First Molecular Characterization of Small Ruminant Lentiviruses Detected in Romania
Abstract
:Simple Summary
Abstract
1. Introduction
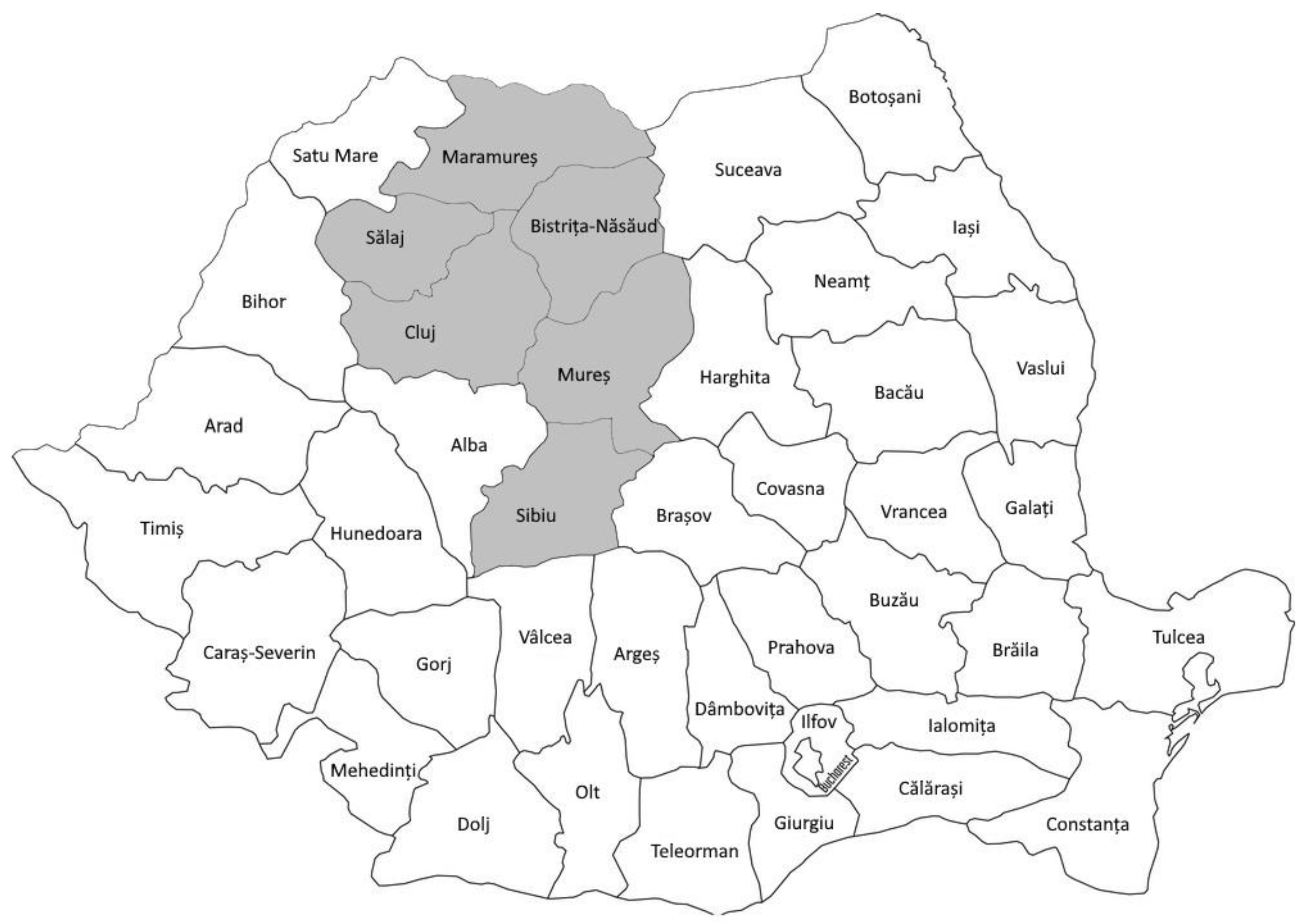
2. Materials and Methods
2.1. Samples
2.2. DNA Extraction
2.3. Nested Real-Time PCR for the Proviral Detection of SRLVs
2.4. PCR Amplification, Sequencing and Sequence Analysis
2.5. Analysis of Recombination
3. Results
3.1. Amplification and SRLV Sequences
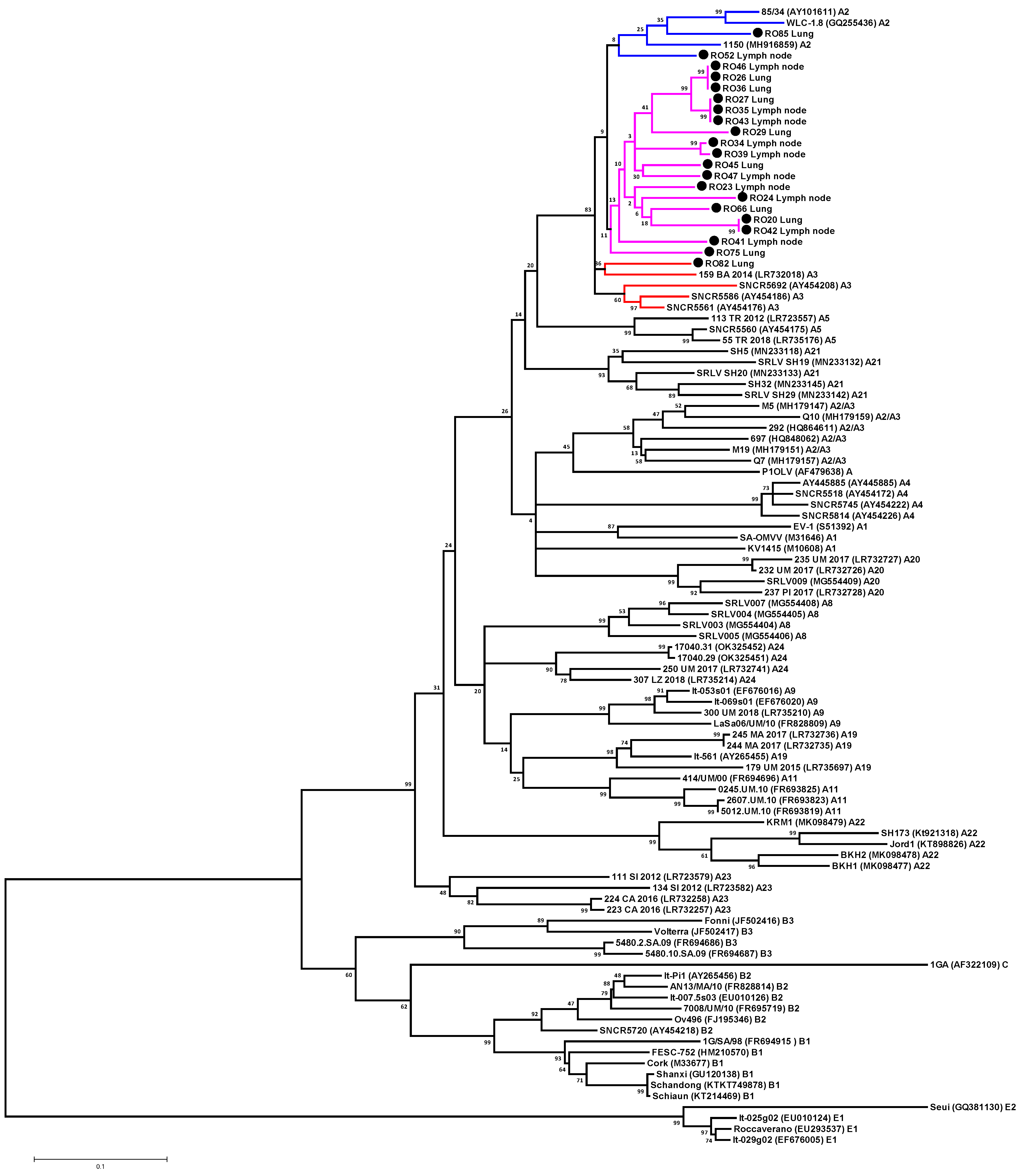
3.2. Phylogenetic Analysis of SRLV Strains Based on Gag-Pol Fragment
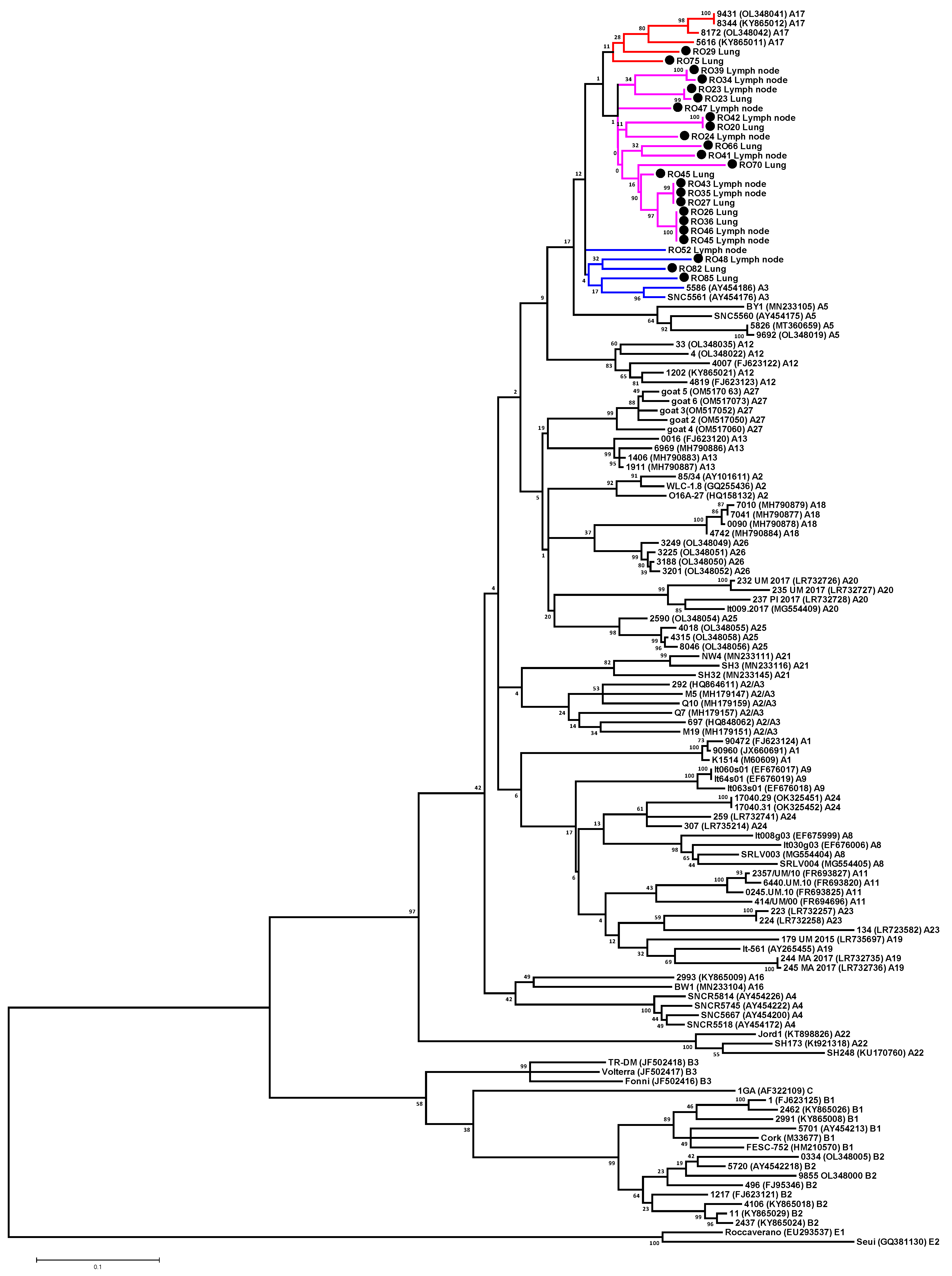
3.3. Phylogenetic Analysis of SRLV Strains Based on Gag Fragment
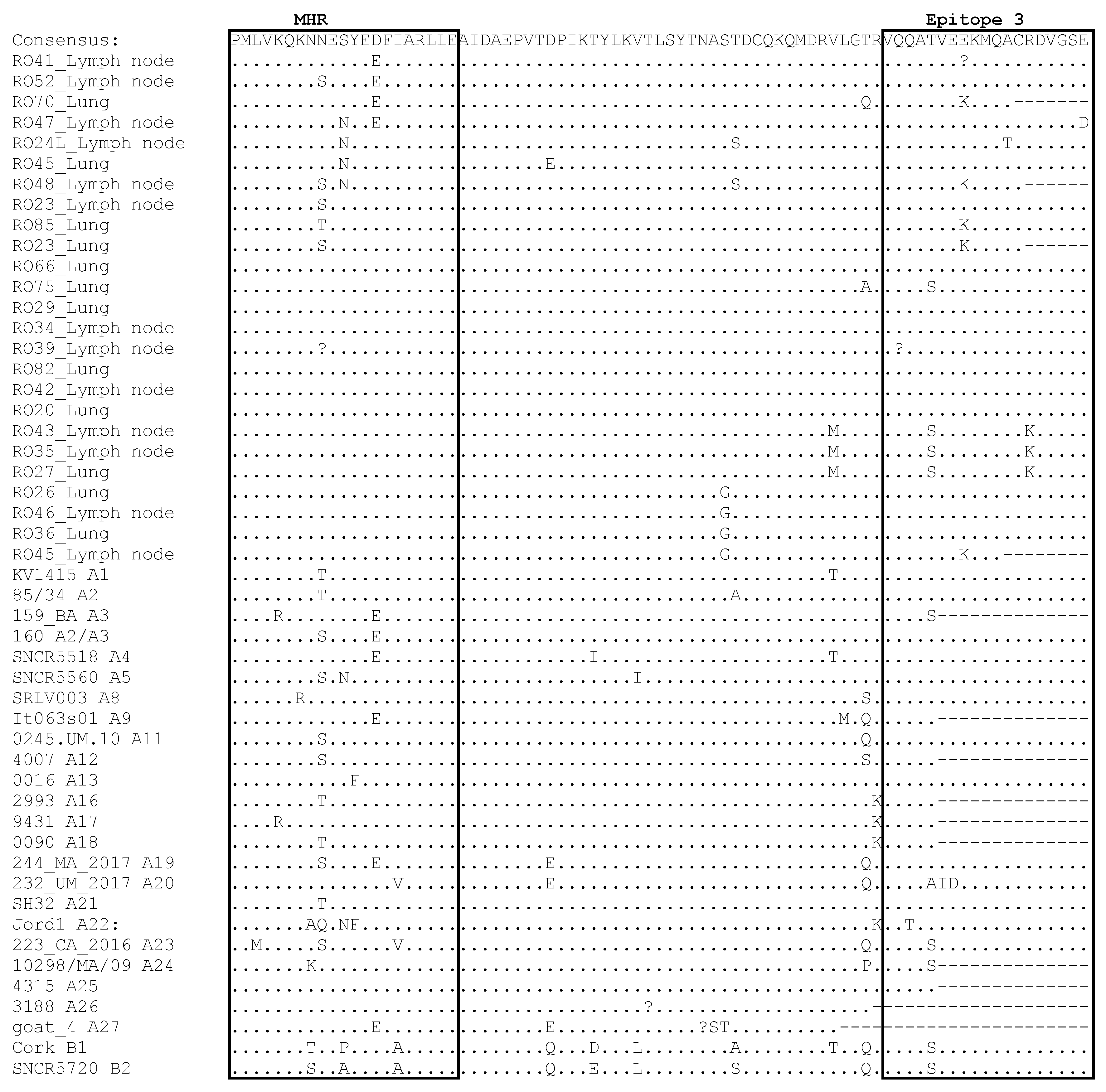
3.4. Comparative Analysis of Immunodominant Regions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blacklaws, B.A. Small ruminant lentiviruses: Immunopathogenesis of visna-maedi and caprine arthritis and encephalitis virus. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Kalogianni, A.I.; Bossis, I.; Ekateriniadou, L.V.; Gelasakis, A.I. Etiology, Epizootiology and Control of Maedi-Visna in Dairy Sheep: A Review. Animals 2020, 10, 616. [Google Scholar] [CrossRef]
- Minguijón, E.; Reina, R.; Pérez, M.; Polledo, L.; Villoria, M.; Ramírez, H.; Leginagoikoa, I.; Badiola, J.J.; García-Marín, J.F.; de Andrés, D.; et al. Small ruminant lentivirus infections and diseases. Vet. Microbiol. 2015, 181, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Bazzucchi, M.; Pierini, I.; Gobbi, P.; Pirani, S.; Torresi, C.; Iscaro, C.; Feliziani, F.; Giammarioli, M. Genomic Epidemiology and Heterogeneity of SRLV in Italy from 1998 to 2019. Viruses 2021, 13, 2338. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Kuźmak, J. Molecular Characterization of Small Ruminant Lentiviruses in Polish Mixed Flocks Supports Evidence of Cross Species Transmission, Dual Infection, a Recombination Event, and Reveals the Existence of New Subtypes within Group A. Viruses 2021, 13, 2529. [Google Scholar] [CrossRef]
- Arcangeli, C.; Torricelli, M.; Sebastiani, C.; Lucarelli, D.; Ciullo, M.; Passamonti, F.; Giammarioli, M.; Biagetti, M. Genetic Characterization of Small Ruminant Lentiviruses (SRLVs) Circulating in Naturally Infected Sheep in Central Italy. Viruses 2022, 14, 686. [Google Scholar] [CrossRef] [PubMed]
- Braz, G.F.; Heinemann, M.B.; Reis, J.K.P.; Teixeira, B.M.; Cruz, J.C.M.; Rajão, D.S.; Oliveira, F.G.; Alves, F.; Castro, R.S.; Leite, R.C.; et al. Genetic and antigenic characterization of Brazilian SRLV strains: Natural small ruminant interspecies transmission from mixed herds. Infect. Genet. Evol. 2022, 103, 105322. [Google Scholar] [CrossRef]
- Mendiola, W.P.S.; Tórtora, J.L.; Martínez, H.A.; García, M.M.; Cuevas-Romero, S.; Cerriteño, J.L.; Ramírez, H. Genotyping Based on the LTR Region of Small Ruminant Lentiviruses from Naturally Infected Sheep and Goats from Mexico. BioMed Res. Int. 2019, 2019, 4279573. [Google Scholar] [CrossRef]
- Gayo, E.; Cuteri, V.; Polledo, L.; Rossi, G.; García Marín, J.F.; Preziuso, S. Genetic Characterization and Phylogenetic Analysis of Small Ruminant Lentiviruses Detected in Spanish Assaf Sheep with Different Mammary Lesions. Viruses 2018, 10, 315. [Google Scholar] [CrossRef]
- Olech, M.; Kycko, A.; Kuźmak, J. Molecular Characterization of Small Ruminant Lentiviruses Isolated from Polish Goats with Arthritis. Viruses 2022, 14, 735. [Google Scholar] [CrossRef]
- Ramírez, H.; Reina, R.; Amorena, B.; de Andrés, D.; Martínez, H.A. Small ruminant lentiviruses: Genetic variability, tropism and diagnosis. Viruses 2013, 5, 1175–1207. [Google Scholar] [CrossRef]
- Cardinaux, L.; Zahno, M.L.; Deubelbeiss, M.; Zanoni, R.; Vogt, H.R.; Bertoni, G. Virological and phylogenetic characterization of attenuated small ruminant lentivirus isolates eluding efficient serological detection. Vet. Microbiol. 2013, 162, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Schaer, J.; Cvetnic, Z.; Sukalic, T.; Dörig, S.; Grisiger, M.; Iscaro, C.; Feliziani, F.; Pfeifer, F.; Origgi, F.; Zanoni, R.G.; et al. Evaluation of Serological Methods and a New Real-Time Nested PCR for Small Ruminant Lentiviruses. Pathogens 2022, 11, 129. [Google Scholar] [CrossRef]
- Grego, E.; Bertolotti, L.; Quasso, A.; Profiti, M.; Lacerenza, D.; Muz, D.; Rosati, S. Genetic characterization of small ruminant lentivirus in Italian mixed flocks: Evidence for a novel genotype circulating in a local goat population. J. Gen. Virol. 2007, 88 Pt 12, 3423–3427. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Kuźmak, J. Compartmentalization of Subtype A17 of Small Ruminant Lentiviruses between Blood and Colostrum in Infected Goats Is Not Exclusively Associated to the env Gene. Viruses 2019, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef] [PubMed]
- L'Homme, Y.; Leboeuf, A.; Arsenault, J.; Fras, M. Identification and characterization of an emerging small ruminant lentivirus circulating recombinant form (CRF). Virology 2015, 475, 159–171. [Google Scholar] [CrossRef]
- Colitti, B.; Coradduzza, E.; Puggioni, G.; Capucchio, M.T.; Reina, R.; Bertolotti, L.; Rosati, S. A new approach for Small Ruminant Lentivirus full genome characterization revealed the circulation of divergent strains. PLoS ONE 2019, 14, e0212585. [Google Scholar] [CrossRef]
- Adjadj, N.R.; Vicca, J.; Michiels, R.; De Regge, N. (Non-)Sense of Milk Testing in Small Ruminant Lentivirus Control Programs in Goats. Comparative Analysis of Antibody Detection and Molecular Diagnosis in Blood and Milk. Viruses 2019, 12, 3. [Google Scholar] [CrossRef]
- Santry, L.A.; de Jong, J.; Gold, A.C.; Walsh, S.R.; Menzies, P.I.; Wootton, S.K. Genetic characterization of small ruminant lentiviruses circulating in naturally infected sheep and goats in Ontario, Canada. Virus Res. 2013, 175, 30–44. [Google Scholar] [CrossRef]
- Tavella, A.; Bettini, A.; Ceol, M.; Zambotto, P.; Stifter, E.; Kusstatscher, N.; Lombardi, R.; Nardeli, S.; Beato, M.S.; Capello, K.; et al. Achievements of an eradication programme against caprine arthritis encephalitis virus in South Tyrol, Italy. Vet. Rec. 2018, 182, 51. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.; van den Brom, R.; Aalberts, M.; Bogt-Kappert, C.T.; Vellema, P. Loss of Caprine Arthritis Encephalitis Virus (CAEV) Herd Accreditation: Characteristics, Diagnostic Approach, and Specific Follow-Up Scenarios on Large Dairy Goat Farms. Pathogens 2022, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Gurău, M.R.; Baraitareanu, S.; Daneş, D. Serological survey of caprine arthritis-encephalitis virus infection in a Southeastern Romanian farm. Sci. Works Ser. C Vet. Med. 2015, 61, 169–171. [Google Scholar]
- Mihai, I.; Crivei, I.C.; Horhogea, C.; Savuţa, G.; Velescu, E. Preliminary serological investigation on caprine arthritis and encephalitis virus infection in a goat Farm from North-Eastern Romanian Region. Bull. UASVM Vet. Med. 2018, 75, 243–245. [Google Scholar] [CrossRef]
- Mihai, I.; Velescu, E.; Tanase, O.I. Epidemiological observations on infectious pathology of goats in the Northeast area of Romania. Agric. Life Life Agric. Conf. Proc. 2018, 1, 449–454. [Google Scholar] [CrossRef]
- Potârniche, A.V.; Cerbu, C.; Olah, D.; Suatean, M.; Peredi, C.; Guranda, S.; Spînu, M. Serological survey of caprine arthritis-encephalitis virus infection in Sibiu county, Romania. Sci. Works Ser. C Vet. Med. 2018, 64, 70–72. [Google Scholar]
- Enache, D.A.; Baraitareanu, S.; Dan, M.; Gurau, M.R.; Otelea, F.; Dobre, A.; Danes, D. Preliminary results of MVV and CAEV seroprevalence in Romanian sheep and goats. Sci. Works Ser. C Vet. Med. 2017, 63, 95–100. [Google Scholar]
- Olech, M.; Croise, B.; Kuźmak, J.; Valas, S. Evidence for interspecies transmission of small ruminant lentiviruses in sheep and goats in Poland. Bull. Vet. Inst. Pulawy. 2009, 53, 165–168. [Google Scholar]
- Minardi da Cruz, J.C.; Singh, D.K.; Lamara, A.; Chebloune, Y. Small ruminant lentiviruses (SRLVs) break the species barrier to acquire new host range. Viruses 2013, 5, 1867–1884. [Google Scholar] [CrossRef]
- Pisoni, G.; Quasso, A.; Moroni, P. Phylogenetic analysis of small-ruminant lentivirus subtype B1 in mixed flocks: Evidence for natural transmission from goats to sheep. Virology 2005, 339, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Fras, M.; Leboeuf, A.; Labrie, F.M.; Laurin, M.A.; Singh Sohal, J.; L'Homme, Y. Phylogenetic analysis of small ruminant lentiviruses in mixed flocks: Multiple evidence of dual infection and natural transmission of types A2 and B1 between sheep and goats. Infect. Genet. Evol. 2013, 19, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Michiels, R.; Adjadj, N.R.; De Regge, N. Phylogenetic Analysis of Belgian Small Ruminant Lentiviruses Supports Cross Species Virus Transmission and Identifies New Subtype B5 Strains. Pathogens 2020, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Huder, J.B.; Böni, J.; Schönmann, M.; Mühlherr, J.; Lutz, H.; Schüpbach, J. Direct evidence for natural transmission of small-ruminant lentiviruses of subtype A4 from goats to sheep and vice versa. J. Virol. 2004, 78, 7518–7522. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.S.; Pinheiro, R.R.; Costa, J.N.; Lima, C.C.; Andrioli, A.; Azevedo, D.A.; Santos, V.W.; Araújo, J.F.; Sousa, A.L.; Pinheiro, D.N.; et al. Interspecific transmission of small ruminant lentiviruses from goats to sheep. Braz. J. Microbiol. 2015, 46, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Potârniche, A.V.; Cerbu, C.G.; Czopowicz, M.; Szalus-Jordanow, O.; Kaba, J.; Spinu, M. The epidemiological background of small ruminant lentivirus infection in goats from Romania. Vet. World 2020, 13, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Cecco, B.S.; Henker, L.C.; Lorenzett, M.P.; Molossi, F.A.; Schwertz, C.I.; Baumbach, L.F.; Weber, M.N.; Canal, C.W.; Driemeier, D.; Pavarini, S.P.; et al. An outbreak of visna-maedi in a flock of sheep in Southern Brazil. Braz. J. Microbiol. 2022, 53, 1723–1730. [Google Scholar] [CrossRef]
- Ooms, M.; Verhoef, K.; Southern, E.; Huthoff, H.; Berkhout, B. Probing alternative foldings of the HIV-1 leader RNA by antisense oligonucleotide scanning arrays. Nucleic Acids Res. 2004, 32, 819–827. [Google Scholar] [CrossRef]
- Shah, C.; Böni, J.; Huder, J.B.; Vogt, H.R.; Mühlherr, J.; Zanoni, R.; Miserez, R.; Lutz, H.; Schüpbach, J. Phylogenetic analysis and reclassification of caprine and ovine lentiviruses based on 104 new isolates: Evidence for regular sheep-to-goat transmission and worldwide propagation through livestock trade. Virology 2004, 319, 12–19. [Google Scholar] [CrossRef]
- Glaria, I.; Reina, R.; Ramírez, H.; de Andrés, X.; Crespo, H.; Jauregui, P.; Salazar, E.; Luján, L.; Pérez, M.M.; Benavides, J.; et al. Visna/Maedi virus genetic characterization and serological diagnosis of infection in sheep from a neurological outbreak. Vet. Microbiol. 2012, 155, 137–146. [Google Scholar] [CrossRef]
- Olech, M.; Murawski, M.; Kuźmak, J. Molecular analysis of small-ruminant lentiviruses in Polish flocks reveals the existence of a novel subtype in sheep. Arch. Virol. 2019, 164, 1193–1198. [Google Scholar] [CrossRef]
- Olech, M.; Kuźmak, J.; Kycko, A.; Junkuszew, A. Phylogenetic Analysis of Small Ruminant Lentiviruses Originating from Naturally Infected Sheep and Goats from Poland Based on the Long Terminal Repeat Sequences. J. Vet. Res. 2022, 66, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Molaee, V.; Bazzucchi, M.; De Mia, G.M.; Otarod, V.; Abdollahi, D.; Rosati, S.; Lühken, G. Phylogenetic analysis of small ruminant lentiviruses in Germany and Iran suggests their expansion with domestic sheep. Sci. Rep. 2020, 10, 2243. [Google Scholar] [CrossRef] [PubMed]
- Michiels, R.; Van Mael, E.; Quinet, C.; Adjadj, N.R.; Cay, A.B.; De Regge, N. Comparative Analysis of Different Serological and Molecular Tests for the Detection of Small Ruminant Lentiviruses (SRLVs) in Belgian Sheep and Goats. Viruses 2018, 10, 696. [Google Scholar] [CrossRef] [PubMed]
- Lacerenza, D.; Giammarioli, M.; Grego, E.; Marini, C.; Profiti, M.; Rutili, D.; Rosati, S. Antibody response in sheep experi-mentally infected with different small ruminant lentivirus genotypes. Vet. Immunol. Immunopathol. 2006, 112, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Brinkhof, J.; van Maanen, C. Evaluation of five enzyme-linked immunosorbent assays and an agar gel immunodiffusion test for detection of antibodies to small ruminant lentiviruses. Clin. Vaccine Immunol. 2007, 14, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Jerre, A.; Nordstoga, A.B.; Dean, K.R.; Holmøy, I.H. Evaluation of three commercial ELISA tests for serological detection of maedi-visna virus using Bayesian latent class analysis. Prev. Vet. Med. 2022, 208, 105765. [Google Scholar] [CrossRef]
- de Andrés, X.; Ramírez, H.; Bertolotti, L.; San Román, B.; Glaria, I.; Crespo, H.; Jáuregui, P.; Minguijón, E.; Juste, R.; Leginagoikoa, I.; et al. An insight into a combination of ELISA strategies to diagnose small ruminant lentivirus infections. Vet. Immunol. Immunopathol. 2013, 152, 277–288. [Google Scholar] [CrossRef]
- de Andrés, D.; Klein, D.; Watt, N.J.; Berriatua, E.; Torsteinsdottir, S.; Blacklaws, B.A.; Harkiss, G.D. Diagnostic tests for small ruminant lentiviruses. Vet. Microbiol. 2005, 107, 49–62. [Google Scholar] [CrossRef]

| Sample No. | Name | GenBank Accession Number | |
|---|---|---|---|
| Gag-Pol | Gag | ||
| 1. | RO20_Lung | OR671960 | OR666886 |
| 2. | RO23_Lung | N/A | OR666885 |
| 3. | RO26_Lung | OR671963 | OR666883 |
| 4. | RO27_Lung | OR671964 | N/A |
| 5. | RO29_Lung | OR671965 | OR666882 |
| 6. | RO36_Lung | OR671959 | OR666880 |
| 7. | RO45_Lung | OR671972 | OR666876 |
| 8. | RO66_Lung | OR671975 | OR666870 |
| 9. | RO70_Lung | N/A | OR666869 |
| 10. | RO75_Lung | OR671976 | N/A |
| 11. | RO82_Lung | OR671977 | OR666868 |
| 12. | RO85_Lung | OR671978 | OR666867 |
| 13. | RO23_Lymph node | OR671961 | OR666884 |
| 14. | RO24_Lymph node | OR671962 | OR666866 |
| 15. | RO34_Lymph node | OR671966 | N/A |
| 16. | RO35_Lymph node | OR671967 | OR666881 |
| 17. | RO39_Lymph node | OR671968 | N/A |
| 18. | RO41_Lymph node | OR671969 | OR666879 |
| 19. | RO42_Lymph node | OR671970 | OR666878 |
| 20. | RO43_Lymph node | OR671971 | OR666877 |
| 21. | RO45_Lymph node | N/A | OR666876 |
| 22. | RO46_Lymph node | OR671958 | OR666874 |
| 23. | RO47_Lymph node | OR671973 | OR666873 |
| 24. | RO48_Lymph node | N/A | OR666872 |
| 25. | RO52_Lymph node | OR671974 | OR666871 |
| A1 | A2 | A3 | A4 | A5 | A8 | A9 | A11 | A12 | A13 | A16 | A17 | A18 | A19 | A20 | A21 | A22 | A23 | A24 | A25 | A26 | A27 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 23L | 13.1 | 11.8 | 8.4 | 14.2 | 10.8 | 16.1 | 12.2 | 15.2 | 10.5 | 11.3 | 13.9 | 9.0 | 11.9 | 16.1 | 14.5 | 13.3 | 18.7 | 15.7 | 12.3 | 11.9 | 11.8 | 11.8 |
| 20L | 15.5 | 14.0 | 10.9 | 14.2 | 12.6 | 16.4 | 14.6 | 16.7 | 13.6 | 12.9 | 15.4 | 10.1 | 12.9 | 16.1 | 16.8 | 14.6 | 18.6 | 18.6 | 14.2 | 15.6 | 14.0 | 12.6 |
| 23LN | 13.3 | 11.5 | 8.3 | 14.4 | 10.9 | 16.0 | 12.1 | 15.2 | 10.4 | 11.6 | 14.1 | 9.0 | 12.5 | 15.9 | 14.7 | 13.3 | 18.8 | 16.0 | 12.1 | 12.0 | 11.9 | 12.0 |
| 24LN | 14.9 | 12.2 | 9.6 | 14.2 | 11.9 | 15.8 | 14.0 | 15.6 | 12.6 | 11.7 | 15.4 | 9.4 | 12.1 | 15.1 | 15.4 | 14.3 | 18.9 | 17.0 | 11.7 | 13.1 | 11.7 | 12.1 |
| 26L | 15.7 | 12.2 | 10.6 | 14.7 | 12.4 | 16.4 | 14.1 | 15.7 | 12.5 | 13.0 | 16.5 | 8.3 | 13.3 | 18.7 | 15.9 | 14.3 | 19.6 | 18.5 | 12.8 | 14.5 | 11.1 | 11.8 |
| 27L | 14.6 | 12.7 | 10.4 | 13.5 | 11.9 | 16.1 | 13.3 | 15.7 | 12.5 | 12.4 | 16.1 | 8.1 | 13.7 | 17.9 | 15.8 | 13.8 | 19.1 | 18.2 | 12.8 | 14.1 | 11.2 | 11.3 |
| 34LN | 14.0 | 11.5 | 10.0 | 12.9 | 10.5 | 15.0 | 11.5 | 14.6 | 12.0 | 11.9 | 15.6 | 9.5 | 13.0 | 16.5 | 13.8 | 12.4 | 18.3 | 17.3 | 12.3 | 13.1 | 11.7 | 11.2 |
| 35LN | 14.6 | 12.7 | 10.4 | 13.5 | 11.9 | 16.1 | 13.3 | 15.7 | 12.5 | 12.4 | 16.1 | 8.1 | 13.7 | 17.9 | 15.8 | 13.8 | 19.1 | 18.2 | 12.8 | 14.1 | 11.2 | 11.3 |
| 36L | 15.7 | 12.2 | 10.6 | 14.7 | 12.4 | 16.4 | 14.1 | 15.7 | 12.5 | 13.0 | 16.5 | 8.3 | 13.3 | 18.7 | 15.9 | 14.3 | 19.6 | 18.5 | 12.8 | 14.5 | 11.1 | 11.8 |
| 39LN | 13.0 | 10.0 | 8.3 | 11.3 | 9.1 | 14.2 | 11.0 | 13.9 | 10.9 | 10.6 | 13.6 | 8.3 | 12.0 | 15.2 | 12.8 | 12.0 | 17.2 | 15.7 | 11.3 | 11.5 | 11.0 | 10.3 |
| 41LN | 15.9 | 12.6 | 10.4 | 15.2 | 12.5 | 17.8 | 14.2 | 16.5 | 12.5 | 12.5 | 16.1 | 10.3 | 13.8 | 17.1 | 16.6 | 13.1 | 20.1 | 18.5 | 13.0 | 13.7 | 13.1 | 13.2 |
| 42LN | 15.5 | 14.0 | 10.9 | 15.2 | 12.6 | 16.4 | 14.6 | 16.7 | 13.6 | 12.9 | 15.4 | 10.1 | 12.9 | 16.1 | 16.8 | 14.6 | 18.6 | 18.6 | 14.2 | 15.6 | 14.0 | 12.6 |
| 43LN | 14.6 | 12.7 | 10.4 | 13.5 | 11.9 | 16.1 | 13.3 | 15.7 | 12.5 | 12.4 | 16.1 | 8.1 | 13.7 | 17.9 | 15.8 | 13.8 | 19.1 | 18.2 | 12.8 | 14.1 | 11.2 | 11.3 |
| 45L | 15.1 | 12.7 | 9.1 | 14.6 | 12.0 | 16.1 | 13.3 | 15.0 | 11.6 | 11.5 | 15.7 | 8.1 | 11.9 | 17.6 | 15.9 | 13.0 | 18.9 | 17.5 | 12.3 | 13.9 | 11.2 | 10.7 |
| 45LN | 15.7 | 12.2 | 10.6 | 14.7 | 12.4 | 16.4 | 14.1 | 15.7 | 12.5 | 13.0 | 16.5 | 8.3 | 13.3 | 18.7 | 15.9 | 14.3 | 19.6 | 18.5 | 12.8 | 14.5 | 11.1 | 11.8 |
| 46LN | 15.7 | 12.2 | 10.6 | 14.7 | 12.4 | 16.4 | 14.1 | 15.7 | 12.5 | 13.0 | 16.5 | 8.3 | 13.3 | 18.7 | 15.9 | 14.3 | 19.6 | 18.5 | 12.8 | 14.5 | 11.1 | 11.8 |
| 47LN | 14.6 | 12.8 | 9.7 | 14.0 | 11.5 | 14.9 | 13.9 | 16.0 | 12.2 | 12.4 | 15.8 | 9.4 | 14.5 | 16.6 | 14.9 | 13.5 | 18.6 | 18.6 | 13.3 | 13.5 | 12.2 | 10.8 |
| 66L | 14.4 | 13.8 | 10.7 | 14.5 | 10.4 | 16.5 | 14.0 | 16.2 | 12.1 | 11.3 | 15.4 | 11.4 | 14.9 | 14.9 | 15.5 | 14.0 | 18.6 | 17.5 | 13.3 | 13.6 | 12.5 | 13.0 |
| 70L | 16.5 | 13.1 | 11.4 | 14.9 | 12.6 | 16.3 | 14.7 | 15.5 | 12.3 | 13.2 | 15.6 | 11.0 | 14.2 | 16.4 | 14.6 | 14.7 | 20.0 | 16.9 | 13.9 | 12.7 | 13.1 | 13.2 |
| 82L | 16.0 | 12.2 | 9.4 | 13.5 | 11.9 | 15.6 | 14.0 | 14.5 | 11.7 | 11.9 | 15.8 | 9.9 | 13.3 | 16.0 | 15.8 | 13.2 | 17.4 | 16.5 | 12.5 | 13.3 | 11.4 | 11.9 |
| 85L | 16.3 | 10.4 | 9.1 | 15.1 | 13.8 | 16.2 | 13.3 | 13.7 | 12.3 | 12.1 | 16.2 | 9.8 | 13.0 | 16.3 | 16.5 | 15.1 | 20.7 | 16.0 | 12.8 | 13.1 | 11.9 | 12.4 |
| 75L | 14.8 | 10.5 | 9.4 | 14.4 | 11.9 | 15.3 | 12.0 | 15.2 | 12.1 | 11.3 | 15.4 | 8.5 | 11.4 | 14.3 | 13.6 | 14.9 | 19.0 | 15.9 | 12.7 | 10.6 | 11.9 | 10.6 |
| 29L | 14.3 | 12.6 | 10.9 | 14.6 | 10.8 | 15.6 | 12.7 | 16.0 | 12.3 | 11.5 | 16.2 | 8.3 | 15.2 | 16.0 | 15.9 | 13.9 | 19.3 | 18.3 | 12.8 | 14.8 | 12.0 | 12.4 |
| 48LN | 16.9 | 13.6 | 9.9 | 16.4 | 13.2 | 18.4 | 15.8 | 16.3 | 14.4 | 14.9 | 15.7 | 12.7 | 14.7 | 17.7 | 16.5 | 14.2 | 19.1 | 17.0 | 15.7 | 14.0 | 16.0 | 14.6 |
| 52LN | 15.6 | 10.6 | 9.5 | 14.9 | 11.8 | 16.6 | 13.1 | 15.8 | 12.4 | 11.8 | 15.8 | 9.4 | 13.3 | 16.4 | 15.8 | 14.0 | 17.7 | 17.9 | 14.9 | 12.8 | 13.3 | 12.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olech, M.; Hodor, D.; Toma, C.; Negoescu, A.; Taulescu, M. First Molecular Characterization of Small Ruminant Lentiviruses Detected in Romania. Animals 2023, 13, 3718. https://doi.org/10.3390/ani13233718
Olech M, Hodor D, Toma C, Negoescu A, Taulescu M. First Molecular Characterization of Small Ruminant Lentiviruses Detected in Romania. Animals. 2023; 13(23):3718. https://doi.org/10.3390/ani13233718
Chicago/Turabian StyleOlech, Monika, Dragoş Hodor, Corina Toma, Andrada Negoescu, and Marian Taulescu. 2023. "First Molecular Characterization of Small Ruminant Lentiviruses Detected in Romania" Animals 13, no. 23: 3718. https://doi.org/10.3390/ani13233718
APA StyleOlech, M., Hodor, D., Toma, C., Negoescu, A., & Taulescu, M. (2023). First Molecular Characterization of Small Ruminant Lentiviruses Detected in Romania. Animals, 13(23), 3718. https://doi.org/10.3390/ani13233718





