Effect of Fermented Artemisia argyi on Egg Quality, Nutrition, and Flavor by Gut Bacterial Mediation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented A. argyi (AAF)
2.2. Chemical Composition of AAF
2.3. Animal Experiment and Treatment
2.4. Sample Collection
2.5. Laying Performance and Egg Quality Assessment
2.6. Determination of the Amino Acids and Fatty Acids of Eggs
2.7. Hematological Analysis
2.8. Safety Inspection of Eggs
2.9. Headspace-Gas Chromatography-Ion Mobility Spectrometry (HS-GC-IMS) Analysis
2.10. Histopathological Examination
2.11. Microbiomic Analysis
2.12. Statistical Analyses
3. Results
3.1. Material Basis of AAF
3.2. Egg Production of Hens
3.3. Safety of Eggs
3.4. Quality of Eggs
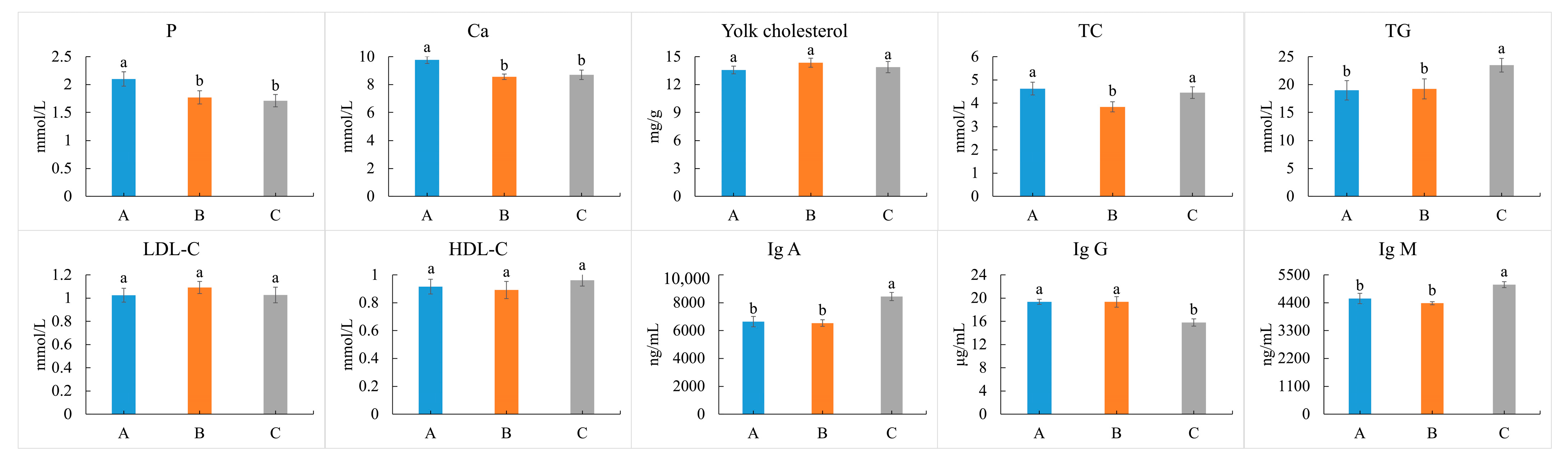
3.5. Cholesterol of Eggs, and Lipid-Related Indices of Blood
3.6. Amino Acids of Eggs
3.7. Fatty Acids of Eggs
3.8. Volatile Components of Eggs
3.9. Intestinal Histology
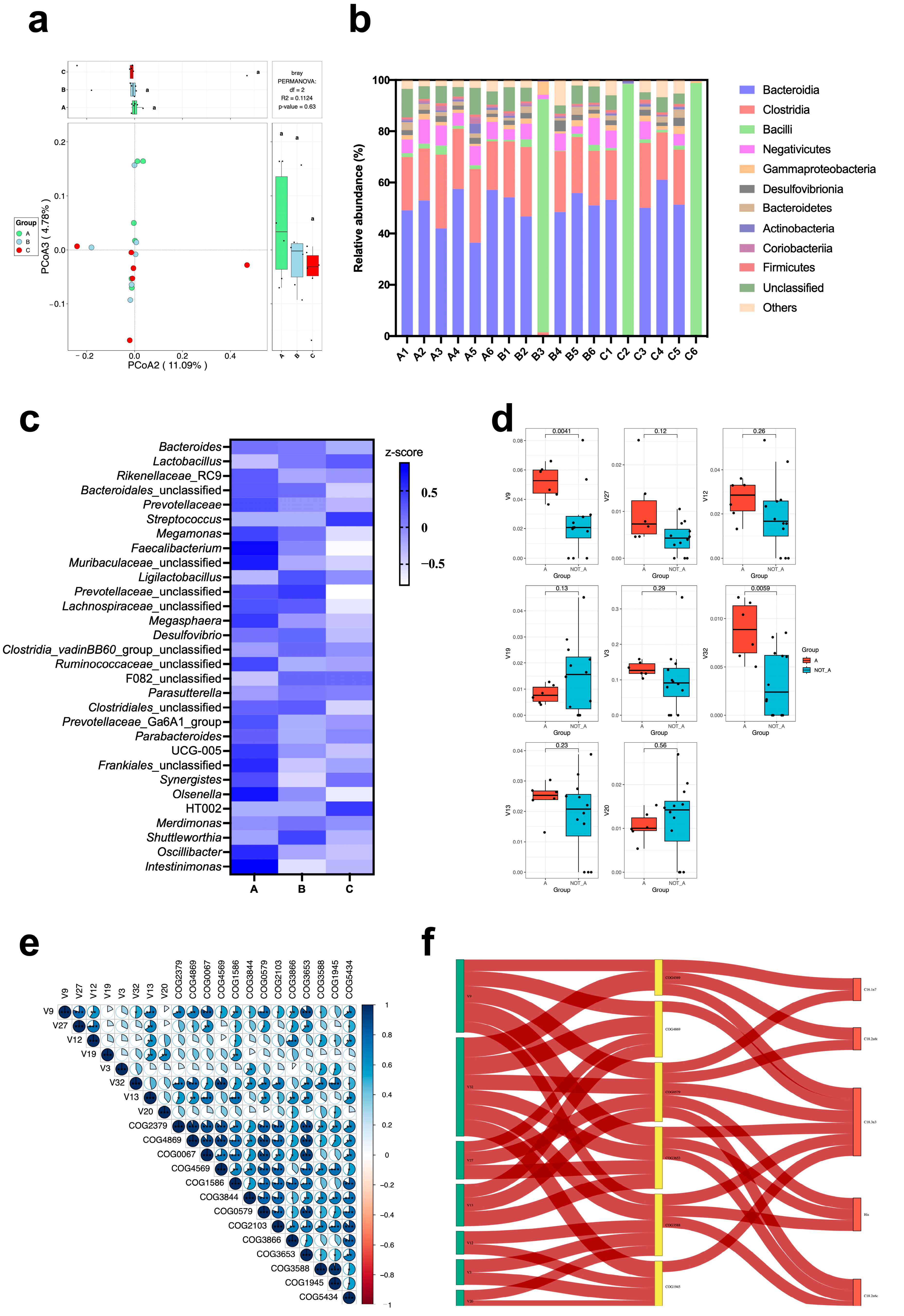
3.10. Gut Microbiome
4. Discussion
4.1. Egg Production Elevation Ascribed to AAF Could Serve as a Feed Attractant in the Hen Industry
4.2. A Relatively Low Dosage of AAF Is Ideal for Maintaining the Normal Quality of Eggs
4.3. Impact of the Fermented Artemisia argyi on the Gut Microbiota
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Lin, X.; Cheng, C.; Mo, K. Analysis of volatile flavor compounds in two types of feed eggs based on GC-IMS. J. Hubei Minzu University (Nat. Sci.) 2023, 41, 178–183+231. (In Chinese) [Google Scholar] [CrossRef]
- Castanon, J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, P.; Bedi, J.S.; Moudgil, A.D.; Gill, J.P.S.; Aulakh, R.S. Emerging issue of antibiotic resistance from food producing animals in India: Perspective and legal framework. Food Rev. Int. 2018, 34, 447–462. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Kirchhelle, C. Pharming animals: A global history of antibiotics in food production (1935–2017). Palgrave. Commun. 2018, 4, 96. [Google Scholar] [CrossRef]
- Bacanli, M.; Başaran, N. Importance of antibiotic residues in animal food. Food. Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.G.; Deng, W.J. Antibiotic residues in food: Extraction, analysis, and human health concerns. J. Agric. Food. Chem. 2019, 27, 7569. [Google Scholar] [CrossRef]
- Dos Santos, A.F.A.; Da Silva, A.S.; Galli, G.M.; Paglia, E.B.; Dacoreggio, M.V.; Kempka, A.P.; Souza, C.F.; Baldissera, M.D.; Da Rosa, G.; Boiago, M.M. Addition of yellow strawberry guava leaf extract in the diet of laying hens had antimicrobial and antioxidant effect capable of improving egg quality. Biocatal. Agric. Biotechnol. 2020, 29, 101788. [Google Scholar] [CrossRef]
- Liu, Z.P.; Chao, J.R.; Xu, P.T.; Lv, H.Y.; Ding, B.Y.; Zhang, Z.F.; Li, L.L.; Guo, S.S. Lonicera flos and Cnicus japonicus extracts improved egg quality partly by modulating antioxidant status, inflammatory-related cytokines and shell matrix protein expression of oviduct in laying hens. Poult. Sci. 2023, 102, 102561. [Google Scholar] [CrossRef]
- Huang, T.; Wang, X.; Yang, Q.; Peng, S.; Peng, M. Effects of dietary supplementation with Ampelopsis grossedentata extract on production performance and body health of hens. Trop. Anim. Health Prod. 2022, 1, 45. [Google Scholar] [CrossRef]
- Peng, M.J.; Huang, T.; Yang, Q.L.; Peng, S.; Jin, Y.X.; Wang, X.S. Dietary supplementation Eucommia ulmoides extract at high content served as a feed additive in the hens industry. Poult. Sci. 2022, 101, 101650. [Google Scholar] [CrossRef]
- Wang, W.W.; Jia, H.J.; Zhang, H.J.; Wang, J.; Lv, H.Y.; Wu, S.G.; Qi, G.H. Supplemental plant extracts from Flos lonicerae in combination with Baikal skullcap attenuate intestinal disruption and modulate gut microbiota in laying hens challenged by Salmonella pullorum. Front. Microbiol. 2019, 10, 1681. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wen, X.; He, J.; Zhao, H.; Li, S.; Wang, M. Phytochemical components and biological activities of Artemisia argyi. J. Funct. Foods 2019, 52, 648–662. [Google Scholar] [CrossRef]
- Miao, Y.; Luo, D.; Zhao, T.; Du, H.; Liu, Z.; Xu, Z.; Guo, L.; Chen, C.; Peng, S.; Li, J.X.; et al. Genome sequencing reveals chromosome fusion and extensive expansion of genes related to secondary metabolism in Artemisia argyi. Plant Biotechnol. 2022, 20, 1902–1915. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Lee, J.; Lee, J.W.; Park, S.H.; Lee, I.K.; Choi, J.A.; Lee, J.S.; Kang, K.S. Anti-inflammatory effect of Artemisia argyi on ethanol-induced gastric ulcer: Analytical, in vitro and in vivo studies for the identification of action mechanism and active compounds. Plants 2021, 10, 332. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Wang, F.; Xu, R.; Yang, M.; Ci, Z.; Wu, Z.; Zhang, D.; Lin, J. From longevity grass to contemporary soft gold: Explore the chemical constituents, pharmacology, and toxicology of Artemisia argyi H.Lév. & vaniot essential oil. J. Ethnopharmacol. 2021, 279, 114404. [Google Scholar] [CrossRef]
- Su, S.; Sundhar, N.; Kuo, W.; Lai, S.; Kuo, C.; Ho, T.; Lin, P.; Lin, S.; Shih, C.Y.; Lin, Y.; et al. Artemisia argyi extract induces apoptosis in human gemcitabine-resistant lung cancer cells via the PI3K/MAPK signaling pathway. J. Ethnopharmacol. 2022, 299, 115658. [Google Scholar] [CrossRef]
- Xu, R.; Ming, Y.; Li, Y.; Li, S.; Zhu, W.; Wang, H.; Guo, J.; Shi, Z.; Shu, S.; Xiong, C.; et al. Full-Length Transcriptomic Sequencing and Temporal Transcriptome Expression Profiling Analyses Offer Insights into Terpenoid Biosynthesis in Artemisia argyi. Molecules 2022, 27, 5948. [Google Scholar] [CrossRef]
- Zhan, H.; Xu, W.; Zhao, X.; Tian, L.; Zhang, F.; Wei, H.; Tao, X. Effects of Lactiplantibacillus plantarum wlpl01 fermentation on antioxidant activities, bioactive compounds, and flavor profile of Artemisia argyi. Food Biosci. 2022, 49, 101908. [Google Scholar] [CrossRef]
- Lee, S.; Won, H.J.; Ban, S.; Park, Y.J.; Kim, S.M.; Kim, H.S.; Choi, J.; Kim, H.; Lee, J.H.; Jung, J.H. Integrative analysis of metabolite and transcriptome reveals biosynthetic pathway and candidate genes for eupatilin and jaceosidin biosynthesis in Artemisia argyi. Front. Plant Sci. 2023, 14, 1186023. [Google Scholar] [CrossRef]
- Wang, W.; Tan, Z.; Gu, L.; Ma, H.; Wang, Z.; Wang, L.; Wu, G.; Qin, G.; Wang, Y.; Pang, H. Dynamics changes of microorganisms community and fermentation quality in soybean meal prepared with lactic acid bacteria and Artemisia argyi through fermentation and aerobic exposure processes. Foods 2022, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Shi, B.; Sun, D.; Chen, H.; Tong, M.; Zhang, P.; Guo, X.; Yan, S. Effects of dietary supplementation of Artemisia argyi aqueous extract on antioxidant indexes of small intestine in broilers. Anim. Nutr. 2016, 2, 198–203. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, D.; Shi, B.; Faucitano, L.; Yan, S. Dietary supplementation with Artemisia argyi extract on inflammatory mediators and antioxidant capacity in broilers challenged with lipopolysaccharide. Ital. J. Anim. Sci. 2020, 19, 1091–1098. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, Y.; Shi, L.; Guo, S.; Jin, X.; Xu, Y.; Yan, S.; Shi, B. The effects of dietary supplementation of Artemisia argyi polysaccharide on immune and antioxidative functions in broilers. J. Appl. Anim. Res. 2022, 50, 587–597. [Google Scholar] [CrossRef]
- Chen, J.; Chen, F.; Peng, S.; Ou, Y.; He, B.; Li, Y.; Lin, Q. Effects of Artemisia argyi powder on egg quality, antioxidant capacity, and intestinal development of roman laying hens. Front. Physiol. 2022, 13, 902568. [Google Scholar] [CrossRef] [PubMed]
- Eliopoulos, C.; Markou, G.; Chorianopoulos, N.; Haroutounian, S.A.; Arapoglou, D. Transformation of mixtures of olive mill stone waste and oat bran or Lathyrus clymenum pericarps into high added value products using solid state fermentation. Waste Manag. 2022, 149, 168–176. [Google Scholar] [CrossRef]
- Niu, Y.; Wan, X.L.; Zhang, L.L.; Wang, C.; He, J.T.; Bai, K.W.; Zhang, X.H.; Zhao, L.G.; Wang, T. Effect of different doses of fermented Gginkgo biloba leaves on serum biochemistry, antioxidant capacity hepatic gene expression in broilers. Anim. Feed Sci. Technol. 2019, 248, 132–140. [Google Scholar] [CrossRef]
- Sugiharto, S.; Ranjitkar, S. Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: A review. Anim. Nutr. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, J.; Wang, Y.; Lv, J.; Li, J.; Guo, L.; Min, Y. Fermented corn–soybean meal mixed feed modulates intestinal morphology, barrier functions and cecal microbiota in laying hens. Animals 2021, 11, 3059. [Google Scholar] [CrossRef]
- Wei, G.; Chitrakar, B.; Regenstein, J.M.; Sang, Y.; Zhou, P. Microbiology, flavor formation, and bioactivity of fermented soybean curd (furu): A review. Food Res. Int. 2023, 163, 112183. [Google Scholar] [CrossRef]
- Fu, H.; Peng, M.; Tang, Q.; Liang, H.; Liang, Y.; Fang, J.; Wang, X. Comprehensive Assessment of the Safety of Eucommia ulmoides Leaf Extract for Consumption as a Traditional Chinese Health Food. J. Renew. Mater. 2023, 11, 3091–3114. [Google Scholar] [CrossRef]
- NHC. Determination of HCH and DDT Residues in Foods; NHC: Beijing, China, 2003; Volume 5009, pp. 19–2003. [Google Scholar]
- NHC. Determination of Quintozene Residues in Vegetable Foods; NHC: Beijing, China, 2003; Volume 5009, pp. 136–2003. [Google Scholar]
- NHC. Determination of Total Bacterial Count; NHC: Beijing, China, 2016; Volume 4789, pp. 2–2016. [Google Scholar]
- NHC. Determination of Staphylococcus aureus; NHC: Beijing, China, 2016; Volume 4789, pp. 10–2016. [Google Scholar]
- NHC. Determination of Multielement in Food; NHC: Beijing, China, 2016; Volume 5009, pp. 268–2016. [Google Scholar]
- NHC. Method for Limit Test of Heavy Metals in Food Additives; NHC: Beijing, China, 2014; Volume 5009, pp. 74–2014. [Google Scholar]
- Kothari, D.; Oh, J.S.; Kim, J.H.; Lee, W.D.; Kim, S.K. Effect of Dietary Supplementation of Fermented Pine Needle Extract on Productive Performance, Egg Quality, and Serum Lipid Parameters in Laying Hens. Animals 2021, 11, 1475. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jin, Y.; Yang, J. Influence of spent ginger yeast cultures on the production performance, egg quality, serum composition, and intestinal microbiota of laying hens. Anim. Biosci. 2022, 35, 1205–1214. [Google Scholar] [CrossRef]
- Park, N.; Lee, T.K.; Nguyen, T.T.H.; An, E.B.; Kim, N.M.; You, Y.H.; Park, T.S.; Kim, D. The effect of fermented buckwheat on producing l-carnitine- and γ-aminobutyric acid (GABA)-enriched designer eggs. J. Sci. Food Agric. 2017, 9, 2891–2897. [Google Scholar] [CrossRef]
- Moon, S.G.; Lee, S.K.; Lee, W.D.; Niu, K.M.; Hwang, W.U.; Oh, J.S.; Kothari, D.; Kim, S.K. Effect of dietary supplementation of a phytogenic blend containing Schisandra chinensis, Pinus densiflora, and Allium tuberosum on productivity, egg quality, and health parameters in laying hens. Anim. Biosci. 2021, 34, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Ege, G.; Bozkurt, M.; Koçer, B.; Tüzün, A.E.; Uygun, M.; Alkan, G. Influence of feed particle size and feed form on productive performance, egg quality, gastrointestinal tract traits, digestive enzymes, intestinal morphology, and nutrient digestibility of laying hens reared in enriched cages. Poult. Sci. 2019, 98, 3787–3801. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, H.; Du, E.; Jin, F.; Zheng, C.; Fan, Q.; Zhao, N.; Guo, W.; Zhang, W.; Huang, S.; et al. Effects of magnolol on egg production, egg quality, antioxidant capacity, and intestinal health of laying hens in the late phase of the laying cycle. Poult. Sci. 2021, 2, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.; Korver, D.R. Long-term effects of Buttiauxella sp. phytase on performance, eggshell quality, apparent ileal Ca and P digestibility, and bone properties of white egg layers. Poult. Sci. 2019, 98, 4848–4859. [Google Scholar] [CrossRef]
- Xiao, Y.Q.; Shao, D.; Sheng, Z.W.; Wang, Q.; Shi, S.R. A mixture of daidzein and Chinese herbs increases egg production and eggshell strength as well as blood plasma Ca, P, antioxidative enzymes, and luteinizing hormone levels in post-peak, brown laying hens. Poult. Sci. 2019, 98, 3298–3303. [Google Scholar] [CrossRef]
- Juan, A.F.D.; Scappaticcio, R.; Aguirre, L.; Fondevila, G.; García, J.; Cámara, L.; Mateos, G.G. Influence of the calcium and nutrient content of the prelay diet on egg production, egg quality, and tibiae mineralization of brown egg-laying hens from 16 to 63 wk of age. Poult. Sci. 2023, 102, 102491. [Google Scholar] [CrossRef]
- Wilson, P.B. Recent advances in avian egg science: A review. Poult. Sci. 2017, 96, 3747–3754. [Google Scholar] [CrossRef]
- Jones, F.T.; Rives, D.V.; Carey, J.B. Salmonella contamination in commercial eggs and an egg production facility. Poult. Sci. 1995, 74, 753–757. [Google Scholar] [CrossRef]
- Fouad, A.M.; Ruan, D.; El-Senousey, H.K.; Chen, W.; Jiang, S.; Zheng, C. Harmful effects and control strategies of aflatoxin B1 produced by Aspergillus flavus and Aspergillus parasiticus strains on poultry: Review. Toxins 2019, 11, 176. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, Y.Z.; Wu, Q.J.; Wang, Y.Q.; Wang, J.P.; Liu, Z.H. Effects of varying dietary intoxication with lead on the performance and ovaries of laying hens. Poult. Sci. 2020, 99, 4505–4513. [Google Scholar] [CrossRef]
- Liu, T.T.; Zeng, Y.; Tang, K.; Chen, X.; Zhang, W.; Xu, X.L. Dihydromyricetin ameliorates atherosclerosis in LDL receptor deficient mice. Atherosclerosis 2017, 262, 39–50. [Google Scholar] [CrossRef]
- Fan, L.; Qu, X.; Yi, T.; Peng, Y.; Jiang, M.; Miao, J.; Xiao, P. Metabolomics of the protective effect of Ampelopsis grossedentata and its major active compound dihydromyricetin on the liver of high-fat diet hamster. Evid. Based Complement. Alternat. Med. 2020, 35, 1–15. [Google Scholar] [CrossRef]
- Ma, Q.; Tan, D.; Gong, X.; Ji, H.; Wang, K.; Lei, Q.; Zhao, G. An Extract of Artemisia argyi Leaves Rich in Organic Acids and Flavonoids Promotes Growth in BALB/c Mice by Regulating Intestinal Flora. Animals 2022, 12, 1519. [Google Scholar] [CrossRef]
- Kaya, A.; Kaya, H.; Macit, M.; Çelebi, Ş.; Esenbuğa, N.; Yörük, M.A.; Karaoglu, M. Effects of dietary inclusion of plant extract mixture and copper into layer diets on egg yield and quality, yolk cholesterol and fatty acid composition. Kafkas Univ. Vet. Fak. Derg. 2013, 19, 673–679. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Fang, J.; Lai, Z.; Li, J.; Peng, M.; Mai, Y. Dietary supplementation Eucommia ulmoides extract at relative low level affect the nutrition, flavor, and crispness of grass carp (Ctenopharyngodon idella) by gut bacterial mediation. LWT Food Sci. Technol. 2023, 177, 114521. [Google Scholar] [CrossRef]
- Mikulski, D.; Jankowski, J.; Naczmanski, J.; Mikulska, M.; Demey, V. Effects of dietary probiotic (Pediococcus acidilactici) supplementation on performance, nutrient digestibility, egg traits, egg yolk cholesterol, and fatty acid profile in laying hens. Poult. Sci. 2012, 10, 2691–2700. [Google Scholar] [CrossRef]
- Park, J.W.; Jeong, J.S.; Lee, S.I.; Kim, I.H. Effect of dietary supplementation with a probiotic (Enterococcus faecium) on production performance, excreta microflora, ammonia emission, and nutrient utilization in isa brown laying hens. Poult. Sci. 2016, 95, 2829–2835. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yan, Y.; Feng, L.; Wang, F.; Guo, Y.; Zhang, X.; Zhang, Z. Bisphenol A alters volatile fatty acids accumulation during sludge anaerobic fermentation by affecting amino acid metabolism, material transport and carbohydrate-active enzymes. Bioresour. Technol. 2021, 11, 124588. [Google Scholar] [CrossRef] [PubMed]

| Group | A | B | C | |
|---|---|---|---|---|
| Laying performance | Laying rate (%) | 87.55 | 93.44 | 92.23 |
| Egg weight (g) | 62.43 ± 2.33 a | 61.63 ± 3.25 a | 61.35 ± 3.36 a | |
| BER (%) | 0.40 | 0.03 | 0.17 | |
| DFC (g) | 99.42 ± 6.65 a | 106.19 ± 9.69 a | 104.82 ± 8.46 a | |
| FCR | 1.83 | 1.85 | 1.86 | |
| Initial BW (kg) | 1.15 ± 0.14 a | 1.14 ± 0.20 a | 1.09 ± 0.15 a | |
| Final BW (kg) | 1.62 ± 0.11 a | 1.71 ± 0.14 a | 1.59 ± 0.14 a | |
| Organ coefficient | Liver (%) | 2.53 ± 0.44 a | 2.43 ± 0.48 a | 2.52 ± 0.29 a |
| Spleen (%) | 0.10 ± 0.01 a | 0.10 ± 0.01 a | 0.12 ± 0.02 a |
| Items | A | B | C |
|---|---|---|---|
| Yolk color | 6.60 ± 0.55 a | 6.60 ± 0.55 a | 6.80 ± 0.45 a |
| Egg shape index | 1.30 ± 0.02 a | 1.30 ± 0.02 a | 1.31 ± 0.04 a |
| Shell strength (N) | 38.79 ± 7.29 a | 36.51 ± 8.67 a | 37.35 ± 5.38 a |
| Shell weight (g) | 5.71 ± 0.33 a | 5.46 ± 0.33 a | 5.43 ± 0.36 a |
| Shell thickness (mm) | 0.32 ± 0.01 a | 0.31 ± 0.01 a | 0.31 ± 0. 01 a |
| Egg albumen height (mm) | 8.72 ± 0.87 a | 8.52± 0.63 a | 8.42 ± 0.93 a |
| Haugh unit | 92.50 ± 4.10 a | 92.34 ± 3.27 a | 88.16 ± 4.39 a |
| Percentage of yolk (%) | 25.86 ± 1.52 a | 26.95 ± 1.68 a | 25.73 ± 0.87 a |
| Yolk moisture content (%) | 48.81 ± 0.33 a | 48.90 ± 0.29 a | 48.16 ± 0.48 a |
| Albumen moisture content (%) | 88.25 ± 0.32 a | 88.32 ± 0.43 a | 87.93 ± 0.46 a |
| Item | Amino Acid (g/100 g) | A | B | C |
|---|---|---|---|---|
| DAA | Asp | 1.38 ± 0.03 a | 1.36 ± 0.02 a | 1.39 ± 0.02 a |
| Glu | 1.98 ± 0.03 a | 1.96 ± 0.03 a | 1.99 ± 0.02 a | |
| Tyr | 0.57 ± 0.01 a | 0.55 ± 0.02 a | 0.57 ± 0.03 a | |
| Gly | 0.47 ± 0.02 a | 0.45 ± 0.02 a | 0.47 ± 0.01 a | |
| Phe | 0.76 ± 0.01 a | 0.73 ± 0.03 a | 0.77 ± 0.02 a | |
| Ala | 0.77 ± 0.02 a | 0.75 ± 0.02 a | 0.79 ± 0.03 a | |
| SAA | Lys | 1.09 ± 0.03 a | 1.05 ± 0.02 a | 1.07 ± 0.02 a |
| Pro | 0.48 ± 0.02 a | 0.46 ± 0.02 a | 0.48 ± 0.01 a | |
| Ser | 1.03 ± 0.02 a | 1.02 ± 0.03 a | 1.04 ± 0.03 a | |
| Thr | 0.67 ± 0.01 a | 0.66 ± 0.02 a | 0.69 ± 0.04 a | |
| BAA | Val | 0.86 ± 0.03 a | 0.83 ± 0.02 a | 0.87 ± 0.03 a |
| Leu | 1.15 ± 0.03 a | 1.12 ± 0.04 a | 1.18 ± 0.04 a | |
| Met | 0.44 ± 0.01 a | 0.41 ± 0.02 a | 0.44 ± 0.01 a | |
| Arg | 0.88 ± 0.02 a | 0.88 ± 0.01 a | 0.88 ± 0.01 a | |
| His | 0.44 ± 0.01 a | 0.36 ± 0.01 b | 0.34 ± 0.01 b | |
| Ile | 0.69 ± 0.02 a | 0.68 ± 0.01 a | 0.70 ± 0.02 a | |
| EAA/TAA (%) | 41.43 | 41.01 | 40.99 | |
| DAA/TAA (%) | 43.41 | 43.58 | 43.91 | |
| Items | A | B | C | |
|---|---|---|---|---|
| Fatty acids (g/100 g) | C14:0 | 0.11 ± 0.02 a | 0.12 ± 0.01 a | 0.12 ± 0.01 a |
| C14:1n5 | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a | |
| C15:0 | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.03 ± 0.01 a | |
| C16:0 | 9.03 ± 0.21 a | 9.01 ± 0.12 a | 9.02 ± 0.17 a | |
| C16:1n7 | 1.31 ± 0.03 a | 1.21 ± 0.02 b | 1.26 ± 0.03 ab | |
| C17:0 | 0.05 ± 0.01 a | 0.05 ± 0.02 a | 0.05 ± 0.01 a | |
| C18:0 | 2.64 ± 0.11 a | 2.71 ± 0.11 a | 2.73 ± 0.13 a | |
| C18:1n9t | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a | |
| C18:1n9c | 13.30 ± 0.12 b | 13.70 ± 0.14 a | 13.72 ± 0.13 a | |
| C18:2n6t | 0.01 ± 0.01 a | 0.003 ± 0.001 b | 0.004 ± 0.001 b | |
| C18:2n6c | 5.40 ± 0.21 a | 4.89 ± 0.16 b | 4.87 ± 0.14 b | |
| C20:0 | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | |
| C18:3n6 | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a | |
| C18:3n3 | 0.25 ± 0.02 a | 0.18 ± 0.02 b | 0.18 ± 0.01 b | |
| C20:1n9 | 0.07 ± 0.01 a | 0.07 ± 0.01 a | 0.07 ± 0.01 a | |
| C20:2n6 | 0.05 ± 0.01 a | 0.05 ± 0.01 a | 0.05 ± 0.02 a | |
| C22:0 | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a | |
| C20:3n6 | 0.06 ± 0.01 a | 0.06 ± 0.02 a | 0.06 ± 0.01 a | |
| C20:4n6 | 0.75 ± 0.08 a | 0.77 ± 0.04 a | 0.76 ± 0.06 a | |
| C24:1n9 | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | |
| C22:6n3 | 0.27 ± 0.02 a | 0.24 ± 0.03 a | 0.24 ± 0.03 a | |
| Total | 33.46 ± 0.22 a | 33.22 ± 0.24 a | 33.30 ± 0.18 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Zheng, L.; Geng, T.; Wang, Y.; Peng, M.; Hu, F.; Zhao, J.; Wang, X. Effect of Fermented Artemisia argyi on Egg Quality, Nutrition, and Flavor by Gut Bacterial Mediation. Animals 2023, 13, 3678. https://doi.org/10.3390/ani13233678
Zhou M, Zheng L, Geng T, Wang Y, Peng M, Hu F, Zhao J, Wang X. Effect of Fermented Artemisia argyi on Egg Quality, Nutrition, and Flavor by Gut Bacterial Mediation. Animals. 2023; 13(23):3678. https://doi.org/10.3390/ani13233678
Chicago/Turabian StyleZhou, Min, Lingyan Zheng, Tuo Geng, Yunfan Wang, Mijun Peng, Fengyang Hu, Jing Zhao, and Xuesong Wang. 2023. "Effect of Fermented Artemisia argyi on Egg Quality, Nutrition, and Flavor by Gut Bacterial Mediation" Animals 13, no. 23: 3678. https://doi.org/10.3390/ani13233678
APA StyleZhou, M., Zheng, L., Geng, T., Wang, Y., Peng, M., Hu, F., Zhao, J., & Wang, X. (2023). Effect of Fermented Artemisia argyi on Egg Quality, Nutrition, and Flavor by Gut Bacterial Mediation. Animals, 13(23), 3678. https://doi.org/10.3390/ani13233678




