36, 923–937. [Google Scholar] [CrossRef]
- Hoogewijs, M.; Morrell, J.; VAN Soom, A.; Govaere, J.; Johannisson, A.; Piepers, S.; DE Schauwer, C.; DE Kruif, A.; DE Vliegher, S. Sperm selection using single layer centrifugation prior to cryopreservation can increase thawed sperm quality. Equine Vet. J. 2011, 43, 35–41. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agarwal, A. Sperm quality improvement in cryopreserved human semen. J. Urol. 1996, 156, 1008–1012. [Google Scholar] [CrossRef]
- Dorado, J.; Gálvez, M.J.; Morrell, J.M.; Alcaráz, L.; Hidalgo, M. Use of single-layer centrifugation with Androcoll-C to enhance sperm quality in frozen-thawed dog semen. Theriogenology 2013, 80, 955–962. [Google Scholar] [CrossRef]
- Dorado, J.; Ortiz, M.; Urbano, M.; Hidalgo, M. Single-layer centrifugation through PureSperm® 80 selects improved quality spermatozoa from forzen-thawed dog semen. Anim. Reprod. Sci. 2013, 140, 232–240. [Google Scholar] [CrossRef]
- Urbano, M.; Dorado, J.; Ortiz, I.; Morrell, J.M.; Demyda-Peyrás, S.; Gálvez, M.J.; Alcaraz, L.; Ramírez, L.; Hidalgo, M. Effect of cryopreservation and single layer centrifugation oncanine sperm DNA fragmentation assessed by the spermchromatin dispersion test. Anim. Reprod. Sci. 2013, 143, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Rabadán, P.; Morrell, J.; Johannisson, A.; Ramón, M.; García-Álvarez, O.; Maroto-Morales, A.; Álvaro-García, P.; Pérez-Guzmán, M.; Fernández-Santos, M.; Garde, J.; et al. Single layer centrifugation (SLC) improves sperm quality of cryopreserved Blanca-Celtibérica buck semen. Amin. Reprod. Sci. 2012, 136, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Alborcia, M.; Morrell, J.; Parrilla, I.; Barranco, I.; Vázquez, J.; Martinez, E.; Roca, J. Improvement of boar sperm cryosurvival by using single-layer colloid centrifugation prior freezing. Theriogenology 2012, 78, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Hoogewijs, M.; Piepers, S.; Govaere, J.; De Schauwer, C.; de Kruif, A.; Morrell, J. Sperm longevity following pre-freeze sperm selection. J. Equine Vet. Sci. 2012, 32, 489. [Google Scholar] [CrossRef]
- Nongbua, T.; Johannisson, A.; Edman, A.; Morrell, J.M. Effects of single layer centrifugation (SLC) on bull spermatozoa prior to freezing on post-thaw semen characteristics. Reprod. Domest. Anim. 2017, 52, 596–602. [Google Scholar] [CrossRef]
- Hidalgo, M.; Ortiz, I.; Dorado, J.; Morrell, J.M.; Gosálvez, J.; Consuegra, C.; Diaz-Jimenez, M.; Pereira, B.; Crespo, F. Stallion sperm selection prior to freezing usigna modified colloid swim-up procedure without centrifugation. Anim. Reprod. Sci. 2017, 185, 83–88. [Google Scholar] [CrossRef]
- Al-Essawe, E.M.; Johannisson, A.; Wulf, M.; Aurich, C.; Morrell, J.M. Improved cryosurvival of stallion spermatozoa after colloid centrifugation is independent of the addition of seminal plasma. Cryobiology 2018, 81, 145–152. [Google Scholar] [CrossRef]
- Macías García, B.; González-Fernández, L.; Gallardo-Bolañosa, J.M.; Peña, F.J.; Johannisson, A.; Morrell, J.M. Androcoll-E large selects a subset of live stallion spermatozoa capable of producing ROS. Anim. Reprod. Sci. 2012, 132, 74–82. [Google Scholar] [CrossRef]
- Morrell, J.M.; Rodriguez-Martinez, H.; Johannisson, A. Single layer centrifugation of stallion spermatozoa consistently selects the most robust spermatozoa from the rest of the ejaculate in a large sample size: Data from 3 breeding seasons. Equine Vet. J. 2010, 42, 579–585. [Google Scholar] [CrossRef]
- Silva, A.R.; Cardoso, R.C.; Silva, L.D.M.; Chirinéa, V.H.; Lopes, M.D.; Souza, F.F. Prognostic value of canine frozen-thawed semen parameters on in vitro sperm–oocyte interactions. Theriogenology 2006, 66, 456–462. [Google Scholar] [CrossRef]
- Holt, C.; Holt, W.V.; Moore, H.D.; Reed, H.C.; Curnock, R.M. Objectively measured boar sperm motility parameters correlate with the outcomes of on-farm inseminations: Results of two fertility trials. J. Androl. 1997, 8, 312–323. [Google Scholar]
- Olds-Clarke, P. How does poor motility alter sperm fertilizing ability. J. Androl. 1996, 17, 183–186. [Google Scholar] [PubMed]
- Robayo, I.; Montenegro, V.; Valdés, C.; Cox, J.F. CASA assessment of kinematic parameters of ram spermatozoa and their relationship to migration efficiency in ruminant cervical mucus. Reprod. Domest. Anim. 2008, 43, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Gillan, L.; Kroetsch, T.; Chis Maxwell, W.M.; Evans, G. Assessment of in vitro sperm characteristics in relation to fertility in dairy bulls. Anim. Reprod. Sci. 2008, 103, 201–214. [Google Scholar] [CrossRef]
- Van den Bergh, M.; Emiliani, S.; Biramane, J.; Vannin, A.S.; Englert, Y. A first prospective study of the individual straight line velocity of the spermatozoon and its influences on the fertilization rate after intracytoplasmic sperm injection. Hum. Reprod. 1998, 13, 3103–3107. [Google Scholar] [CrossRef]
- Liu, I.K.; Scott, M.A. Sperm in the oviduct. J. Equine Vet. Sci. 2000, 20, 836. [Google Scholar] [CrossRef]
- Alvarenga, M.A.; Papa, F.O.; Landim-Alvarenga, F.C.; Medeiros, A.S.L. Amides as cryoprotectants for freezing stallion semen: A review. Anim. Reprod. Sci. 2005, 89, 105–113. [Google Scholar] [CrossRef]
- Álvarez, C.; Gil, L.; González, N.; Olaciregui, M.; Luño, V. Equine sperm post-thaw evaluation after the addition of different cryoprotectants added to INRA 96 extender. Cryobiology 2014, 69, 144–148. [Google Scholar] [CrossRef]
- Melo, C.M.; Zahn, F.S.; Martin, I.; Orlandi, C.; Dell’Aqua, J.A., Jr.; Alvarenga, M.A.; Papa, F.O. Influence of semen storage and crioprotectant on post-thaw viability and fertility of stallion. J. Equine Vet. Sci. 2007, 27, 171–175. [Google Scholar] [CrossRef]
- Hoffmann, N.; Oldenhof, H.; Morandini, C.; Rohn, K.; Sieme, H. Optimal concentrations of cryoprotective agents for semen from stallions that are classified ‘good’ or ‘poor’ for freezing. Anim. Reprod. Sci. 2011, 25, 112–118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).