Resveratrol Improves the Frozen-Thawed Ram Sperm Quality
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ethical Approval
2.3. Animals
2.4. Collection of Semen
2.5. Semen Freezing and Thawing
2.6. Evaluation of Sperm Motility
2.7. Evaluation of Sperm Acrosome Integrity and Plasma Membrane Integrity
2.8. Evaluation of Mitochondrial Activity
2.9. Evaluation of ATP Content
2.10. Evaluation of NADH/NAD+
2.11. Evaluation of Sperm ROS Content
2.12. Evaluation of Sperm MDA Content
2.13. Evaluation of Sperm LPO Content
2.14. Evaluation of GSH Content, GPx, SOD, and CAT Activity
2.15. Evaluation of Sperm Oxidative DNA Damage
2.16. Western Blotting
2.17. Statistical Analysis
3. Result
3.1. Addition of Resveratrol Improved Sperm Motility Parameters
3.2. Addition of Resveratrol Improved the Sperm Acrosome Integrity and Plasma Membrane Integrity
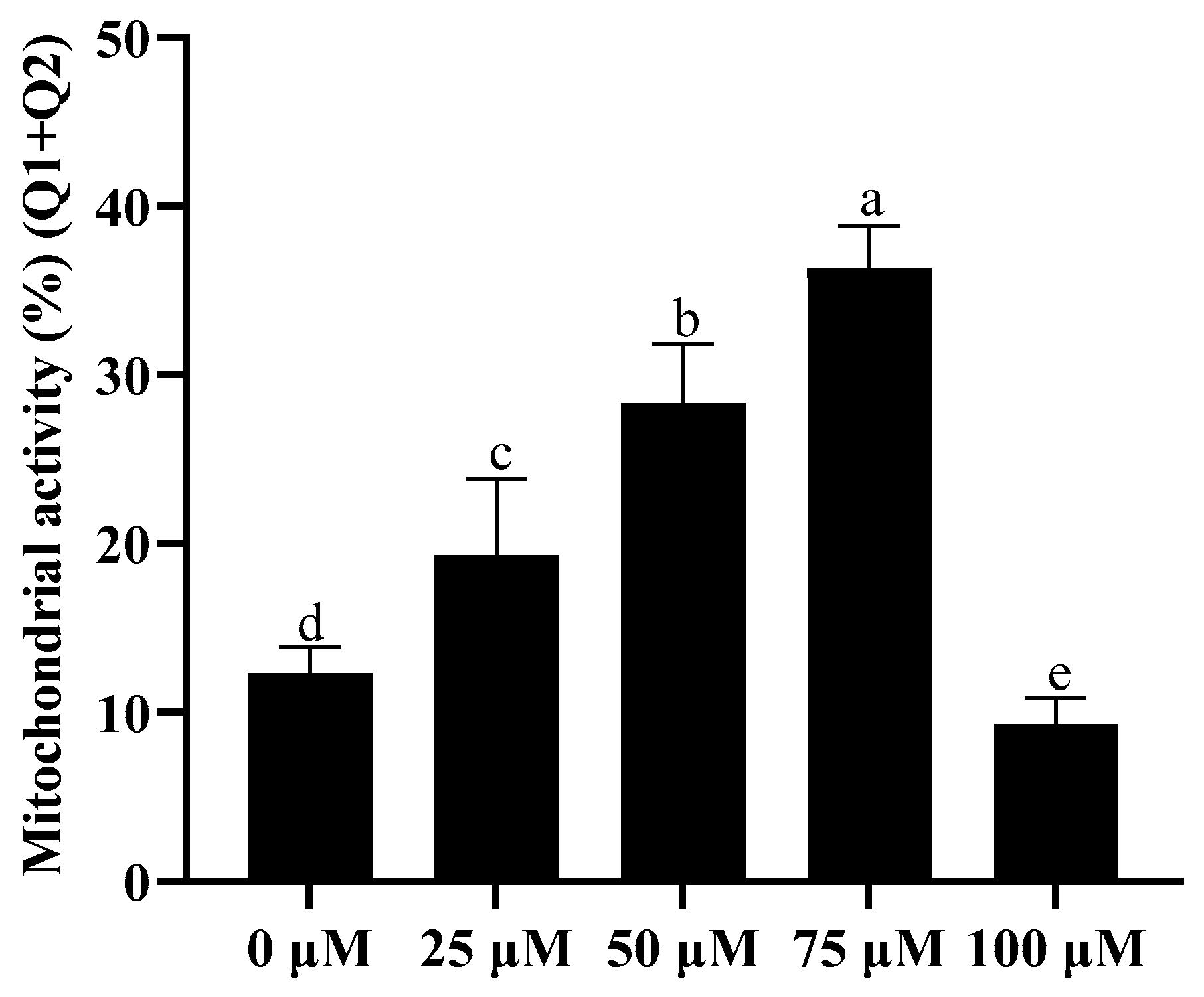
3.3. Addition of Resveratrol Improved Sperm Mitochondrial Activity
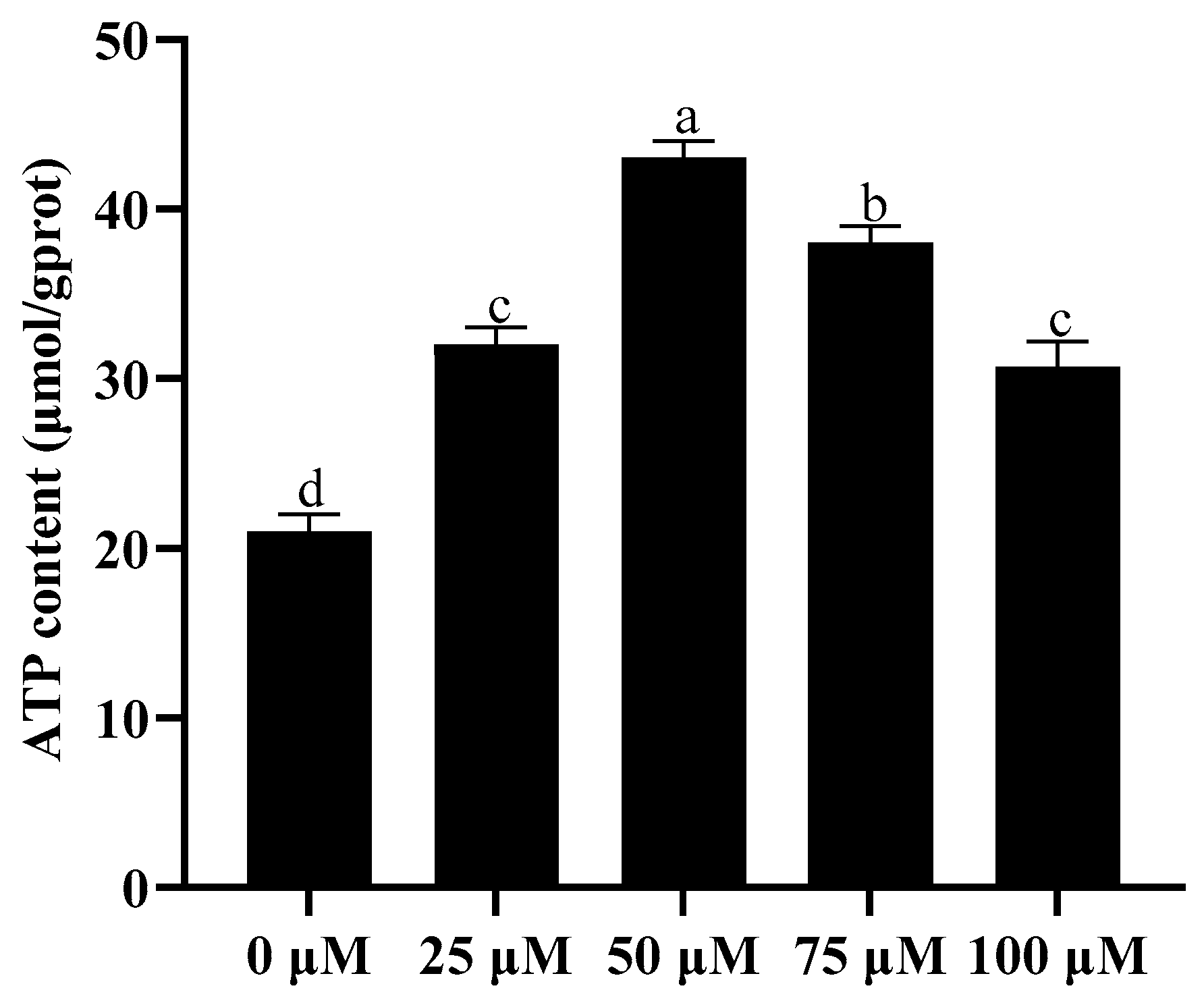
3.4. Addition of Resveratrol Improved Sperm ATP Content
3.5. Addition of Resveratrol Improved Sperm NAD+ Content and Reduced Sperm NADH/NAD+
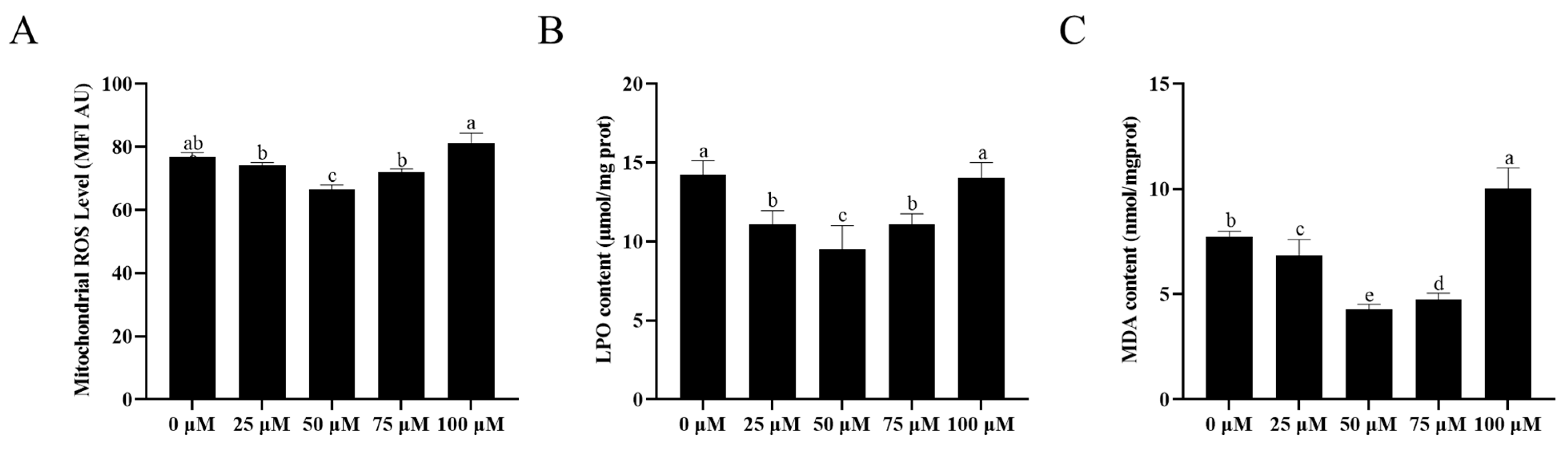
3.6. Addition of Resveratrol Reduced Sperm LPO, ROS Level, and MDA Content
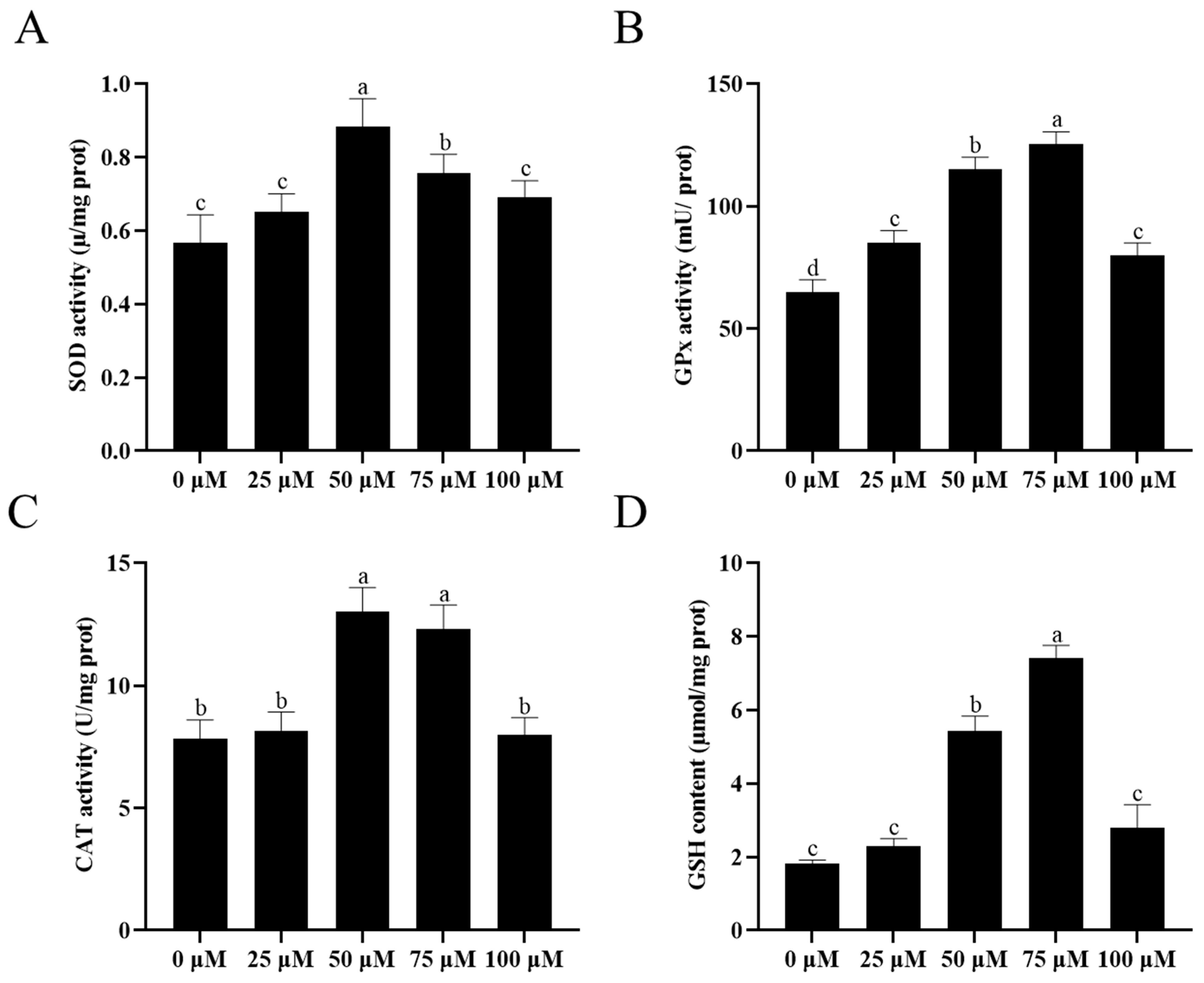
3.7. Addition of Resveratrol Improved the Sperm Antioxidative Ability
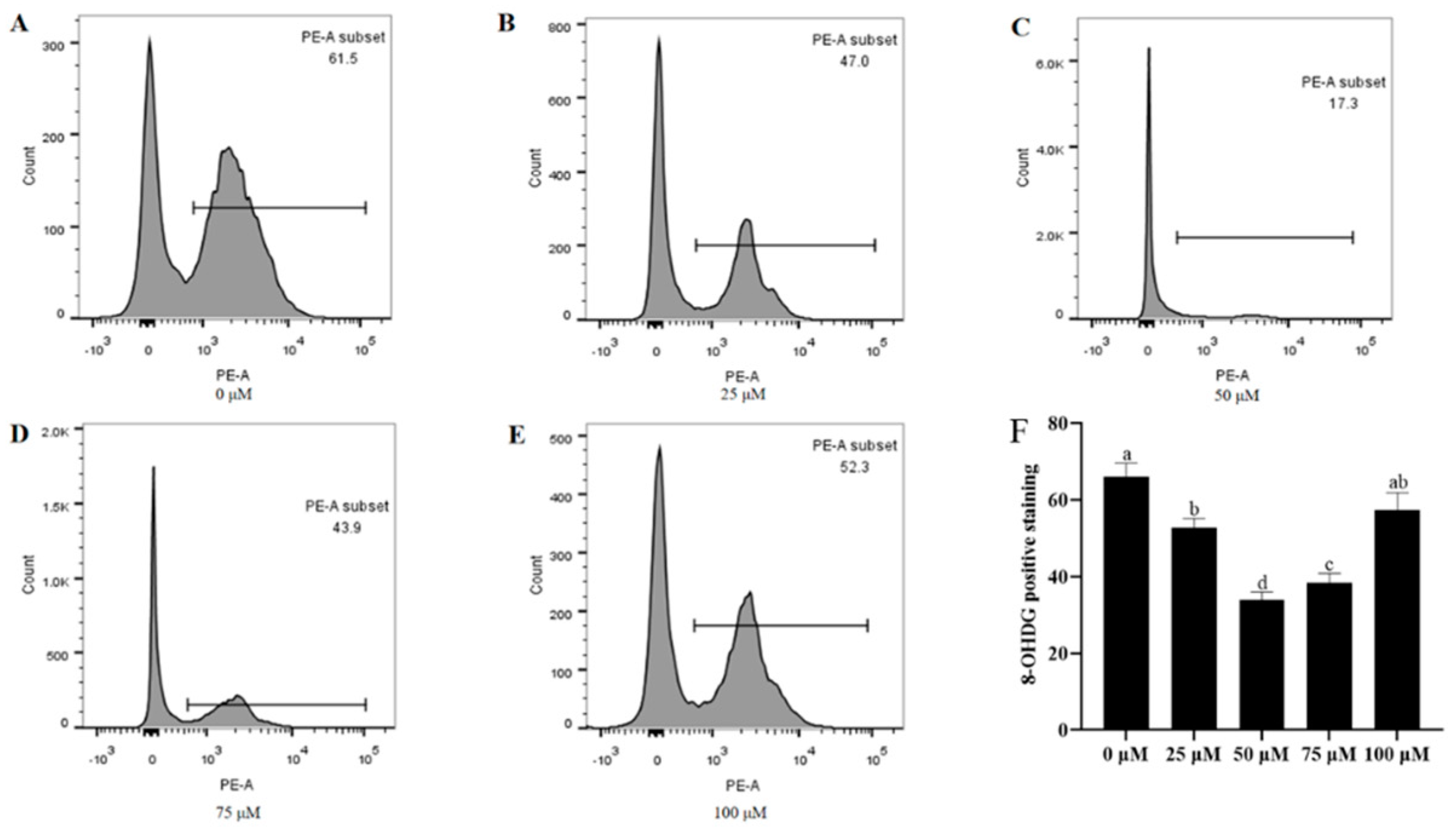
3.8. Addition of Resveratrol Reduced the Oxidative DNA Damage
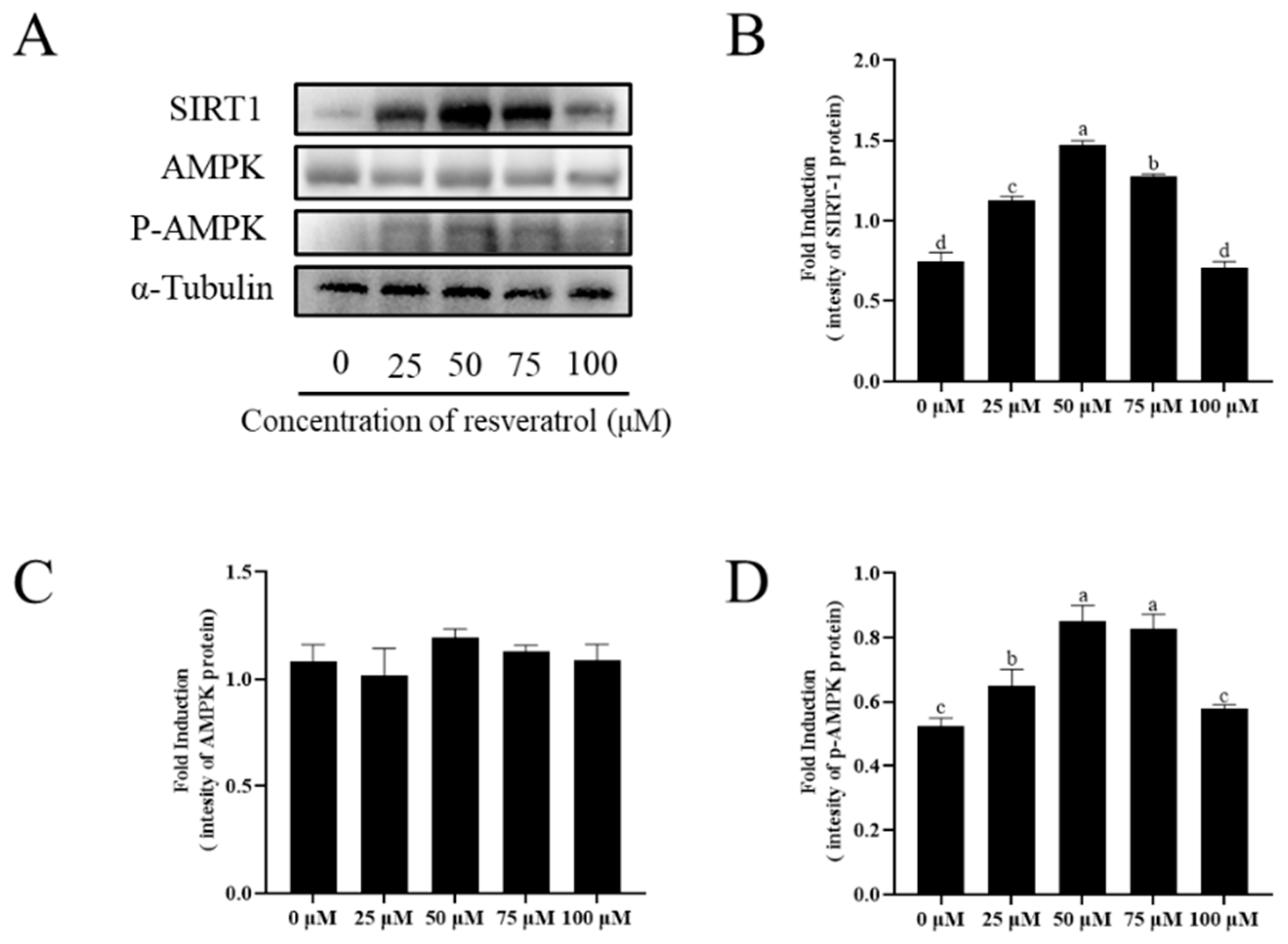
3.9. Addition of Resveratrol Promotes AMPK Phosphorylation against ROS Damage by Activating SIRT1
3.10. Addition of Resveratrol Attenuated the Sperm Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yanez-Ortiz, I.; Catalan, J.; Rodriguez-Gil, J.E.; Miro, J.; Yeste, M. Advances in sperm cryopreservation in farm animals: Cattle, horse, pig and sheep. Anim. Reprod. Sci. 2022, 246, 106904. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Wu, G.; Hong, Q.; Quan, G. Spermatozoa Cryopreservation: State of Art and Future in Small Ruminants. Biopreserv. Biobank. 2019, 17, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Anel-Lopez, L.; Boixo, J.C.; Chamorro, C.; Neila-Montero, M.; Montes-Garrido, R.; de Paz, P.; Anel, L. Current challenges in sheep artificial insemination: A particular insight. Reprod. Domest. Anim. 2019, 54 (Suppl. 4), 32–40. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.; Hollinshead, F.K.; Maxwell, W.M. Preservation and artificial insemination of sexed semen in sheep. Reprod. Fertil. Dev. 2004, 16, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Khosravizadeh, Z.; Khodamoradi, K.; Rashidi, Z.; Jahromi, M.; Shiri, E.; Salehi, E.; Talebi, A. Sperm cryopreservation and DNA methylation: Possible implications for ART success and the health of offspring. J. Assist. Reprod. Genet. 2022, 39, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Peris, S.I.; Bilodeau, J.F.; Dufour, M.; Bailey, J.L. Impact of cryopreservation and reactive oxygen species on DNA integrity, lipid peroxidation, and functional parameters in ram sperm. Mol. Reprod. Dev. 2007, 74, 878–892. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef]
- Beygi, Z.; Forouhari, S.; Mahmoudi, E.; Hayat, S.M.G.; Nourimand, F. Role of Oxidative Stress and Antioxidant Supplementation in Male Fertility. Curr. Mol. Med. 2021, 21, 265–282. [Google Scholar] [CrossRef]
- Nazari, P.; Farshad, A.; Hosseini, Y. Protective Effects of Trehalose and Pentoxifylline on Goat Sperm Exposed to Chilling-Freezing Process. Biopreserv. Biobank. 2022, 20, 540–550. [Google Scholar] [CrossRef]
- Makris, A.; Alevra, A.I.; Exadactylos, A.; Papadopoulos, S. The Role of Melatonin to Ameliorate Oxidative Stress in Sperm Cells. Int. J. Mol. Sci. 2023, 24, 15056. [Google Scholar] [CrossRef]
- Rubinstein, S.; Breitbart, H. Role of spermine in mammalian sperm capacitation and acrosome reaction. Biochem. J. 1991, 278 Pt 1, 25–28. [Google Scholar] [CrossRef]
- Liman, M.S.; Hassen, A.; McGaw, L.J.; Sutovsky, P.; Holm, D.E. Potential Use of Tannin Extracts as Additives in Semen Destined for Cryopreservation: A Review. Animals 2022, 12, 1130. [Google Scholar] [CrossRef] [PubMed]
- Parameswari, R.; Rao, K.A.; Manigandan, P.; Vickram, A.S.; Archana, A.; Sridharan, T.B. Tea polyphenol-T. arjuna bark as sperm antioxidant extender in infertile smokers. Cryoletters 2017, 38, 95–99. [Google Scholar] [PubMed]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.L.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The Role of Resveratrol in Mammalian Reproduction. Molecules 2020, 25, 4554. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutary, M.G.; Al-Ghadi, M.Q.; Ammari, A.A.; Al-Himadi, A.R.; Al-Jolimeed, A.H.; Arafah, M.W.; Amran, R.A.; Aleissa, M.S.; Swelum, A.A. Effect of different concentrations of resveratrol on the quality and in vitro fertilizing ability of ram semen stored at 5 degrees C for up to 168 h. Theriogenology 2020, 152, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Su, W.D.; Qiu, L.J.; Wang, X.; Liu, J. Resveratrol protects human sperm against cryopreservation-induced injury. Zhonghua Nan Ke Xue 2018, 24, 499–503. [Google Scholar] [PubMed]
- Kaeoket, K.; Chanapiwat, P. The Beneficial Effect of Resveratrol on the Quality of Frozen-Thawed Boar Sperm. Animals 2023, 13, 2829. [Google Scholar] [CrossRef]
- Longobardi, V.; Zullo, G.; Salzano, A.; De Canditiis, C.; Cammarano, A.; De Luise, L.; Puzio, M.V.; Neglia, G.; Gasparrini, B. Resveratrol prevents capacitation-like changes and improves in vitro fertilizing capability of buffalo frozen-thawed sperm. Theriogenology 2017, 88, 1–8. [Google Scholar] [CrossRef]
- Najafi, A.; Daghigh Kia, H.; Hamishehkar, H.; Moghaddam, G.; Alijani, S. Effect of resveratrol-loaded nanostructured lipid carriers supplementation in cryopreservation medium on post-thawed sperm quality and fertility of roosters. Anim. Reprod. Sci. 2019, 201, 32–40. [Google Scholar] [CrossRef]
- Shabani Nashtaei, M.; Amidi, F.; Sedighi Gilani, M.A.; Aleyasin, A.; Bakhshalizadeh, S.; Naji, M.; Nekoonam, S. Protective features of resveratrol on human spermatozoa cryopreservation may be mediated through 5′ AMP-activated protein kinase activation. Andrology 2017, 5, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Dong, H.; Zhao, P.; Shang, C.; Qi, H.; Ma, Y.; Gao, C.; Zhang, D.; Shen, J.; Lei, Y.; et al. Resveratrol and lycium barbarum polysaccharide improve Qinling giant panda (Ailuropoda melanoleuca Qinlingensis) sperm quality during cryopreservation. BMC Vet. Res. 2022, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Zhu, Z. Carboxylated epsilon-Poly-L-Lysine Supplementation of the Freezing Extender Improves the Post-Thawing Boar Sperm Quality. Animals 2022, 12, 1726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Min, L.; Li, Y.; Lang, Y.; Hoque, S.A.M.; Adetunji, A.O.; Zhu, Z. Beneficial Effect of Proline Supplementation on Goat Spermatozoa Quality during Cryopreservation. Animals 2022, 12, 2626. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, R.; Ma, G.; Bai, W.; Fan, X.; Lv, Y.; Luo, J.; Zeng, W. 5′-AMP-Activated Protein Kinase Regulates Goat Sperm Functions via Energy Metabolism In Vitro. Cell Physiol. Biochem. 2018, 47, 2420–2431. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Fan, X.; Zeng, Y.; Wang, L.; Zhu, Z.; Li, R.; Tian, X.; Wang, Y.; Lin, Y.; Wu, D.; et al. Resveratrol protects boar sperm in vitro via its antioxidant capacity. Zygote 2020, 28, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wu, X.; Zhu, Z.; Lv, Y.; Zheng, Y.; Lu, H.; Zhou, K.; Wu, D.; Zeng, W.; Dong, W.; et al. Polyamines protect boar sperm from oxidative stress in vitro. J. Anim. Sci. 2022, 100, skac069. [Google Scholar] [CrossRef] [PubMed]
- Wulster-Radcliffe, M.C.; Wang, S.; Lewis, G.S. Transcervical artificial insemination in sheep: Effects of a new transcervical artificial insemination instrument and traversing the cervix on pregnancy and lambing rates. Theriogenology 2004, 62, 990–1002. [Google Scholar] [CrossRef]
- Gogol, P. The quality and fertilizing ability of the ram semen cryopreserved around 40 years ago. Pol. J. Vet. Sci. 2022, 25, 631–633. [Google Scholar] [CrossRef]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Fan, X.; Lv, Y.; Zheng, Y.; Hoque, S.A.M.; Wu, D.; Zeng, W. Resveratrol Improves Boar Sperm Quality via 5′AMP-Activated Protein Kinase Activation during Cryopreservation. Oxid. Med. Cell Longev. 2019, 2019, 5921503. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Lourenco, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, D.; Bornman, M.S.; Van Der Merwe, M.P.; Du Plessis, D.J.; Oosthuizen, J.M. Differential sperm motility scoring and sperm ATP concentrations. Arch. Androl. 1993, 30, 69–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Pierantoni, R. Mitochondrial Reactive Oxygen Species (ROS) Production Alters Sperm Quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Jahan, S.; Ullah, H.; Ullah, F.; Salman, M.M. The addition of resveratrol in tris citric acid extender ameliorates post-thaw quality parameters, antioxidant enzymes levels, and fertilizing capability of buffalo (Bubalus bubalis) bull spermatozoa. Theriogenology 2020, 152, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.T.; Wang, J.R.; Sun, L.Z.; Jin, X.H.; Shi, X.Y.; Lin, J.Y.; Yue, S.L.; Zhou, J.B. Effects of astaxanthin on plasma membrane function and fertility of boar sperm during cryopreservation. Theriogenology 2021, 164, 58–64. [Google Scholar] [CrossRef]
- Shahat, A.M.; Thundathil, J.C.; Kastelic, J.P. Melatonin improves post-thaw sperm quality after mild testicular heat stress in rams. Reprod. Domest. Anim. 2023, 58, 423–430. [Google Scholar] [CrossRef]
- Oldenhof, H.; Gojowsky, M.; Wang, S.; Henke, S.; Yu, C.; Rohn, K.; Wolkers, W.F.; Sieme, H. Osmotic stress and membrane phase changes during freezing of stallion sperm: Mode of action of cryoprotective agents. Biol. Reprod. 2013, 88, 68. [Google Scholar] [CrossRef]
- Garcez, M.E.; dos Santos Branco, C.; Lara, L.V.; Pasqualotto, F.F.; Salvador, M. Effects of resveratrol supplementation on cryopreservation medium of human semen. Fertil. Steril. 2010, 94, 2118–2121. [Google Scholar] [CrossRef]
- Silva, E.C.; Cajueiro, J.F.; Silva, S.V.; Soares, P.C.; Guerra, M.M. Effect of antioxidants resveratrol and quercetin on in vitro evaluation of frozen ram sperm. Theriogenology 2012, 77, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Falchi, L.; Pau, S.; Pivato, I.; Bogliolo, L.; Zedda, M.T. Resveratrol supplementation and cryopreservation of buck semen. Cryobiology 2020, 95, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Tvrda, E.; Kovacik, A.; Tusimova, E.; Massanyi, P.; Lukac, N. Resveratrol offers protection to oxidative stress induced by ferrous ascorbate in bovine spermatozoa. J. Environ. Sci. Health A Toxic/Hazard. Subst. Environ. Eng. 2015, 50, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A. The Role of the Natural Antioxidant Mechanism in Sperm Cells. Reprod. Sci. 2022, 29, 1387–1394. [Google Scholar] [CrossRef]
- Ofosu, J.; Nartey, M.A.; Mo, X.; Ye, J.; Zhang, Y.; Zeng, C.; Zhang, M.; Fang, Y.; Zhou, G. Ram sperm cryopreservation disrupts metabolism of unsaturated fatty acids. Theriogenology 2023, 204, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Di Emidio, G.; Vitti, M.; Di Carlo, M.; Santini, S., Jr.; D’Alessandro, A.M.; Falone, S.; Amicarelli, F. Sirtuin Functions in Female Fertility: Possible Role in Oxidative Stress and Aging. Oxid. Med. Cell Longev. 2015, 2015, 659687. [Google Scholar] [CrossRef]
- Morita, Y.; Wada-Hiraike, O.; Yano, T.; Shirane, A.; Hirano, M.; Hiraike, H.; Koyama, S.; Oishi, H.; Yoshino, O.; Miyamoto, Y.; et al. Resveratrol promotes expression of SIRT1 and StAR in rat ovarian granulosa cells: An implicative role of SIRT1 in the ovary. Reprod. Biol. Endocrinol. 2012, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L. Sirt1 and the Mitochondria. Mol. Cells 2016, 39, 87–95. [Google Scholar] [CrossRef]
- Iniesta-Cuerda, M.; Havrankova, J.; Rimnacova, H.; Garcia-Alvarez, O.; Nevoral, J. Male SIRT1 insufficiency leads to sperm with decreased ability to hyperactivate and fertilize. Reprod. Domest. Anim. 2022, 57 (Suppl. 5), 72–77. [Google Scholar] [CrossRef]
- Han, J.; Wang, S.; Wang, H.; Zhang, T.; Yang, Y.; Zhao, T.; Chen, Z.; Xia, G.; Wang, C. SIRT1 reduction contributes to doxorubicin-induced oxidative stress and meiotic failure in mouse oocytes. Toxicol. Appl. Pharmacol. 2023, 476, 116671. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Cao, Q.; Wang, F.; Huang, L.Y.; Sang, T.T.; Liu, F.; Chen, S.Y. SIRT1 Protects Against Oxidative Stress-Induced Endothelial Progenitor Cells Apoptosis by Inhibiting FOXO3a via FOXO3a Ubiquitination and Degradation. J. Cell Physiol. 2015, 230, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, Z.; Wei, J.; Lu, L.; Huang, Y.; Luo, L.; Xie, H. Protective effect of SIRT1 on toxicity of microglial-derived factors induced by LPS to PC12 cells via the p53-caspase-3-dependent apoptotic pathway. Neurosci. Lett. 2013, 553, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, Q.; Zeng, Z.; Wu, J.; Zhang, Y.; Chen, Z. Sirt1 Inhibits Oxidative Stress in Vascular Endothelial Cells. Oxid. Med. Cell Longev. 2017, 2017, 7543973. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.R.; Ariu, F.; Maltana, A.; Leoni, G.G.; Martino, N.A.; Mastrorocco, A.; Dell’Aquila, M.E.; Bogliolo, L. Protective effect of resveratrol against cadmium-induced toxicity on ovine oocyte in vitro maturation and fertilization. J. Anim. Sci. Biotechnol. 2022, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Martin-Hidalgo, D.; Hurtado de Llera, A.; Calle-Guisado, V.; Gonzalez-Fernandez, L.; Garcia-Marin, L.; Bragado, M.J. AMPK Function in Mammalian Spermatozoa. Int. J. Mol. Sci. 2018, 19, 3293. [Google Scholar] [CrossRef]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Chini, C.C.S.; Zeidler, J.D.; Kashyap, S.; Warner, G.; Chini, E.N. Evolving concepts in NAD(+) metabolism. Cell Metab. 2021, 33, 1076–1087. [Google Scholar] [CrossRef]
- Waddell, J.; Khatoon, R.; Kristian, T. Cellular and Mitochondrial NAD Homeostasis in Health and Disease. Cells 2023, 12, 1329. [Google Scholar] [CrossRef]
- Ke, R.; Xu, Q.; Li, C.; Luo, L.; Huang, D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol. Int. 2018, 42, 384–392. [Google Scholar] [CrossRef]
- Feng, T.Y.; Lv, D.L.; Zhang, X.; Du, Y.Q.; Yuan, Y.T.; Chen, M.J.; Xi, H.M.; Li, Y.; Han, N.; Hu, J.H. Rosmarinic acid improves boar sperm quality, antioxidant capacity and energy metabolism at 17 degrees C via AMPK activation. Reprod. Domest. Anim. 2020, 55, 1714–1724. [Google Scholar] [CrossRef]
- Rezaie, F.S.; Hezavehei, M.; Sharafi, M.; Shahverdi, A. Improving the post-thaw quality of rooster semen using the extender supplemented with resveratrol. Poult. Sci. 2021, 100, 101290. [Google Scholar] [CrossRef]
- Lu, Y.; Pan, Y.; Sheng, N.; Zhao, A.Z.; Dai, J. Perfluorooctanoic acid exposure alters polyunsaturated fatty acid composition, induces oxidative stress and activates the AKT/AMPK pathway in mouse epididymis. Chemosphere 2016, 158, 143–153. [Google Scholar] [CrossRef]
- Hurtado de Llera, A.; Martin-Hidalgo, D.; Gil, M.C.; Garcia-Marin, L.J.; Bragado, M.J. AMPK up-activation reduces motility and regulates other functions of boar spermatozoa. Mol. Hum. Reprod. 2015, 21, 31–45. [Google Scholar] [CrossRef]



| Concentration | 0 μM | 25 μM | 50 μM | 75 μM | 100 μM | |
|---|---|---|---|---|---|---|
| Sperm Parameters | ||||||
| TM (%) | 37.6 ± 1.1 b | 40.8 ± 1.8 b | 69.2 ± 4.2 a | 59.6 ± 5.0 a | 33.2 ± 2.4 b | |
| PM (%) | 23.6 ± 0.6 b | 24.0 ± 3.5 b | 48.4 ± 7.0 a | 30.4 ± 3.4 b | 19.1 ± 0.7 c | |
| VCL (μm/s) | 36.7 ± 6.8 b | 44.1 ± 13.0 b | 61.1 ± 13.1 a | 47.1 ± 9.1 b | 43.0 ± 5.4 c | |
| VSL (μm/s) | 19.3 ± 2.9 b | 24.4 ± 7.3 b | 34.1 ± 3.6 a | 22.3 ± 2.6 b | 22.3 ± 5.2 b | |
| VAP (μm/s) | 24.2 ± 3.5 d | 30.8 ± 9.1 b | 35.8 ± 8.8 a | 25.2 ± 5.2 c | 28.4 ± 5.6 c | |
| BCF (HZ) | 7.8 ± 1.7 a | 6.4 ± 1.4 b | 6.2 ± 1.2 b | 6.1 ± 1.1 b | 5.4 ± 0.8 c | |
| ALH (μm) | 3.0 ± 0.2 | 3.0 ± 0.4 | 3.4 ± 0.7 | 3.8 ± 0.6 | 3.7 ± 0.2 | |
| STR (%) | 79.6 ± 0.7 | 79.1 ± 1.0 | 77.5 ± 1.3 | 75.4 ± 1.2 | 76.6 ± 3.9 | |
| LIN (%) | 53.7 ± 2.2 | 56.4 ± 3.9 | 54.3 ± 3.9 | 51.1 ± 4.5 | 51.9 ± 5.3 | |
| WOB (%) | 67.1 ± 3.1 b | 71.1 ± 2.0 a | 69.8 ± 3.8 a | 67.6 ± 5.5 b | 67.2 ± 4.1 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Zhao, H.; Cui, H.; Adetunji, A.O.; Min, L. Resveratrol Improves the Frozen-Thawed Ram Sperm Quality. Animals 2023, 13, 3887. https://doi.org/10.3390/ani13243887
Zhu Z, Zhao H, Cui H, Adetunji AO, Min L. Resveratrol Improves the Frozen-Thawed Ram Sperm Quality. Animals. 2023; 13(24):3887. https://doi.org/10.3390/ani13243887
Chicago/Turabian StyleZhu, Zhendong, Haolong Zhao, Haixiang Cui, Adedeji O. Adetunji, and Lingjiang Min. 2023. "Resveratrol Improves the Frozen-Thawed Ram Sperm Quality" Animals 13, no. 24: 3887. https://doi.org/10.3390/ani13243887
APA StyleZhu, Z., Zhao, H., Cui, H., Adetunji, A. O., & Min, L. (2023). Resveratrol Improves the Frozen-Thawed Ram Sperm Quality. Animals, 13(24), 3887. https://doi.org/10.3390/ani13243887





