Effects of High-Lipid Dietary Protein Ratio on Growth, Antioxidant Parameters, Histological Structure, and Expression of Antioxidant- and Immune-Related Genes of Hybrid Grouper
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Method
2.1. Experimental Conditions
2.2. Experimental Diets
2.3. Sample Collection
2.4. The Methods of Analysis
2.5. Antioxidant and Immunity Indexes in Serum and Liver
2.6. Hepatic Histopathology
2.7. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Statistical Analysis
3. Results
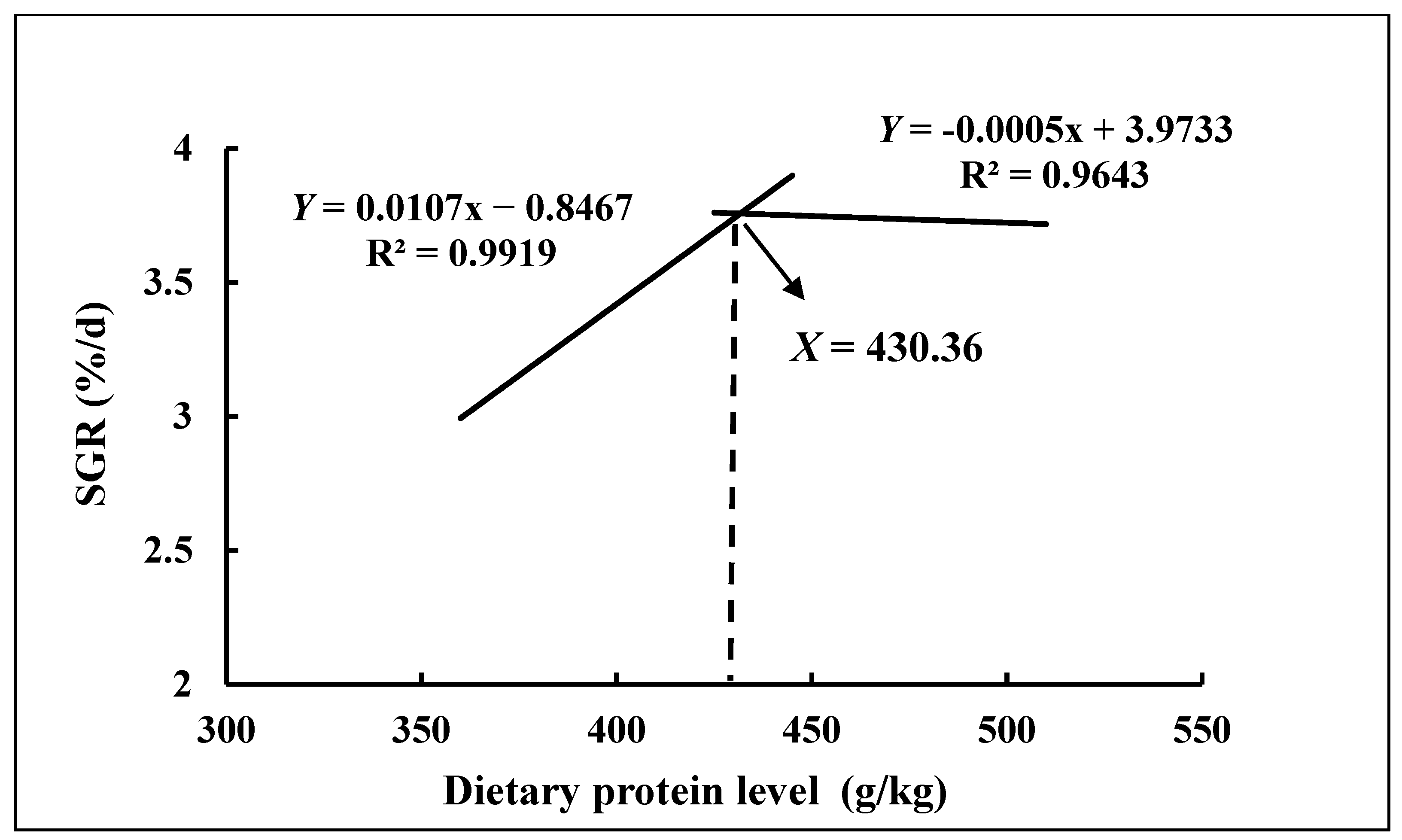
3.1. Growth Performance
3.2. Antioxidant Parameters in Serum
3.3. Antioxidant and Non-Specific Immunity Parameters in Liver
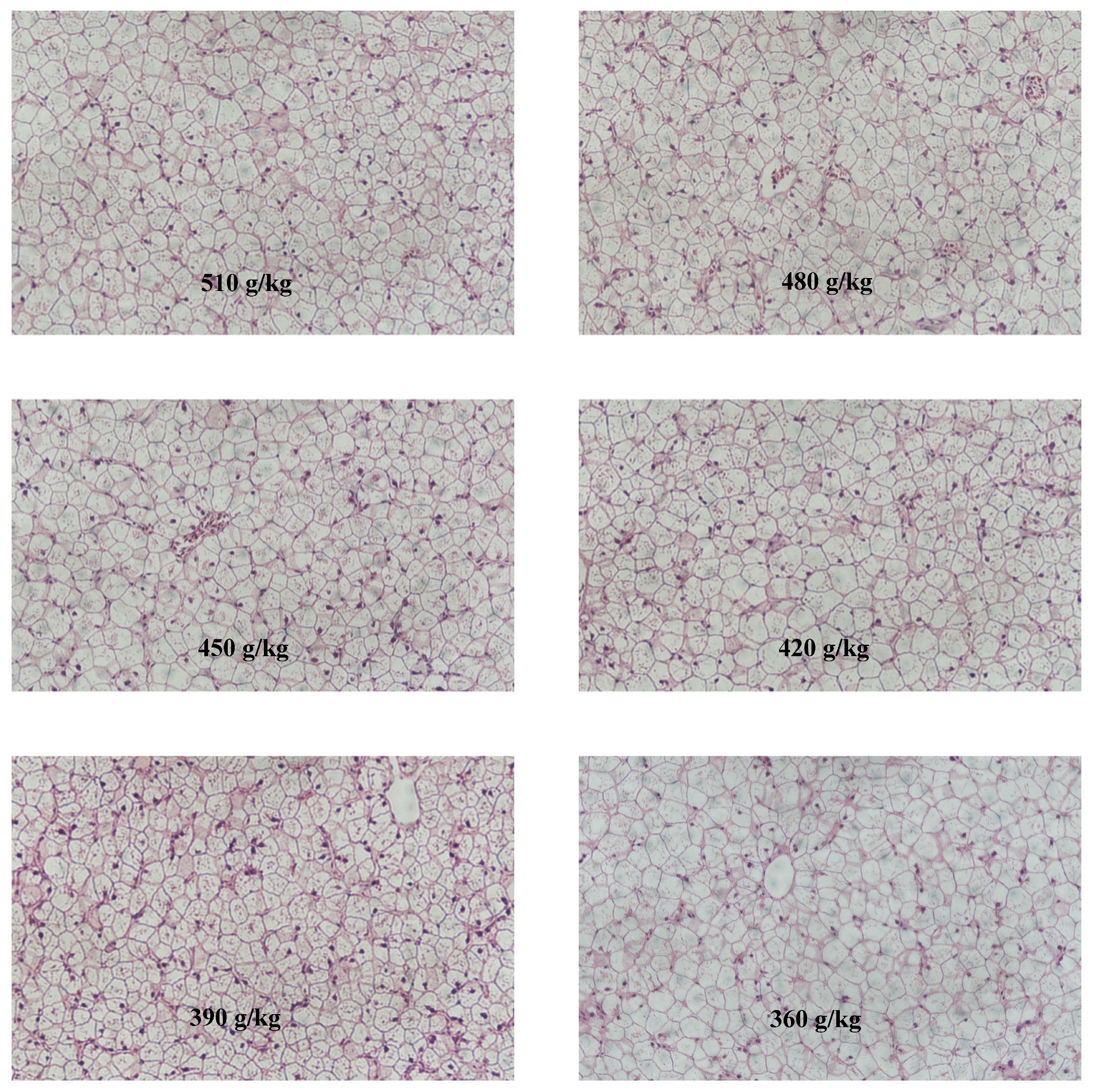
3.4. Histological Structure of Liver
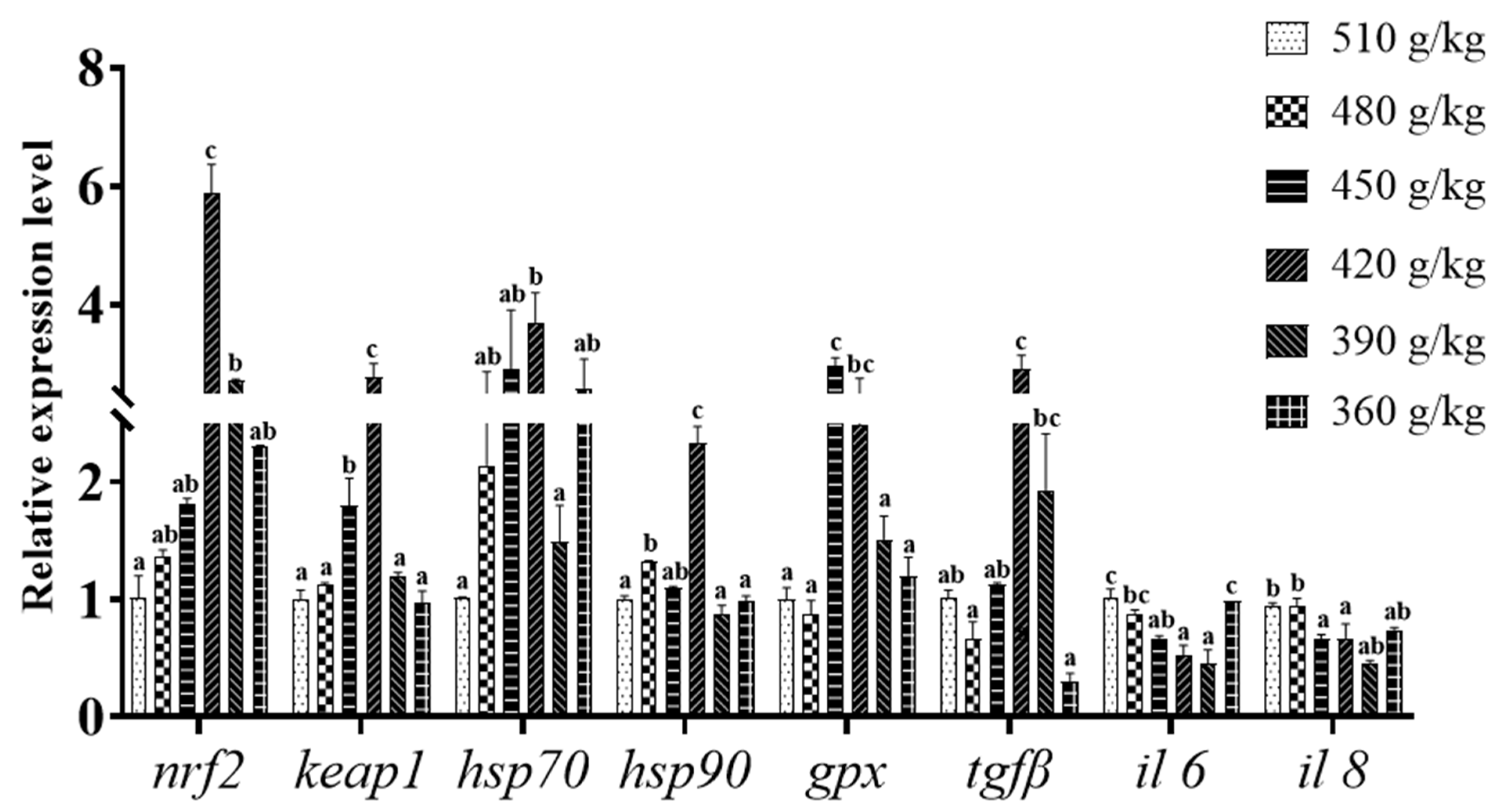
3.5. Expression of Antioxidant- and Immune-Related Genes in Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, W.; Yan, X.; Liu, H.; Tan, B.; Suo, X.; Pan, S.; Li, T.; Yang, Y.; Dong, X. Effects of Vitamin E supplementation of a high-lipid diet on the growth and biochemical parameters of hybrid groupers (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatus). Front. Mar. Sci. 2022, 9, 924018. [Google Scholar] [CrossRef]
- Pan, S.; Yan, X.; Dong, X.; Li, T.; Suo, X.; Liu, H.; Tan, B.; Zhang, S.; Li, Z.; Yang, Y.; et al. Influence of dietary inositol supplementation on growth, liver histology, lipid metabolism, and related genes expression on juvenile hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatus) fed high-lipid diets. Aquac. Nutr. 2022, 2022, 6743690. [Google Scholar] [CrossRef]
- Chen, G.; Yin, B.; Liu, H.; Tan, B.; Dong, X.; Yang, Q.; Chi, S.; Zhang, S. Effects of fishmeal replacement with cottonseed protein concentrate on growth, digestive proteinase, intestinal morphology and microflora in pearl gentian grouper (♀ Epinephelus fuscoguttatus × ♂ Epinephelus lanceolatu). Aquac. Res. 2020, 51, 2870–2884. [Google Scholar] [CrossRef]
- Cai, L.S.; Wang, L.; Song, K.; Lu, K.L.; Zhang, C.X.; Rahimnejad, S. Evaluation of protein requirement of spotted seabass (Lateolabrax maculatus) under two temperatures, and the liver transcriptome response to thermal stress. Aquaculture 2020, 516, 734615. [Google Scholar] [CrossRef]
- Singh, R.K.; Balange, A.K.; Ghughuskar, M.M. Protein sparing effect of carbohydrates in the diet of Cirrhinus mrigala (Hamilton, 1822) fry. Aquaculture 2006, 258, 680–684. [Google Scholar] [CrossRef]
- Jiang, W.D.; Xu, J.; Zhou, X.Q.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A. Dietary protein levels regulated antibacterial activity, inflammatory response and structural integrity in the head kidney, spleen and skin of grass carp (Ctenopharyngodon idella) after challenged with Aeromonas hydrophila. Fish Shellfish Immunol. 2017, 68, 154–172. [Google Scholar] [CrossRef]
- Xu, J.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A. Effects of dietary protein levels on the disease resistance, immune function and physical barrier function in the gill of grass carp (Ctenopharyngodon idella) after challenged with Flavobacterium columnare. Fish Shellfish Immunol. 2016, 57, 1–16. [Google Scholar] [CrossRef]
- Singh, R.K.; Chavan, S.L.; Desai, A.S.; Khandagale, P.A. Influence of dietary protein levels and water temperature on growth, body composition and nutrient utilization of Cirrhinus mrigala (Hamilton, 1822) fry. J. Therm. Biol. 2008, 33, 20–26. [Google Scholar] [CrossRef]
- Watanabe, T. Lipid nutrition in fish. Comp. Biochem. Physiol. Part B Biochem. 1982, 73, 3–15. [Google Scholar] [CrossRef]
- Lee, S.M.; Im, G.J.; Jong, Y.L. Effects of digestible protein and lipid levels in practical diets on growth, protein utilization and body composition of juvenile rockfish (Sebastes schlegeli). Aquaculture 2002, 211, 227–239. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Wang, Q.; Zhang, X. Effects of digestible crude protein and lipid levels on growth performance, metabolism of energy substances and antioxidant capacity of juvenile GIFI tilapia, Oreochromis niloticus. Chin. Agric. Sci. Bull. 2021, 37, 126–135. [Google Scholar]
- Suo, X.; Yan, X.; Tan, B.; Pan, S.; Li, T.; Liu, H.; Huang, W.; Zhang, S.; Yang, Y.; Dong, X. Lipid metabolism disorders of hybrid grouper (♀ Epinephelus Fuscointestinestatus × ♂ E. lanceolatu) induced by high-lipid diet. Front. Mar. Sci. 2022, 9, 990193. [Google Scholar] [CrossRef]
- Wang, C.; Hu, G.; Sun, P.; Gu, W.; Wang, B.; Xu, Q. Effects of dietary protein and lipid levels on growth performance, digestive enzyme activities and serum indices of Salmo trutta fario broodstock. Chin. J. Anim. Nutr. 2017, 29, 571–582. [Google Scholar]
- Li, T.; Yan, X.; Dong, X.; Pan, S.; Tan, B.; Zhang, S.; Suo, X.; Li, Z.; Huang, W.; Yang, Y. Choline alleviates disorders of lipid metabolism in hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatus) caused by high-lipid diet. Aquac. Nutr. 2022, 2022, 8998849. [Google Scholar] [CrossRef]
- Long, S.; Dong, X.; Tan, B.; Zhang, S.; Xie, S.; Yang, Q.; Chi, S.; Liu, H.; Deng, J.; Yang, Y.; et al. Growth performance, antioxidant ability, biochemical index in serum, liver histology and hepatic metabolomics analysis of juvenile hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ Epinephelus lanceolatus) fed with oxidized fish oil. Aquaculture 2021, 545, 737261. [Google Scholar] [CrossRef]
- Jobling, M. National Research Council (NRC): Nutrient requirements of fish and shrimp. Aquac. Int. 2012, 20, 601–602. [Google Scholar] [CrossRef]
- Hirwitz, W.; Latimer, G. Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995; Volume 6, p. 382. [Google Scholar]
- GB/T 6435-2006; Determination of Moisture in Feed. China National Standardization Administration: Beijing, China, 2006.
- GB/T 6432-2018; Determination of Crude Protein in Feeds—Kjeldahl Method. China National Standardization Administration: Beijing, China, 2018.
- GB/T 5512-2008; Grain and Oil Test Determination of Crude Fat Content in Grain. China National Standardization Administration: Beijing, China, 2008.
- Yin, B.; Liu, H.; Tan, B.; Dong, X.; Chi, S.; Yang, Q.; Zhang, S.; Chen, L. Cottonseed protein concentrate (CPC) suppresses immune function in different intestinal segments of hybrid grouper ♀ Epinephelus fuscoguttatus × ♂ Epinephelus lanceolatu via TLR-2/MyD88 signaling pathways. Fish Shellfish Immunol. 2018, 81, 318–328. [Google Scholar] [CrossRef]
- Pan, S.; Yan, X.; Li, T.; Suo, X.; Liu, H.; Tan, B.; Huang, W.; Yang, Y.; Zhang, H.; Dong, X. Impacts of tea polyphenols on growth, antioxidant capacity and immunity in juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂) fed high-lipid diets. Fish Shellfish Immunol. 2022, 128, 348–350. [Google Scholar] [CrossRef]
- Yan, X.; Yang, J.; Dong, X.; Tan, B.; Zhang, S.; Chi, S.; Yang, Q.; Liu, H.; Yang, Y. The optimal dietary protein level of large-size grouper Epinephelus coioides. Aquac. Nutr. 2020, 26, 705–714. [Google Scholar] [CrossRef]
- Jiang, S.; Wu, X.; Luo, Y.; Wu, M.; Lu, S.; Jin, Z.; Yao, W. Optimal dietary protein level and protein to energy ratio for hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) juveniles. Aquaculture 2016, 465, 28–36. [Google Scholar] [CrossRef]
- Jayant, M.; Muralidhar, A.P.; Sahu, N.P.; Jain, K.K.; Pal, A.K.; Srivastava, P.P. Protein requirement of juvenile striped catfish, Pangasianodon hypophthalmus. Aquac. Int. 2018, 26, 375–389. [Google Scholar] [CrossRef]
- Lee, C.; Lee, K.J. Dietary protein requirement of pacific white shrimp Litopenaeus vannamei in three different growth stages. Fish. Aquat. Sci. 2018, 21, 30. [Google Scholar] [CrossRef]
- McGoogan, B.B.; Gatlin, D.M. Dietary manipulations affecting growth and nitrogenous waste production of red drum, Sciaenops ocellatus II. Effects of energy levels. Aquaculture 2000, 182, 271–285. [Google Scholar] [CrossRef]
- Cho, C.Y.; Hynes, J.D.; Wood, K.R.; Yoshida, H.K. Development of high-nutrient-dense, low-pollution diets and prediction of aquaculture wastes using biological approaches. Aquaculture 1994, 124, 293–305. [Google Scholar] [CrossRef]
- Zhou, J.B.; Zhou, Q.C.; Chi, S.Y.; Yang, Q.H.; Liu, C.W. Optimal dietary protein requirement for juvenile ivory shell, Babylonia areolate. Aquaculture 2007, 270, 186–192. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, L.; Xia, S.; Liu, S.; Ru, X.; Xu, Q.; Zhang, T.; Yang, H. Effects of dietary protein levels on the growth, energy budget, and physiological and immunological performance of green, white and purple color morphs of sea cucumber, Apostichopus japonicus. Aquaculture 2016, 450, 375–382. [Google Scholar] [CrossRef]
- Peres, H.; Oliva-Teles, A. Influence of temperature on protein utilization in juvenile European seabass (Dicentrarchus labrax). Aquaculture 1999, 170, 337–348. [Google Scholar] [CrossRef]
- Keembiyehetty, C.N.; Wilson, R.P. Effect of water temperature on growth and nutrient utilization of sunshine bass (Morone chrysops ♀ × Morone saxatilis ♂) fed diets containing different energy/protein ratios. Aquaculture 1998, 166, 151–162. [Google Scholar] [CrossRef]
- Vergara, J.M.; Fernández-Palacios, H.; Robainà, L.; Jauncey, K.; De La Higuera, M.; Izquierdo, M. The effects of varying dietary protein level on the growth, feed efficiency, protein utilization and body composition of gilthead sea bream fry. Fish. Sci. 1996, 62, 620–623. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Huang, X.; Zhu, Y.; Kuang, X.; Yi, G. Effects of dietary protein levels on the growth, digestive enzyme activity and fecundity in the oriental river prawn, Macrobrachium nipponense. Aquac. Res. 2022, 53, 2886–2894. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.J.; Mai, K.S.; Tian, L.X.; Liu, D.H.; Tan, X.Y. Optimal dietary protein requirement of grouper Epinephelus coioides Juveniles fed isoenergetic diets in floating net cages. Aquac. Nutr. 2004, 10, 247–252. [Google Scholar] [CrossRef]
- Zhao, S.; Lin, H.; Huang, Z.; Zhou, C.; Wang, J.; Wang, Y.; Qi, C. Effect of dietary protein level on growth performance plasma biochemical indices and flesh quality of grouper Epinephelus lanceolatus × E. fuscoguttatus at two growth stages. S. China Fish. Sci. 2017, 13, 87–96. [Google Scholar]
- Kanak, E.G.; Dogan, Z.; Eroglu, A.; Atli, G.; Canli, M. Effects of fish size on the response of antioxidant systems of Oreochromis niloticus following metal exposures. Fish Physiol. Biochem. 2014, 40, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Peng, S.; Chen, C.; Gao, Q.; Shi, Z. Effects of dietary protein and lipid levels on immune and antioxidant function of juvenile Epinehelus moara. Mar. Fish. 2016, 38, 634–644. [Google Scholar]
- Tang, Y. Effects of Dietary Protein Levels on Digestive Enzymes, Non-Specific Immunity and Intestinal Contents Bacteria of Tranchinotus ovatus (Linn). Ph.D. Thesis, Xiamen University, Xiamen, China, 2014. [Google Scholar]
- Hu, B.; Song, L.; Mao, S.; Xu, P. Effects of four Chinese herbal preparations on growth performance and antioxidant activity in juvenile Micropterus salmoides. J. Guangdong Ocean Univ. 2019, 39, 101–107. [Google Scholar]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative Eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Kucukbay, F.Z.; Yazlak, H.; Karaca, I.; Sahin, N.; Tuzcu, M.; Cakmak, M.N.; Sahin, K. The Effects of dietary organic or inorganic selenium in rainbow Trout (Oncorhynchus mykiss) under crowding conditions. Aquac. Nutr. 2009, 15, 569–576. [Google Scholar] [CrossRef]
- Wang, L.; Xu, B.; Sagada, G.; Ng, W.K.; Chen, K.; Zhang, J.; Shao, Q. Dietary berberine regulates lipid metabolism in muscle and liver of black sea bream (Acanthopagrus schlegelii) fed normal or high-lipid diets. Br. J. Nutr. 2021, 125, 481–493. [Google Scholar] [CrossRef]
- Yan, X.; Pan, S.; Dong, X.; Tan, B.; Li, T.; Huang, W.; Suo, X.; Li, Z.; Yang, Y. Vitamin E amelioration of oxidative stress and low immunity induced by high-lipid diets in hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatu). Fish Shellfish Immunol. 2022, 124, 156–163. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Q.; Yue, Y.; Zhu, J.; Xiao, W.; Li, D.; Zou, Z. Effects of dietary protein level on growth performance body composition hematological indexes and hepatic non-specific immune indexes of juvenile Nile tilapia Oreochromis niloticus. Chin. J. Anim. Nutr. 2012, 24, 2384–2392. [Google Scholar]
- Sun, J.; Fan, Z.; Zhang, M.; Cheng, Z.; Bai, D.; Qiao, X. Effect of dietary protein level on hepatic function and antioxidant capacity of juvenile common carps (Cyprinus carpio). S. China Fish. Sci. 2017, 13, 113–119. [Google Scholar]
- Reyes-Becerril, M.; Ascencio, F.; Gracia-Lopez, V.; Macias, M.E.; Roa, M.C.; Esteban, M.Á. Single or combined effects of Lactobacillus sakei and inulin on growth, non-specific immunity and IgM expression in leopard grouper (Mycteroperca rosacea). Fish Physiol. Biochem. 2014, 40, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Chen, X.; Ao, J. The up-regulation of large yellow croaker secretory IgM heavy chain at early phase of immune response. Fish Physiol. Biochem. 2010, 36, 483–490. [Google Scholar] [CrossRef]
- de Andrade, J.I.A.; Ono, E.A.; de Menezes, G.C.; Brasil, E.M.; Roubach, R.; Urbinati, E.C.; Tavares-Dias, M.; Marcon, J.L.; Affonso, E.G. Influence of diets supplemented with Vitamins C and E on pirarucu (Arapaima gigas) blood parameters. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 146, 576–580. [Google Scholar] [CrossRef]
- Wang, M. Effects of Dietary Protein Levels on Growth, Serum Biochemical Index and Histological Structure of GIFT under Ammonia-Nitrogen Stress; Shanghai Ocean University: Shanghai, China, 2018. [Google Scholar]
- Xu, Q.; Li, C.; Yang, P.; Xu, H.; Wang, C. Effects of partial replacement of fishmeal with soy protein isolated and meat bonemeal on grotwh and non-specific immunity in rainbow trout (Oncorhynchus mykis). J. Dalian Fish. Univ. 2008, 23, 8–12. [Google Scholar]
- Zhao, L.; Liang, J.; Liu, H.; Gong, C.; Huang, X.; Hu, Y.; Liu, Q.; He, Z.; Zhang, X.; Yang, S. Yinchenhao decoction ameliorates the high-carbohydrate diet induced suppression of immune response in largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2022, 125, 141–151. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, B.; Liu, L.; Yang, R.; Liu, H. Effects of feed protein levels on growth, digestive enzyme activities, non-specific immunity and protein metabolism of Schizothorax o’connori. Acta Hydrobiol. Sin. 2020, 44, 693–706. [Google Scholar]
- Li, W.; Pan, X.; Cheng, W.; Cheng, Y.; Yin, Y.; Chen, J.; Xu, G.; Xie, L. Serum biochemistry, histology and transcriptomic profile analysis reflect liver inflammation and damage following dietary histamine supplementation in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2018, 77, 83–90. [Google Scholar] [CrossRef]
- Shuqin, L.; Lin, C.; Wenbing, Z.; Wei, X.; Kangsen, M.; Jikang, S. Effect of dietary protein content on the protein retention and nitrogenous metabolism of large yellow croaker. Period. Ocean Univ. China 2014, 44, 32–39. [Google Scholar]
- Ruijian, S.; Wenbing, Z.; Wei, X.; Kangsen, M. Effects of dietary protein level and feeding frequency on the growth performance, body composition and protein metabolism of juvenile large yellow croakers, Pseudosciaena crocea R. Acta Hydrobilolgica Sin. 2013, 37, 281–289. [Google Scholar]
- Magnadóttir, B. Innate immunity of fish. Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sun, Z.; Liu, Q.; Ye, H.; Zou, C.; Ye, C.; Wang, A.; Lin, H. Effects of dietary ginkgo biloba leaf extract on growth performance, plasma biochemical parameters, fish composition, immune responses, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatus). Fish Shellfish Immunol. 2018, 72, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Pan, T.; Cheng, X.; Zhu, T.T.; Sun, P.; Zhou, F.; Ding, X.; Zhou, Q.C. Effects of supplemental dietary l-carnitine and bile acids on growth performance, antioxidant and immune ability, histopathological changes and inflammatory response in juvenile black seabream (Acanthopagrus schlegelii) fed high-fat diet. Aquaculture 2019, 504, 199–209. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, S.; Ge, X.; Xia, S.; Zhu, J.; Miao, L.; Lin, Y.; Liang, H.; Pan, W.; Su, Y. Acute effects of ammonia exposure on the plasma and haematological parameters and histological structure of the juvenile blunt snout bream, Megalobrama amblycephala, and post-exposure recovery. Aquac. Res. 2018, 49, 1008–1019. [Google Scholar] [CrossRef]
- Barja-Fernández, S.; Míguez, J.M.; Álvarez-Otero, R. Histopathological effects of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in the gills, intestine and liver of Turbot (Psetta maxima). Ecotoxicol. Environ. Saf. 2013, 95, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Gu, Z.; Fu, H.; Zhu, J.; Ge, X.; Xuan, F. Molecular cloning, characterization, and expression analysis of P53 from the oriental river prawn, Macrobrachium nipponense, in response to hypoxia. Fish Shellfish Immunol. 2016, 54, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Welker, A.F.; Moreira, D.C.; Campos, É.G.; Hermes-Lima, M. Role of redox metabolism for adaptation of aquatic animals to drastic changes in oxygen availability. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 165, 384–404. [Google Scholar] [CrossRef] [PubMed]
- Asea, A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Baré, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel signal transduction pathway utilized by extracellular HSP70. role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002, 277, 15028–15034. [Google Scholar] [CrossRef]
- Vabulas, R.M.; Ahmad-Nejad, P.; Ghose, S.; Kirschning, C.J.; Issels, R.D.; Wagner, H. HSP70 as endogenous stimulus of the Toll/Interleukin-1 receptor signal pathway. J. Biol. Chem. 2002, 277, 15107–15112. [Google Scholar] [CrossRef]
- Zeng, F.; Tee, C.; Liu, M.; Sherry, J.P.; Dixon, B.; Duncker, B.P.; Bols, N.C. The P53/HSP70 inhibitor, 2phenylethynesulfonamide, causes oxidative stress, unfolded protein response and apoptosis in rainbow trout cells. Aquat. Toxicol. 2014, 146, 45–51. [Google Scholar] [CrossRef]
- Habte-Tsion, H.M.; Ren, M.C.; Ge, X.P.; Kumar, V.; Liu, B.; Xie, J.; Chen, R.L. Adequate dietary protein level enhances stress resistance and immune status of blunt snout bream (Megalobrama amblycephala Yih, 1955). J. Appl. Ichthyol. 2017, 33, 75–83. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Niu, D.; Li, Y.; Li, X. Immunological effects of paraquat on common carp, Cyprinus carpio L. Fish Shellfish Immunol. 2014, 37, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gallagher, E.P. Role of Nrf2 antioxidant defense in mitigating cadmium-induced oxidative stress in the olfactory system of zebrafish. Toxicol. Appl. Pharmacol. 2013, 266, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Zheng, Q.; Yang, Q.; Tan, B.; Dong, X.; Chi, S.; Liu, H.; Zhang, S. Alterations on growth performance, antioxidant responses and lipid metabolism in liver for juvenile hybrid Grouper (♀ Epinephelus fuscoguttatus × ♂ Epinephelus lanceolatus) fed dietary Vitamin E. Aquac. Rep. 2021, 21, 100862. [Google Scholar] [CrossRef]
- Sun, S.; Wu, Y.; Yu, H.; Su, Y.; Ren, M.; Zhu, J.; Ge, X. Serum biochemistry, liver histology and transcriptome profiling of bighead carp Aristichthys nobilis following different dietary protein levels. Fish Shellfish Immunol. 2019, 86, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.J.; Pan, L.Q. Evaluation of dietary protein level on selected parameters of immune and antioxidant systems, and growth performance of juvenile Litopenaeus vannamei reared in zero-water exchange biofloc-based culture tanks. Aquaculture 2014, 426–427, 181–188. [Google Scholar] [CrossRef]
- Xie, S.; Lin, Y.Y.; Wu, T.; Tian, L.; Liang, J.; Tan, B. Dietary lipid levels affected growth performance, lipid accumulation, inflammatory response and apoptosis of Japanese seabass (Lateolabrax japonicus). Aquac. Nutr. 2021, 27, 807–816. [Google Scholar] [CrossRef]
- Silswal, N.; Singh, A.K.; Aruna, B.; Mukhopadhyay, S.; Ghosh, S.; Ehtesham, N.Z. Human resistin stimulates the pro-inflammatory cytokines TNF-α and IL-12 in macrophages by NF-ΚB-dependent pathway. Biochem. Biophys. Res. Commun. 2005, 334, 1092–1101. [Google Scholar] [CrossRef]
- Costa, M.M.; Maehr, T.; Diaz-Rosales, P.; Secombes, C.J.; Wang, T. Bioactivity studies of rainbow trout (Oncorhynchus mykiss) interleukin-6: Effects on macrophage growth and antimicrobial peptide gene expression. Mol. Immunol. 2011, 48, 1903–1916. [Google Scholar] [CrossRef]
| Ingredients | Dietary Protein Level (%) | |||||
|---|---|---|---|---|---|---|
| 51 | 48 | 45 | 42 | 39 | 36 | |
| Fish meal | 43 | 39 | 35 | 31 | 27 | 23 |
| Wheat gluten powder | 5 | 5 | 5 | 5 | 5 | 5 |
| Wheat flour | 17 | 17 | 17 | 17 | 17 | 17 |
| CAP | 15 | 15 | 15 | 15 | 15 | 15 |
| Soybean lecithin | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Fish oil | 5 | 5 | 5 | 5 | 5 | 5 |
| Corn oil | 7 | 7.28 | 7.57 | 7.86 | 8.15 | 8.44 |
| Dextrinized starch | 2 | 2 | 2 | 2 | 2 | 2 |
| Premix a | 1 | 1 | 1 | 1 | 1 | 1 |
| Vitamin C | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Choline chloride | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Ca(H2PO4)2 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Ethoxyquin | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Attractant | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Sodium carboxymethyl cellulose | 1 | 1 | 1 | 1 | 1 | 1 |
| Cellulose microcrystalline | 0.2 | 3.92 | 7.63 | 11.34 | 15.05 | 18.76 |
| Lysine | 0.00 | 0.16 | 0.31 | 0.47 | 0.62 | 0.78 |
| Methionine | 0.00 | 0.06 | 0.12 | 0.18 | 0.24 | 0.30 |
| Proximate composition b | ||||||
| Crude protein | 50.9 | 47.61 | 44.47 | 41.15 | 38.79 | 35.77 |
| Crude lipid | 16.04 | 16.13 | 16.15 | 16.25 | 15.8 | 16.4 |
| Primer Names | Forward and Reverse Primer Sequence (5′ to 3′) | GenBank Accession No. |
|---|---|---|
| β-action-F /R | GGCTACTCCTTCACCACCACA /TCTGGGCAACGGAACCTCT | AY510710.2 |
| hsp70-F /R | AAAAGCACGGCTCATACTCAC /GCTCTGGCAGCAGTGGAA | FJ196235.1 |
| hsp90-F /R | GCTACAGAGCAGCACGACA /CTCCTCATCTTCGCTTTCC | DQ232867.1 |
| keap1-F /R | GAAGTCTGAGAGGGAGAGCGGTAG /GTGTGACAGGGTTGAGCGATGAG | XM-033623805.1 |
| gpx-F /R | TCCTCTGTGGAAGTGGCTGA /TCATCCAGGGGTCCGTATCT | HQ441085.1 |
| il-6-F /R | AGGAAGTCTGGCTGTCAGGA /GCCCTGAGGCCTTCAAGATT | JN806222.1 |
| il-8-F /R | AAGTTTGCCTTGACCCCGAA /AAGCAGATCTCTCCCGGTCT | GU988706.1 |
| tgfβ-F /R nrf2-F /R | CGATGTCACTGACGCCCTGC /AGCCGCGGTCATCACTTATC GAAGGAGCGTCTGTTGAGTGA /GAAGATGCTGCCGTTAGTTGA | GQ205390.1 KU892416.1 |
| Dietary Protein Levels (g/kg) | |||||||
|---|---|---|---|---|---|---|---|
| Diets | 510 | 480 | 450 | 420 | 390 | 360 | p Value |
| SGR (%/d) | 3.72 ± 0.03 a | 3.73 ± 0.06 a | 3.75 ± 0.05 a | 3.65 ± 0.02 a | 3.28 ± 0.02 b | 3.01 ± 0.07 c | 0.03 |
| FCR | 0.76 ± 0.01 a | 0.80 ± 0.05 a | 0.81 ± 0.01 a | 0.84 ± 0.01 ab | 1.15 ± 0.02 b | 1.14 ± 0.06 b | 0.01 |
| SR (%) | 94.44 ± 2.94 a | 92.22 ± 2.94 a | 96.67 ± 1.93 a | 96.67 ± 1.93 a | 91.67 ± 2.89 a | 81.11 ± 2.94 b | 0.01 |
| Diets | SOD (mg/mL) | CAT (mg/mL) | T-AOC (U/mL) | ROS (U/mL) | MDA (mg/mL) |
|---|---|---|---|---|---|
| 510 | 6.22 ± 0.29 a | 11.40 ± 0.53 a | 13.42 ± 0.70 a | 490.50 ± 11.80 b | 7.30 ± 0.72 |
| 480 | 9.03 ± 0.34 ab | 12.50 ± 0.66 ab | 14.80 ± 0.62 ab | 434.95 ± 13.43 ab | 6.76 ± 0.68 |
| 450 | 10.25 ± 1.04 b | 15.52 ± 0.85 ab | 16.11 ± 0.87 abc | 438.02 ± 15.32 ab | 6.63 ± 1.08 |
| 420 | 10.11 ± 0.84 b | 17.01 ± 1.46 b | 19.58 ± 0.19 c | 319.28 ± 5.83 a | 4.98 ± 0.52 |
| 390 | 14.59 ± 0.94 c | 14.95 ± 0.18 ab | 18.54 ± 0.08 bc | 317.93 ± 49.56 a | 5.54 ± 0.51 |
| 360 | 10.53 ± 0.34 b | 14.73 ± 1.02 ab | 16.52 ± 1.26 abc | 332.43 ± 45.29 ab | 5.60 ± 0.81 |
| p value | <0.01 | 0.01 | 0.01 | 0.02 | 0.40 |
| Dietary Protein Levels (g/kg) | |||||||
|---|---|---|---|---|---|---|---|
| Diets | 510 | 480 | 450 | 420 | 390 | 360 | p Value |
| SOD (ng/mg.pro) | 13.40 ± 0.59 a | 14.43 ± 1.03 a | 12.92 ± 0.90 a | 20.38 ± 1.42 b | 16.65 ± 0.22 ab | 14.89 ± 0.98 a | 0.01 |
| CAT (ng/mg.pro) | 15.95 ± 1.58 a | 17.53 ± 0.75 a | 22.26 ± 3.78 ab | 23.20 ± 1.03 ab | 26.30 ± 0.96 b | 21.83 ± 1.48 ab | 0.01 |
| T-AOC (U/mg.pro) | 17.70 ± 1.39 a | 18.10 ± 1.11 a | 18.10 ± 0.37 a | 27.30 ± 3.66 b | 22.55 ± 1.18 ab | 17.80 ± 0.64 a | 0.01 |
| ROS (U/mL) | 333.12 ± 22.00 | 316.72 ± 15.06 | 287.33 ± 86.73 | 217.53 ± 33.95 | 192.58 ± 35.37 | 221.30 ± 23.76 | 0.08 |
| IgM (μg/mg.pro) | 25.29 ± 0.69 a | 36.92 ± 0.67 ab | 32.53 ± 4.12 ab | 43.29 ± 1.80 b | 39.06 ± 0.72 ab | 36.98 ± 3.34 ab | 0.01 |
| LYS (mU/mg.pro) | 3.28 ± 0.47 a | 4.35 ± 0.49 ab | 5.35 ± 0.61 ab | 6.52 ± 1.00 b | 6.36 ± 0.24 b | 4.46 ± 0.58 ab | 0.03 |
| LYS (mU/mg.pro) | 3.28 ± 0.47 a | 4.35 ± 0.49 ab | 5.35 ± 0.61 ab | 6.52 ± 1.00 b | 6.36 ± 0.24 b | 4.46 ± 0.58 ab | 0.03 |
| ACP (mU/mg.pro) | 12.96 ± 0.68 | 11.5 ± 0.75 | 11.46 ± 1.42 | 10.66 ± 1.12 | 13.41 ± 0.74 | 9.83 ± 0.57 | 0.11 |
| AKP (miU/mg.pro) | 11.72 ± 0.81 a | 12.03 ± 1.31 a | 12.37 ± 1.84 a | 19.3 ± 1.09 b | 16.16 ± 1.53 ab | 11.7 ± 1.73 a | 0.01 |
| AST (mU/mg.pro) | 25.52 ± 0.64 b | 23.48 ± 0.44 b | 24.77 ± 1.17 b | 22.28 ± 0.72 ab | 18.91 ± 1.23 a | 18.55 ± 0.53 a | <0.01 |
| ALT (mU/mg.pro) | 13.22 ± 0.07 b | 11.65 ± 0.99 ab | 10.82 ± 0.92 ab | 10.07 ± 0.29 ab | 9.58 ± 0.20 a | 11.5 ± 0.34 ab | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Liu, H.; Yang, S.; Zhou, M.; Zhang, S.; Tan, B.; Yang, Y.; Zhang, H.; Xie, R.; Dong, X. Effects of High-Lipid Dietary Protein Ratio on Growth, Antioxidant Parameters, Histological Structure, and Expression of Antioxidant- and Immune-Related Genes of Hybrid Grouper. Animals 2023, 13, 3710. https://doi.org/10.3390/ani13233710
Huang W, Liu H, Yang S, Zhou M, Zhang S, Tan B, Yang Y, Zhang H, Xie R, Dong X. Effects of High-Lipid Dietary Protein Ratio on Growth, Antioxidant Parameters, Histological Structure, and Expression of Antioxidant- and Immune-Related Genes of Hybrid Grouper. Animals. 2023; 13(23):3710. https://doi.org/10.3390/ani13233710
Chicago/Turabian StyleHuang, Weibin, Hao Liu, Shipei Yang, Menglong Zhou, Shuang Zhang, Beiping Tan, Yuanzhi Yang, Haitao Zhang, Ruitao Xie, and Xiaohui Dong. 2023. "Effects of High-Lipid Dietary Protein Ratio on Growth, Antioxidant Parameters, Histological Structure, and Expression of Antioxidant- and Immune-Related Genes of Hybrid Grouper" Animals 13, no. 23: 3710. https://doi.org/10.3390/ani13233710
APA StyleHuang, W., Liu, H., Yang, S., Zhou, M., Zhang, S., Tan, B., Yang, Y., Zhang, H., Xie, R., & Dong, X. (2023). Effects of High-Lipid Dietary Protein Ratio on Growth, Antioxidant Parameters, Histological Structure, and Expression of Antioxidant- and Immune-Related Genes of Hybrid Grouper. Animals, 13(23), 3710. https://doi.org/10.3390/ani13233710





