Probiotic-Fermented Distillers Grain Alters the Rumen Microbiome, Metabolome, and Enzyme Activity, Enhancing the Immune Status of Finishing Cattle
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PFDG
2.2. Animals, Diets, and Experimental Design
2.3. Sample Collection and Pre-Treatment
2.4. Serum Parameter Analysis
2.5. Microbial Analysis of the Rumen Fluid
2.6. LC-MS Analysis of the Rumen Fluid
2.7. Rumen Enzyme Activity Analysis
2.8. Statistical Analysis
3. Results
3.1. Effect of Feeding Varying Levels of Probiotic-Fermented Distillers Grain on the ADG of Finishing Cattle
3.2. Effect of Feeding with Varying Levels of Probiotic-Fermented Distillers Grain on the Serum Antioxidant Indexes of Finishing Cattle
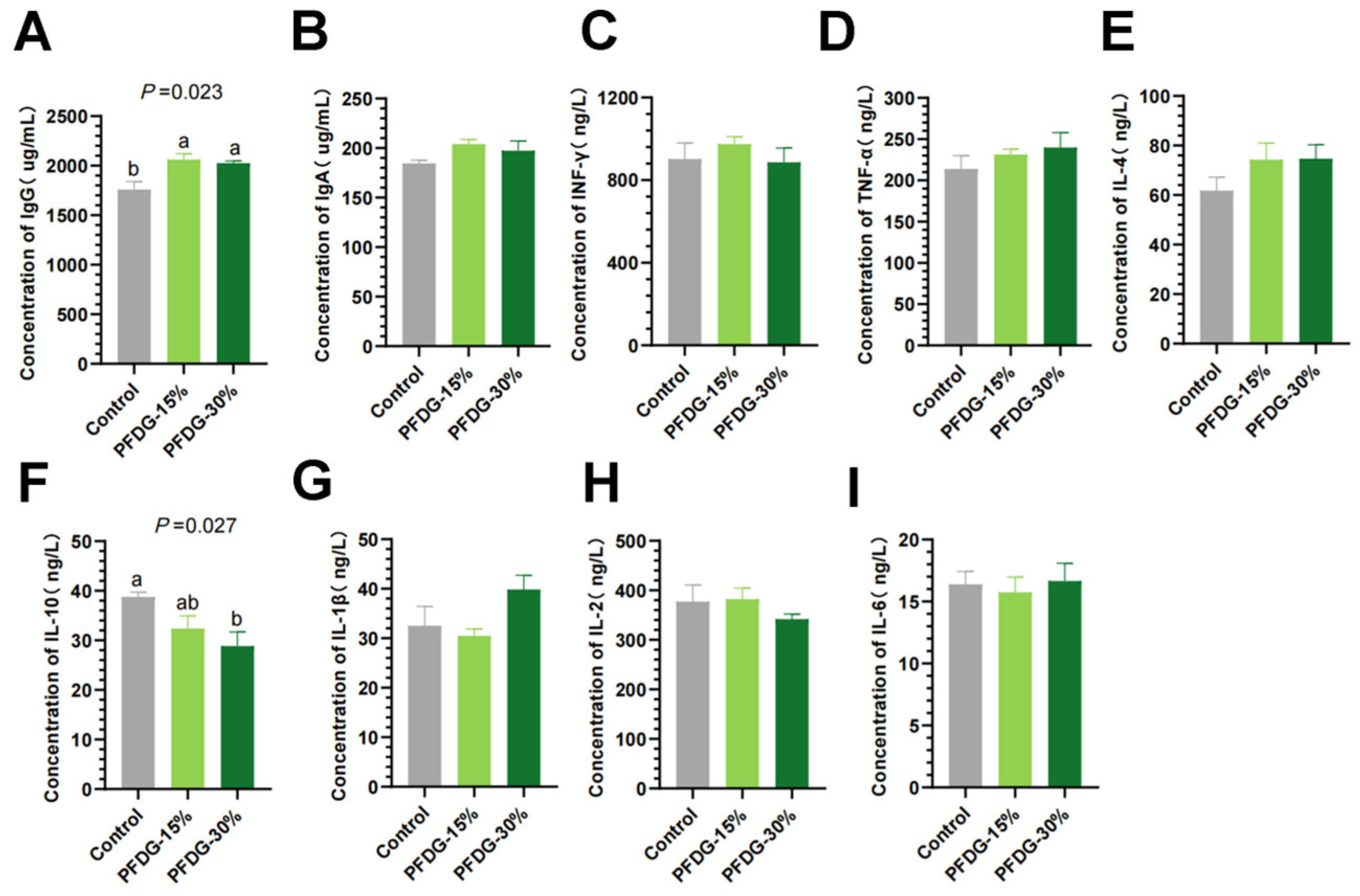
3.3. Effect of Feeding with Varying Levels of Probiotic-Fermented Distillers Grain on the Immune Status of Finishing Cattle
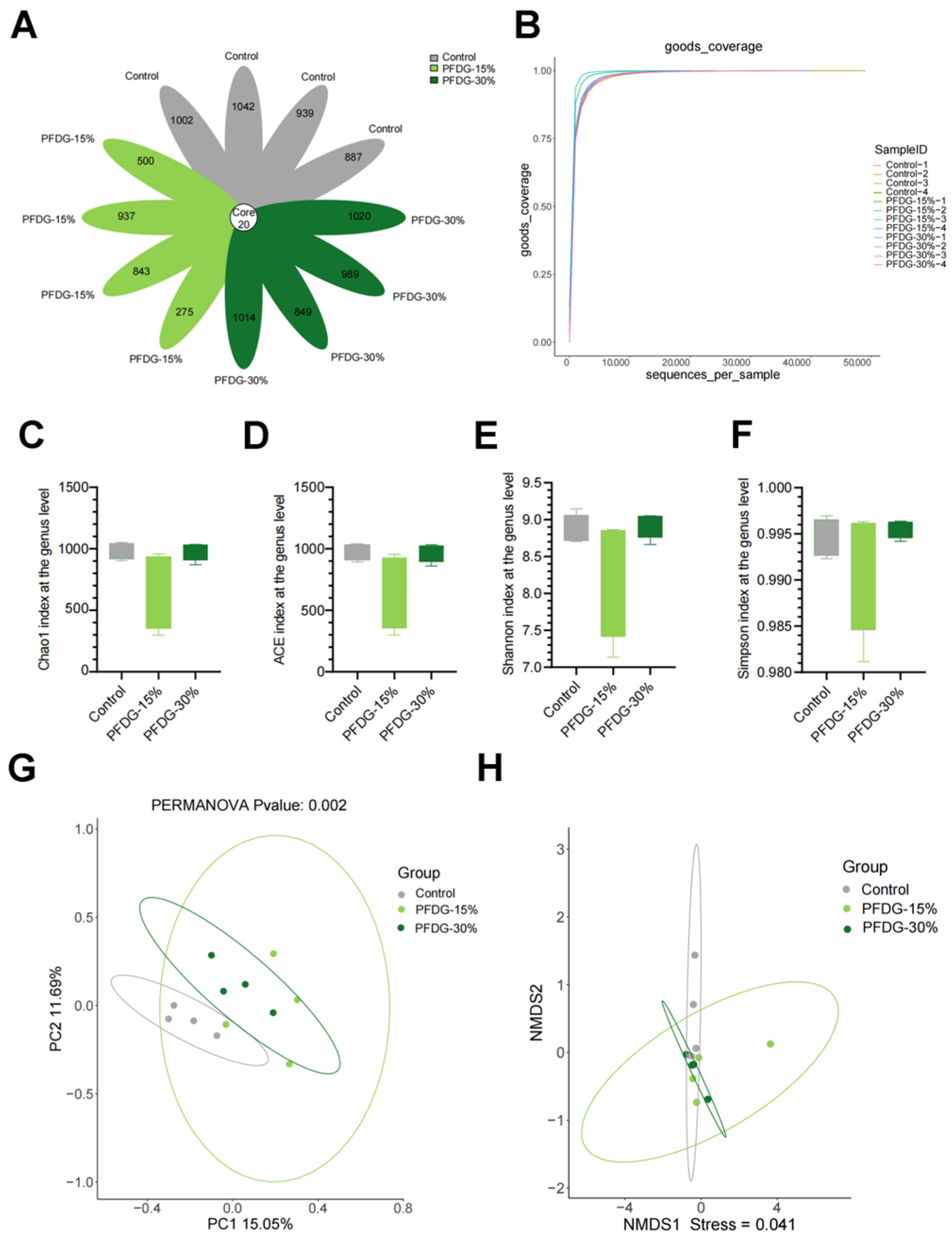
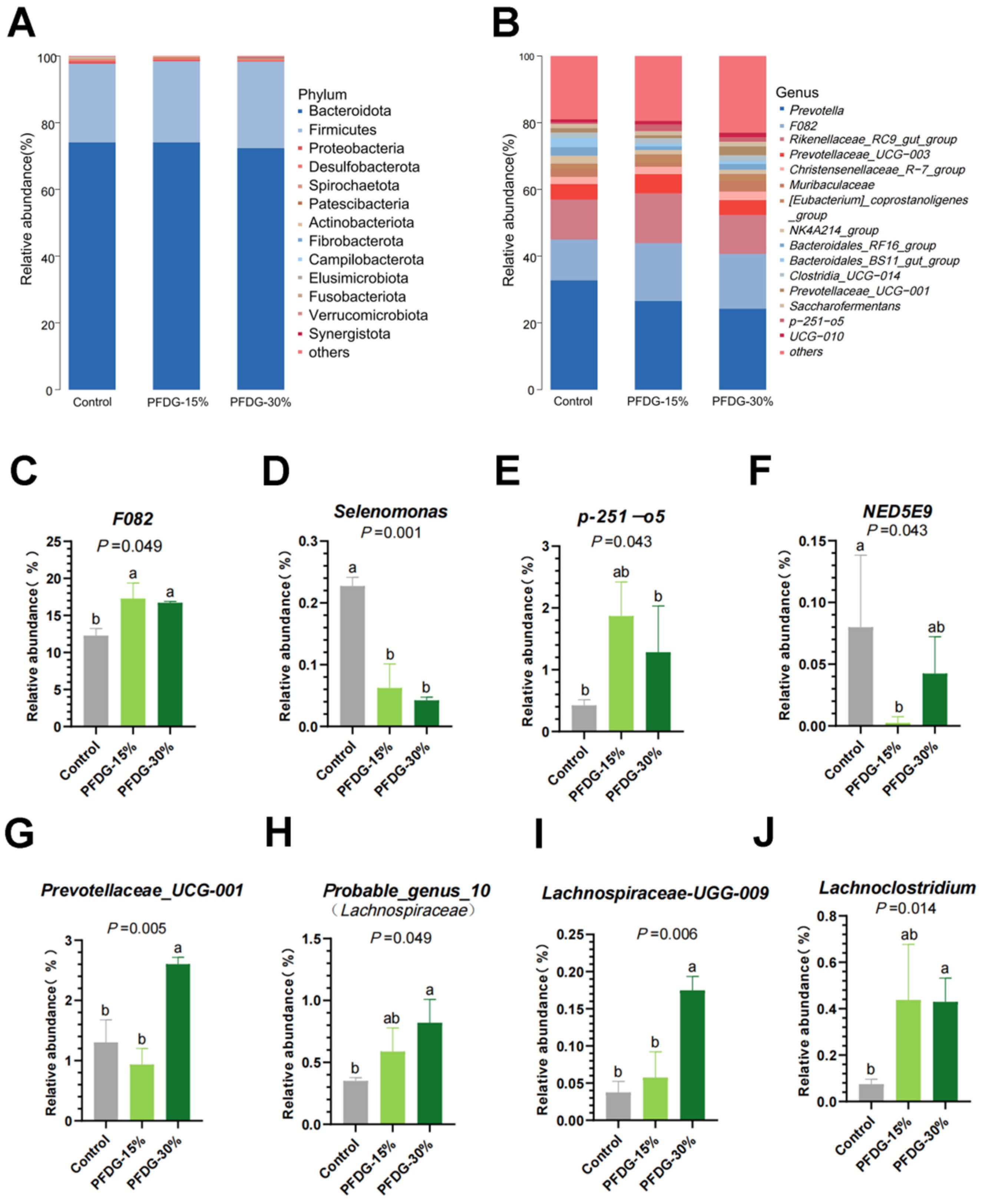
3.4. Effect of Feeding with Varying Levels of Probiotic-Fermented Distillers Grain on the Composition of Rumen Microbiota in Finishing Cattle
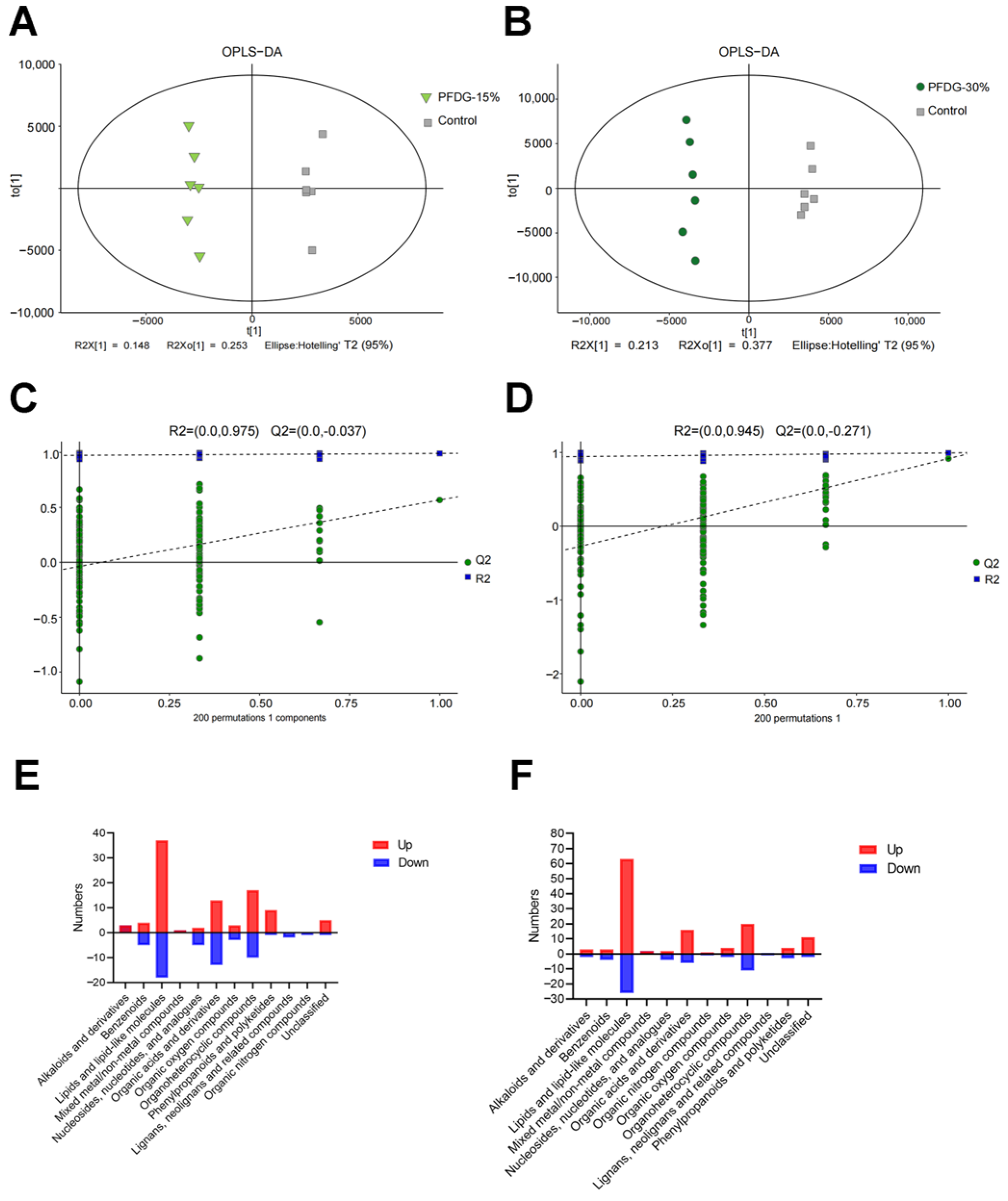
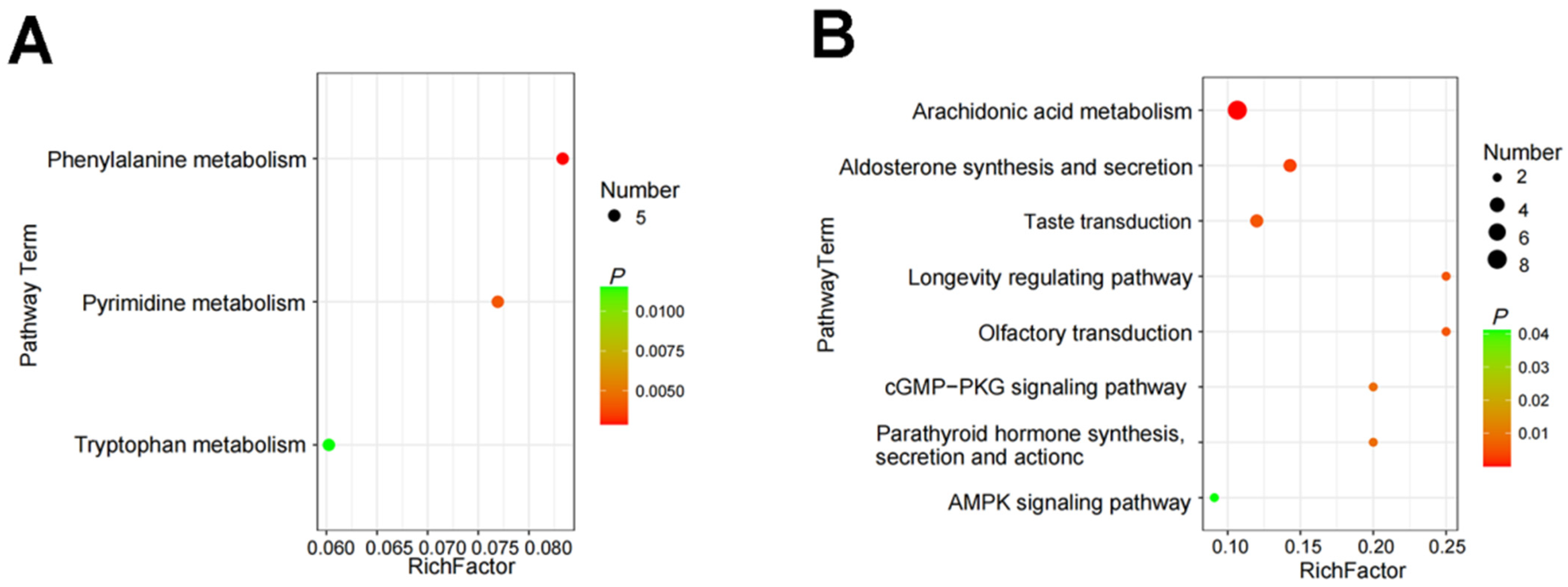
3.5. Effect of Feeding with Varying Levels of Probiotic-Fermented Distillers Grain on hte Rumen Metabolome in Finishing Cattle
3.6. Effect of Feeding with Varying Levels of Probiotic-Fermented Distillers Grain on Rumen Enzyme Activity of Finishing Cattle
3.7. Correlation Analysis of Rumen Microbiota according to ADG, Enzyme Activity, Antioxidant Indexes, Immune Indexes, and Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Q.; Gao, F.; Yu, Z.; Tao, Y.; Zhao, S.; Cai, Y. Fermentation quality and chemical composition of shrub silage treated with lactic acid bacteria inoculants and cellulase additives. Anim. Sci. J. 2012, 83, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Selaledi, L.; Mbajiorgu, C.A.; Mabelebele, M. The use of yellow mealworm (T. molitor) as alternative source of protein in poultry diets: A review. Trop. Anim. Health Prod. 2020, 52, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Less, J.F.; Wang, L.; Yan, T.; Kiron, V.; Kaushik, S.J.; Lei, X.G. Meeting Global Feed Protein Demand: Challenge, Opportunity, and Strategy. Annu. Rev. Anim. Biosci. 2019, 7, 221–243. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Ao, X.; Li, Q.; Cao, Y.; Chen, Q.; Xie, Y. Steam co-gasification of different ratios of spirit-based distillers’ grains and anthracite coal to produce hydrogen-rich gas. Bioresour. Technol. 2019, 283, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, K. Chemical composition of distillers grains, a review. J. Agric. Food. Chem. 2011, 59, 1508–1526. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T.; Przybylo, M.; Zapletal, P.; Turek, A.; Pabianczyk, M.; Bartlewski, P.M. Effects of Using Corn Dried Distillers’ Grains with Solubles (cDDGS) as a Partial Replacement for Soybean Meal on the Outcomes of Pig Fattening, Pork Slaughter Value and Quality. Animals 2021, 11, 2956. [Google Scholar] [CrossRef] [PubMed]
- Alimkulo, Z.; Velyamov, M.; Potoroko, I.; Kuanysh, S.; Zhumaliyeva, T.; Shauliyeva, K. Development of Resource-Saving Biotechnology for the Production of a Feed Additive from Distiller’s Wastes with Probiotic Properties. Arch. Razi Inst. 2022, 77, 2281–2289. [Google Scholar]
- Clark, P.W.; Armentano, L.E. Effectiveness of neutral detergent fiber in whole cottonseed and dried distillers grains compared with alfalfa haylage. J. Dairy Sci. 1993, 76, 2644–2650. [Google Scholar] [CrossRef]
- Fan, W.; Sun, X.; Cui, G.; Li, Q.; Xu, Y.; Wang, L.; Li, X.; Hu, B.; Chi, Z. A strategy of co-fermentation of distillers dried grains with solubles (DDGS) and lignocellulosic feedstocks as swine feed. Crit. Rev. Biotechnol. 2023, 43, 212–226. [Google Scholar] [CrossRef]
- Wang, C.; Su, W.; Zhang, Y.; Hao, L.; Wang, F.; Lu, Z.; Zhao, J.; Liu, X.; Wang, Y. Solid-state fermentation of distilled dried grain with solubles with probiotics for degrading lignocellulose and upgrading nutrient utilization. AMB Express 2018, 8, 188. [Google Scholar] [CrossRef]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Dia, V.P.; Wang, Z.; West, M.; Singh, V.; West, L.; de Mejia, E.G. Processing method and corn cultivar affected anthocyanin concentration from dried distillers grains with solubles. J. Agric. Food Chem. 2015, 63, 3205–3218. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.Y.; Zhang, T.Z.; Wang, H.F.; Liu, J.X. Upgrading of by-product from beverage industry through solid-state fermentation with Candida utilis and Bacillus subtilis. Lett. Appl. Microbiol. 2018, 67, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Paganelli, A.; Righi, V.; Tarentini, E.; Magnoni, C. Current Knowledge in Skin Metabolomics: Updates from Literature Review. Int. J. Mol. Sci. 2022, 23, 8776. [Google Scholar] [CrossRef]
- Zengin, M.; Sur, A.; İlhan, Z.; Azman, M.A.; Tavşanlı, H.; Esen, S.; Bacaksız, O.K.; Demir, E. Effects of fermented distillers grains with solubles, partially replaced with soybean meal, on performance, blood parameters, meat quality, intestinal flora, and immune response in broiler. Res. Vet. Sci. 2022, 150, 58–64. [Google Scholar] [CrossRef]
- Wiseman, M.; McBride, B.; Li, J.; Wey, D.; Zhu, J.; de Lange, C.F.M. Effects of steeped or fermented distillers dried grains with solubles on growth performance in weanling pigs. J. Anim. Sci. 2017, 95, 3563–3578. [Google Scholar] [CrossRef]
- GB 4789.15-2016; National Food Safety Standard—Food Microbiological Examination—Enumeration of Moulds and Yeasts. China Health Standard Management: Beijing, China, 2018; pp. 1–3.
- GB 4789.35-2016; National Food Safety Standard—Microbiological Examination of Food—Examination of Lactic Acid Bacteria. China Health Standard Management: Beijing, China, 2017; pp. 1–3.
- Fang, S.; Chen, X.; Pan, J.; Chen, Q.; Zhou, L.; Wang, C.; Xiao, T.; Gan, Q.F. Dynamic distribution of gut microbiota in meat rabbits at different growth stages and relationship with average daily gain (ADG). BMC Microbiol. 2020, 20, 116. [Google Scholar] [CrossRef]
- Reis, V.A.; Reis, R.A.; Araujo, T.; Lage, J.F.; Teixeira, P.D.; Gionbelli, T.; Lanna, D.P.; Ladeira, M.M. Performance, beef quality and expression of lipogenic genes in young bulls fed low-fat dried distillers grains. Meat Sci. 2020, 160, 107962. [Google Scholar] [CrossRef]
- Larson, H.E.; Jaderborg, J.P.; Paulus-Compart, D.M.; Crawford, G.I.; DiCostanzo, A. Effect of substitution of distillers grains and glycerin for steam-flaked corn in finishing cattle diets on growth performance and carcass characteristics. J. Anim. Sci. 2023, 101, skac348. [Google Scholar] [CrossRef]
- Sarturi, J.O.; Erickson, G.E.; Klopfenstein, T.J.; Vasconcelos, J.T.; Griffin, W.A.; Rolfe, K.M.; Benton, J.R.; Bremer, V.R. Effect of sulfur content in wet or dry distillers grains fed at several inclusions on cattle growth performance, ruminal parameters, and hydrogen sulfide. J. Anim. Sci. 2013, 91, 4849–4860. [Google Scholar] [CrossRef]
- Estell, R.; Tellez, M.; Fredrickson, E.; Anderson, D.; Havstad, K.; Remmenga, M. Extracts of Flourensia cernua reduce consumption of alfalfa pellets by sheep. J. Chem. Ecol. 2001, 27, 2275–2285. [Google Scholar] [CrossRef]
- Singh, V.; Ahlawat, S.; Mohan, H.; Gill, S.S.; Sharma, K.K. Balancing reactive oxygen species generation by rebooting gut microbiota. J. Appl. Microbiol. 2022, 132, 4112–4129. [Google Scholar] [CrossRef]
- Lu, Q.; Luo, Q.; Li, J.; Wang, X.; Ban, C.; Qin, J.; Tian, Y.; Tian, X.; Chen, X. Evaluation of the Chemical Composition, Bioactive Substance, Gas Production, and Rumen Fermentation Parameters of Four Types of Distiller’s Grains. Molecules 2022, 27, 6134. [Google Scholar] [CrossRef]
- He, R.; Yang, Y.; Li, Y.; Yang, M.; Kong, L.; Yang, F. Recent Progress in Distiller’s Grains: Chemical Compositions and Biological Activities. Molecules 2023, 28, 7492. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.N.; Fang, X.P.; Zhao, C.; Wu, H.Y.; Lan, Y.X.; Che, L.; Sun, Y.K.; Lv, J.Y.; Zhang, Y.G.; et al. Effects of replacing soybean meal with pumpkin seed cake and dried distillers grains with solubles on milk performance and antioxidant functions in dairy cows. Animal 2021, 15, 100004. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Liu, Z.; Guo, X.; Deng, Y.; Ouyang, Q.; Liu, H.; Hu, S.; Hu, B.; Li, L.; et al. Effects of rearing systems on production performance, antioxidant capacity and immune status of meat ducks at different ages. Animal 2021, 15, 100199. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Wang, T.; Li, W. Core Fucosylation Regulates the Function of Pre-BCR, BCR and IgG in Humoral Immunity. Front. Immunol. 2022, 13, 844427. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Huang, H.; Zheng, P.; Xue, M.; Ma, J.; Zhan, Z.; Gan, H.; Zeng, Y.; Lin, R.; Li, S.; et al. Humoral immune response of BBIBP COVID-19 vaccination before and after the booster immunization. Allergy 2022, 77, 2404–2414. [Google Scholar] [CrossRef]
- Gazzinelli-Guimaraes, A.C.; Gazzinelli-Guimaraes, P.H.; Nogueira, D.S.; Oliveira, F.; Barbosa, F.S.; Amorim, C.; Cardoso, M.S.; Kraemer, L.; Caliari, M.V.; Akamatsu, M.A.; et al. IgG Induced by Vaccination with Ascaris suum Extracts Is Protective Against Infection. Front. Immunol. 2018, 9, 2535. [Google Scholar] [CrossRef]
- Cheng, Q.; Xu, D.; Chen, Y.; Zhu, M.; Fan, X.; Li, M.; Tang, X.; Liao, C.; Li, P.; Chen, C. Influence of Fermented-Moutai Distillers’ Grain on Growth Performance, Meat Quality, and Blood Metabolites of Finishing Cattle. Front. Vet. Sci. 2022, 9, 874453. [Google Scholar] [CrossRef]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef]
- Yazdi, A.S.; Ghoreschi, K. The Interleukin-1 Family. Adv. Exp. Med. Biol. 2016, 941, 21–29. [Google Scholar] [CrossRef]
- Barekatain, M.R.; Antipatis, C.; Rodgers, N.; Walkden-Brown, S.W.; Iji, P.A.; Choct, M. Evaluation of high dietary inclusion of distillers dried grains with solubles and supplementation of protease and xylanase in the diets of broiler chickens under necrotic enteritis challenge. Poult. Sci. 2013, 92, 1579–1594. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Yang, F. Improving the Anaerobic Digestion of Switchgrass via Cofermentation of Rumen Microorganisms (Rumen Bacteria, Protozoa, and Fungi) and a Biogas Slurry. J. Agric. Food. Chem. 2019, 33, 1185–1195. [Google Scholar] [CrossRef]
- Song, C.; Zhang, T.; Xu, D.; Zhu, M.; Mei, S.; Zhou, B.; Wang, K.; Chen, C.; Zhu, E.; Cheng, Z. Impact of feeding dried distillers’ grains with solubles diet on microbiome and metabolome of ruminal and cecal contents in Guanling yellow cattle. Front. Microbiol. 2023, 14, 1171563. [Google Scholar] [CrossRef]
- Yi, S.; Dai, D.; Wu, H.; Chai, S.; Liu, S.; Meng, Q.; Zhou, Z. Dietary Concentrate-to-Forage Ratio Affects Rumen Bacterial Community Composition and Metabolome of Yaks. Front. Nutr. 2022, 9, 927206. [Google Scholar] [CrossRef]
- Han, H.; Zhang, L.; Shang, Y.; Wang, M.; Phillips, C.; Wang, Y.; Su, C.; Lian, H.; Fu, T.; Gao, T. Replacement of Maize Silage and Soyabean Meal with Mulberry Silage in the Diet of Hu Lambs on Growth, Gastrointestinal Tissue Morphology, Rumen Fermentation Parameters and Microbial Diversity. Animals 2022, 12, 1406. [Google Scholar] [CrossRef]
- Cibis, K.G.; Gneipel, A.; König, H. Isolation of acetic, propionic and butyric acid-forming bacteria from biogas plants. J. Biotechnol. 2016, 220, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, T.; Enishi, O.; Mitsumori, M.; Higuchi, K.; Kobayashi, Y.; Takenaka, A.; Nagashima, K.; Mochizuki, M.; Kobayashi, Y. Mitigation of methane production from cattle by feeding cashew nut shell liquid. J. Dairy. Sci. 2012, 95, 5308–5316. [Google Scholar] [CrossRef] [PubMed]
- Jize, Z.; Zhuoga, D.; Xiaoqing, Z.; Na, T.; Jiacuo, G.; Cuicheng, L.; Bandan, P. Different feeding strategies can affect growth performance and rumen functions in Gangba sheep as revealed by integrated transcriptome and microbiome analyses. Front. Microbiol. 2022, 13, 908326. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wu, W.; Tu, Y.; Zhang, N.; Diao, Q. Resveratrol affects in vitro rumen fermentation, methane production and prokaryotic community composition in a time- and diet-specific manner. Microb. Biotechnol. 2020, 13, 1118–1131. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, C.; Chen, Y.; He, X.; Gao, F.; Xue, D. Quantitative increase in short-chain fatty acids, especially butyrate protects kidney from ischemia/reperfusion injury. J. Invest. Med. 2022, 70, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.R.; Chang, P.V. Deciphering the Chemical Lexicon of Host-Gut Microbiota Interactions. Trends Pharmacol. Sci. 2019, 40, 430–445. [Google Scholar] [CrossRef]
- Hawkes, C.G.; Hinson, A.N.; Vashishta, A.; Read, C.B.; Carlyon, J.A.; Lamont, R.J.; Uriarte, S.M.; Miller, D.P. Selenomonas sputigena Interactions with Gingival Epithelial Cells That Promote Inflammation. Infect. Immun. 2023, 91, e31922. [Google Scholar] [CrossRef]
- Pomeroy, C.; Shanholtzer, C.J.; Peterson, L.R. Selenomonas bacteraemia—Case report and review of the literature. J. Infect. 1987, 15, 237–242. [Google Scholar] [CrossRef]
- Pratama, R.; Schneider, D.; Böer, T.; Daniel, R. First Insights into Bacterial Gastrointestinal Tract Communities of the Eurasian Beaver (Castor fiber). Front. Microbiol. 2019, 10, 1646. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, X.; Li, N.; Zhao, J.; Ye, H.; Zhang, S.; Yang, H.; Pi, Y.; Tao, S.; Han, D.; et al. In Vitro Fermentation Characteristics and Fiber-Degrading Enzyme Kinetics of Cellulose, Arabinoxylan, β-Glucan and Glucomannan by Pig Fecal Microbiota. Microorganisms 2021, 9, 71. [Google Scholar] [CrossRef]
- Schofield, B.J.; Lachner, N.; Le, O.T.; McNeill, D.M.; Dart, P.; Ouwerkerk, D.; Hugenholtz, P.; Klieve, A.V. Beneficial changes in rumen bacterial community profile in sheep and dairy calves as a result of feeding the probiotic Bacillus amyloliquefaciens H57. J. Appl. Microbiol. 2018, 124, 855–866. [Google Scholar] [CrossRef]
- Jiang, L.; Shang, M.; Yu, S.; Liu, Y.; Zhang, H.; Zhou, Y.; Wang, M.; Wang, T.; Li, H.; Liu, Z.; et al. A high-fiber diet synergizes with Prevotella copri and exacerbates rheumatoid arthritis. Cell. Mol. Immunol. 2022, 19, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xie, B.; Gao, J.; Zhao, G. Dietary Supplementation with Sodium Sulfate Improves Rumen Fermentation, Fiber Digestibility, and the Plasma Metabolome through Modulation of Rumen Bacterial Communities in Steers. Appl. Environ. Microbiol. 2020, 86, e01412-20. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Li, W.B.; Wang, H.Y.; Ma, Y.M.; Zhao, X.H.; Yang, H.; Qian, J.M.; Li, J.N. VSL#3 can prevent ulcerative colitis-associated carcinogenesis in mice. World J. Gastroenterol. 2018, 24, 4254–4262. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, W.; Wang, H.; Ma, Y.; Zhao, X.; Zhang, X.; Yang, H.; Qian, J.; Li, J. Saccharomyces boulardii alleviates ulcerative colitis carcinogenesis in mice by reducing TNF-alpha and IL-6 levels and functions and by rebalancing intestinal microbiota. BMC Microbiol. 2019, 19, 246. [Google Scholar] [CrossRef]
- Redding, L.E.; Berry, A.S.; Indugu, N.; Huang, E.; Beiting, D.P.; Pitta, D. Gut microbiota features associated with Clostridioides difficile colonization in dairy calves. PLoS ONE 2021, 16, e251999. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, Z.; Tian, X.; Wu, D.; Dai, Q. Multi-omics reveals host metabolism associated with the gut microbiota composition in mice with dietary epsilon-polylysine. Food Funct. 2022, 13, 4069–4085. [Google Scholar] [CrossRef]
- Tang, Z.; Song, B.; Zheng, C.; Zheng, J.; Yin, Y.; Chen, J. Dietary Beta-Hydroxy-Beta-Methyl Butyrate Supplementation Affects Growth, Carcass Characteristics, Meat Quality, and Serum Metabolomics Profile in Broiler Chickens. Front. Physiol. 2021, 12, 633964. [Google Scholar] [CrossRef]
- Elango, R. Tolerable Upper Intake Level for Individual Amino Acids in Humans: A Narrative Review of Recent Clinical Studies. Adv. Nutr. 2023, 14, 885–894. [Google Scholar] [CrossRef]
- Jiao, M.; He, W.; Ouyang, Z.; Shi, Q.; Wen, Y. Progress in structural and functional study of the bacterial phenylacetic acid catabolic pathway, its role in pathogenicity and antibiotic resistance. Front. Microbiol. 2022, 13, 964019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jiang, M.; Zhao, J.; Song, Y.; Du, W.; Shi, J. The Mechanism Underlying the Influence of Indole-3-Propionic Acid: A Relevance to Metabolic Disorders. Front. Endocrinol. 2022, 13, 841703. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Clement, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Gu, X.; Tohme, R.; Tomlinson, B.; Sakre, N.; Hasipek, M.; Durkin, L.; Schuerger, C.; Grabowski, D.; Zidan, A.M.; Radivoyevitch, T.; et al. Decitabine- and 5-azacytidine resistance emerges from adaptive responses of the pyrimidine metabolism network. Leukemia 2021, 35, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Kaur, K.; Biharee, A.; Jaitak, V. Recent Development in Indole Derivatives as Anticancer Agent: A Mechanistic Approach. Anti-Cancer Agents Med. Chem. 2021, 21, 1802–1824. [Google Scholar] [CrossRef] [PubMed]
- Cansev, M.; Watkins, C.J.; van der Beek, E.M.; Wurtman, R.J. Oral uridine-5’-monophosphate (UMP) increases brain CDP-choline levels in gerbils. Brain Res. 2005, 1058, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Albrecht, M.A.; Wurtman, R.J. Dietary supplementation with uridine-5′-monophosphate (UMP), a membrane phosphatide precursor, increases acetylcholine level and release in striatum of aged rat. Brain Res. 2007, 1133, 42–48. [Google Scholar] [CrossRef][Green Version]
- Martin, S.A.; Brash, A.R.; Murphy, R.C. The discovery and early structural studies of arachidonic acid. J. Lipid. Res. 2016, 57, 1126–1132. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Komorniak, N.; Bilicki, J.; Celewicz, Z.; Zietek, M. The Role of Arachidonic and Linoleic Acid Derivatives in Pathological Pregnancies and the Human Reproduction Process. Int. J. Mol. Sci. 2020, 21, 9628. [Google Scholar] [CrossRef]
- Cheng, Q.; Tian, L.; Liang, H.; Luo, Y. Research progress of 12-HETE in the inflammation and oxidative stress. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019, 31, 1555–1558. [Google Scholar]
- Gagnon, K.J.; Lefort, N.; Poirier, S.J.; Barnett, D.A.; Surette, M.E. 5-lipoxygenase-dependent biosynthesis of novel 20:4 n-3 metabolites with anti-inflammatory activity. Prostaglandins Leukot. Essent. Fat. Acids 2018, 138, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Latorre, J.D.; Merino-Guzman, R.; Morales, R.M.; Ausland, C.; Hernandez-Velasco, X.; Ortiz, H.O.; Delgado, R.; Hargis, B.M.; et al. Whole-Genome Sequence and Interaction Analysis in the Production of Six Enzymes From the Three Bacillus Strains Present in a Commercial Direct-Fed Microbial (Norum) Using a Bliss Independence Test. Front. Vet. Sci. 2022, 9, 784387. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.E.; Al-Khalaifah, H.S.; Ismail, T.A.; Abd, E.R.; El-Mandrawy, S.; Shalaby, S.I.; Ibrahim, D. Performance, Serum Biochemical and Immunological Parameters, and Digestive Enzyme and Intestinal Barrier-Related Gene Expression of Broiler Chickens Fed Fermented Fava Bean By-Products as a Substitute for Conventional Feed. Front. Vet. Sci. 2021, 8, 696841. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.H.; Shang, B.J.; Shao, Y.C.; Li, W.Y.; Sun, J.S. Screening of intestinal probiotics and the effects of feeding probiotics on the growth, immune, digestive enzyme activity and intestinal flora of Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 86, 160–168. [Google Scholar] [CrossRef]



| Items | Control | PFDG-15% | PFDG-30% |
|---|---|---|---|
| Ingredient | |||
| Silo corn | 55.00 | 55.00 | 55.00 |
| PFDG | 0.00 | 15.00 | 30.00 |
| Corn | 15.00 | 11.63 | 7.70 |
| Wheat bran | 11.50 | 6.64 | 2.00 |
| Soybean meal | 5.50 | 2.78 | 0.00 |
| Rapeseed meal | 9.90 | 6.0 | 2.40 |
| Calcium carbonate, 1% | 0.80 | 0.73 | 0.70 |
| Calcium hydrogen phosphate | 0.20 | 0.12 | 0.10 |
| Sodium chloride | 0.10 | 0.10 | 0.10 |
| Sodium bicarbonate | 1.00 | 1.00 | 1.00 |
| Premix 2 | 1.00 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrition levels 3 | |||
| Dry matter, DM, % | 36.73 | 36.83 | 36.34 |
| Metabolic energy, ME, MJ/kg | 9.73 | 9.83 | 9.84 |
| Crude protein, CP, % | 12.82 | 12.53 | 12.45 |
| Neutral detergent fiber, NDF, % | 39.92 | 40.38 | 40.81 |
| Acid detergent fiber, ADF, % | 21.51 | 21.85 | 22.02 |
| Calcium, Ca, /% | 0.82 | 0.79 | 0.84 |
| Phosphorus, P, % | 0.43 | 0.48 | 0.45 |
| Items | Control | PFDG-15% | PFDG-30% | p |
|---|---|---|---|---|
| IBW, kg | 262.900 ± 7.009 | 267.100 ± 8.059 | 265.000 ± 6.909 | 0.922 |
| FBW, kg | 302.950 ± 7.707 | 309.800 ± 7.206 | 299.550 ± 7.193 | 0.611 |
| ADG, kg/d | 0.890 ± 0.033 a | 0.949 ± 0.045 a | 0.768 ± 0.028 b | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, S.; He, G.; Chen, Z.; Zhang, R.; Liao, Y.; Zhu, M.; Xu, D.; Shen, Y.; Zhou, B.; Wang, K.; et al. Probiotic-Fermented Distillers Grain Alters the Rumen Microbiome, Metabolome, and Enzyme Activity, Enhancing the Immune Status of Finishing Cattle. Animals 2023, 13, 3774. https://doi.org/10.3390/ani13243774
Mei S, He G, Chen Z, Zhang R, Liao Y, Zhu M, Xu D, Shen Y, Zhou B, Wang K, et al. Probiotic-Fermented Distillers Grain Alters the Rumen Microbiome, Metabolome, and Enzyme Activity, Enhancing the Immune Status of Finishing Cattle. Animals. 2023; 13(24):3774. https://doi.org/10.3390/ani13243774
Chicago/Turabian StyleMei, Shihui, Guangxia He, Ze Chen, Rong Zhang, Yixiao Liao, Mingming Zhu, Duhan Xu, Yanjuan Shen, Bijun Zhou, Kaigong Wang, and et al. 2023. "Probiotic-Fermented Distillers Grain Alters the Rumen Microbiome, Metabolome, and Enzyme Activity, Enhancing the Immune Status of Finishing Cattle" Animals 13, no. 24: 3774. https://doi.org/10.3390/ani13243774
APA StyleMei, S., He, G., Chen, Z., Zhang, R., Liao, Y., Zhu, M., Xu, D., Shen, Y., Zhou, B., Wang, K., Wang, C., Zhu, E., & Chen, C. (2023). Probiotic-Fermented Distillers Grain Alters the Rumen Microbiome, Metabolome, and Enzyme Activity, Enhancing the Immune Status of Finishing Cattle. Animals, 13(24), 3774. https://doi.org/10.3390/ani13243774




