Inflammatory Status and Chronic Kidney Disease in Cats: Old and New Inflammatory Markers—A Pilot Prospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Sample Collection
2.3. Laboratory Tests
2.4. Statistical Analysis
3. Results
3.1. Study Groups
3.2. Laboratory Analysis
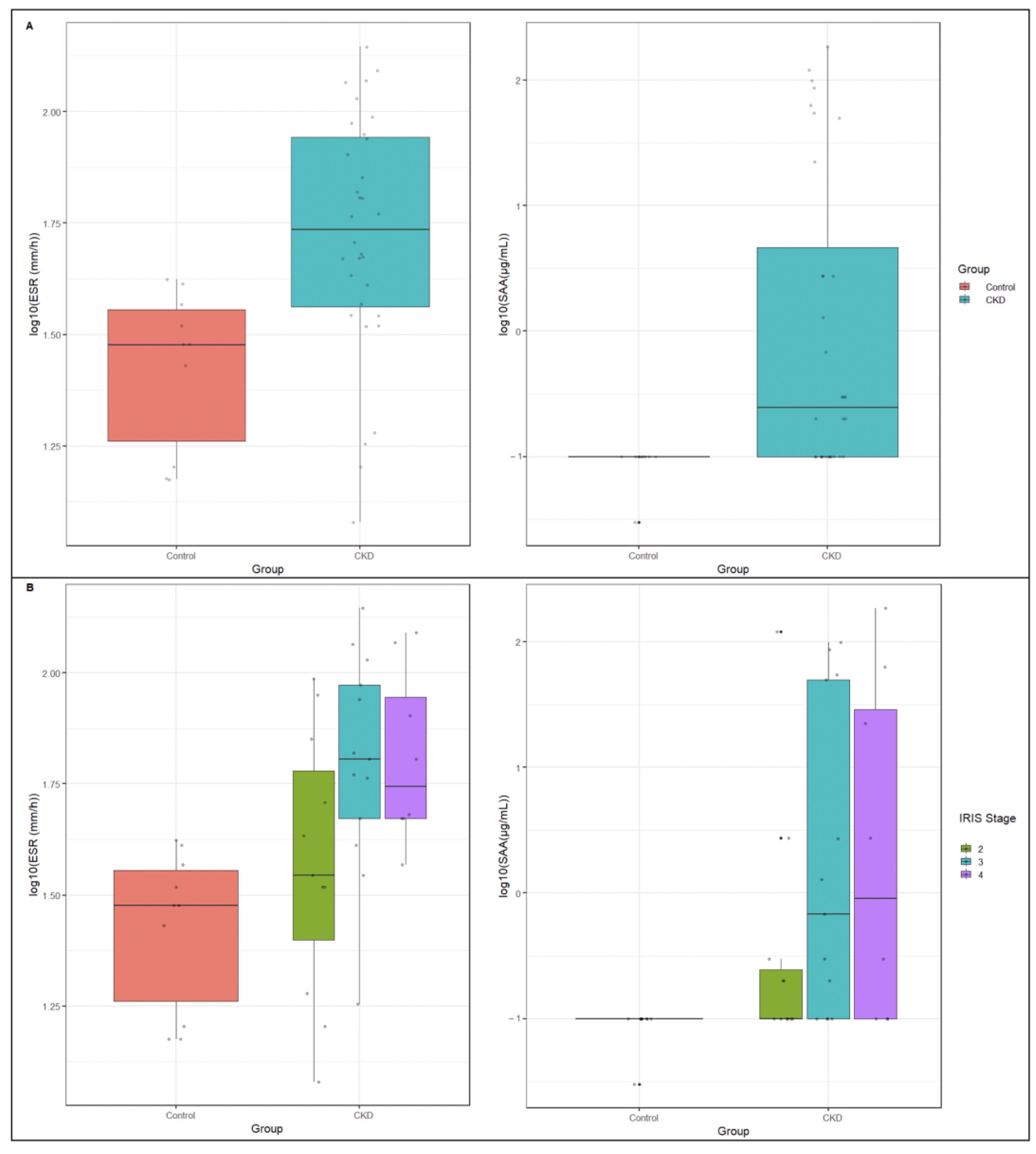
3.2.1. Comparison between Control and CKD Groups
3.2.2. Evaluation of ESR in CKD Cats
3.2.3. Evaluation of SAA in CKD Cats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polzin, D.J. Chronic kidney disease in small animals. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Lulich, J.P.; Osborne, C.A.; O’Brien, T.D.; Polzin, D.J. Feline renal failure: Questions, answers, questions. Compend. Contin. Educ. Pract. Vet. 1992, 14, 127–152. [Google Scholar]
- Ross, S.J.; Osborne, C.A.; Kirk, C.A.; Lowry, S.R.; Koehler, L.A.; Polzin, D.J. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J. Am. Vet. Med. Assoc. 2006, 229, 949–957. [Google Scholar] [CrossRef]
- DiBartola, S.P.; Rutgers, H.C.; Zack, P.M.; Tarr, M.J. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). J. Am. Vet. Med. Assoc. 1987, 190, 1196–1202. [Google Scholar] [PubMed]
- Lawler, D.F.; Evans, R.H.; Chase, K.; Ellersieck, M.; Li, Q.; Larson, B.T.; Satyaraj, E.; Heininger, K. The aging feline kidney: A model mortality antagonist? J. Feline Med. Surg. 2006, 8, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B.S.; Lefebvre, H.P. Feline CKD: Pathophysiology and risk factor—What do we know? J. Feline Med. Surg. 2013, 15 (Suppl. 1), 3–14. [Google Scholar] [CrossRef] [PubMed]
- Marino, C.L.; Lascelles, B.D.X.; Vaden, S.L.; Gruen, M.E.; Marks, S.L. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J. Feline Med. Surg. 2014, 6, 465–472. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Longevity and mortality of cats attending primary care veterinary practices in England. J. Feline Med. Surg. 2015, 17, 125–133. [Google Scholar] [CrossRef]
- IRIS (International Renal Interest Society). 2023. Available online: http://www.iris-kidney.com (accessed on 12 October 2023).
- Zimmermann, J.; Herrlinger, S.; Pruy, A.; Metzger, T.; Wanner, C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999, 55, 648–658. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Wanner, C.; Metzger, T.; Heimbürger, O.; Mallamaci, F.; Tripepi, G.; Malatino, L.; Zoccali, C. Inflammation and outcome in end-stage renal failure: Does female gender constitute a survival advantage? Kidney Int. 2002, 62, 1791–1798. [Google Scholar] [CrossRef]
- Tripepi, G.; Mallamaci, F.; Zoccali, C. Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: Searching for the best risk marker by multivariate modeling. J. Am. Soc. Nephrol. 2005, 16 (Suppl. 1), 83–88. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.D.; Phillips, T.M.; Khetpal, P.; Kimmel, P.L. Cytokine patterns and survival in haemodialysis patients. Nephrol. Dial. Transplant. 2010, 25, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Bazeley, J.; Bieber, B.; Li, Y.; Morgenstern, H.; De Sequera, P.; Combe, C.; Yamamoto, H.; Gallagher, M.; Port, F.K.; Robinson, B.M. C-reactive protein and prediction of 1-year mortality in prevalent hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2452–2461. [Google Scholar] [CrossRef] [PubMed]
- Aveles, P.R.; Criminácio, C.R.; Gonçalves, S.; Bignelli, A.T.; Claro, L.M.; Siqueira, S.S.; Nakao, L.S.; Pecoits-Filho, R. Association between biomarkers of carbonyl stress with increased systemic inflammatory response in different stages of chronic kidney disease and after renal transplantation. Nephron Clin. Pract. 2010, 116, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Vaziri, N.D. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am. J. Physiol. Renal Physiol. 2010, 298, 662–671. [Google Scholar] [CrossRef]
- Ori, Y.; Bergman, M.; Bessler, H.; Zingerman, B.; Levy-Drummer, R.S.; Gafter, U.; Salman, H. Cytokine secretion and markers of inflammation in relation to acidosis among chronic hemodialysis patients. Blood Purif. 2013, 35, 181–186. [Google Scholar] [CrossRef]
- Prats, M.; Font, R.; Garcia, C.; Munoz-Cortes, M.; Cabre, C.; Jariod, M.; Romeu, M.; Giralt, M.; Martinez-Vea, A. Oxidative stress markers in predicting response to treatment with ferric carboxymaltose in nondialysis chronic kidney disease patients. Clin. Nephrol. 2014, 81, 419–426. [Google Scholar] [CrossRef]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef]
- Honda, H.; Qureshi, A.R.; Heimbürger, O.; Barany, P.; Wang, K.; Pecoits-Filho, R.; Stenvinkel, P.; Lindholm, B. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am. J. Kidney Dis. 2006, 47, 139–148. [Google Scholar] [CrossRef]
- Fallahzadeh, M.K.; Roozbeh, J.; Geramizadeh, B.; Namazi, M.R. Interleukin-2 serum levels are elevated in patients with uremic pruritus: A novel finding with practical implications. Nephrol. Dial. Transplant. 2011, 26, 3338–3344. [Google Scholar] [CrossRef]
- Gupta, J.; Mitra, N.; Kanetsky, P.A.; Devaney, J.; Wing, M.R.; Reilly, M.; Shah, V.O.; Balakrishnan, V.S.; Guzman, N.J.; Girndt, M.; et al. CRIC Study Investigators. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 2012, 7, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Buckenmayer, A.; Dahmen, L.; Hoyer, J.; Kamalanabhaiah, S.; Haas, C.S. Erythrocyte Sedimentation Rate in Patients with Renal Insufficiency and Renal Replacement Therapy. Lab. Med. 2022, 53, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Shusterman, N.; Kimmel, P.L.; Kiechle, F.L.; Williams, S.; Morrison, G.; Singer, I. Factors influencing erythrocyte sedimentation in patients with chronic renal failure. Arch. Intern. Med. 1985, 145, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Bathon, J.; Graves, J.; Jens, P.; Hamrick, R.; Mayes, M. The erythrocyte sedimentation rate in end-stage renal failure. Am. J. Kidney Dis. 1987, 10, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, M.; Reade, R.; Boulanger, E.; Cardon, G.; Dracon, M.; Dequiedt, P.; Pagniez, D. Erythrocyte sedimentation rate, an underestimated tool in chronic renal failure. Nephrol. Dial. Transplant. 1996, 11, 2244–2247. [Google Scholar] [CrossRef] [PubMed]
- Levay, P.F.; Retief, J.H. Causes of high erythrocyte sedimentation rates in an inpatient population. S Afr. Med. J. 2005, 95, 45–46. [Google Scholar] [PubMed]
- Alsomaili, M.I.; Yousuf, M.; Hejaili, F.; Almotairi, W.; Al-Sayyari, A.A. Erythrocyte sedimentation rate in stable patients on chronic hemodiaysis. Saudi J. Kidney Dis. Transpl. 2015, 26, 1149–1153. [Google Scholar] [CrossRef]
- Das, A.; Barman, B.; Bhattacharya, P.; Lynrah, K.G.; Tiewsoh, I.; Phukan, P. Inflammation and Its Determinants in Patients With Chronic Kidney Disease: A Study From North Eastern Region of India. Cureus 2022, 14, e20917. [Google Scholar] [CrossRef]
- Kottke-Marchant, K.; Davis, B.H. Laboratory Hematology Practice; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Sasaki, K.; Ma, Z.; Khatlani, T.S.; Okuda, M.; Inokuma, H.; Onishi, T. Evaluation of feline serum amyloid A (SAA) as an inflammatory marker. J. Vet. Med. Sci. 2003, 65, 545–548. [Google Scholar] [CrossRef]
- Javard, R.; Grimes, C.; Bau-Gaudreault, L.; Dunn, M. Acute-Phase Proteins and Iron Status in Cats with Chronic Kidney Disease. J. Vet. Intern. Med. 2017, 31, 457–464. [Google Scholar] [CrossRef]
- Paltrinieri, S. The feline acute phase reaction. Vet. J. 2008, 177, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kann, R.K.C.; Seddon, J.M.; Henning, J.; Meers, J. Acute phase proteins in healthy and sick cats. Res. Vet. Sci. 2012, 93, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Dubova, O.; Feshchenko, D.; Bakhur, T.; Zghozinska, O.; Antipov, A.; Rublenko, S.; Goncharenko, V.; Shahanenko, R.; Shahanenko, V. Disseminated intravascular coagulation syndrome as a complication in acute spontaneous canine babesiosis. Maced. Vet. Rev. 2020, 43, 141–149. [Google Scholar] [CrossRef]
- Ajadi, R.A.; Adebiyi, A.A.; Otesile, E.B.; Kasali, O.B. Erythrocyte sedimentation rates and leukogram changes in canine model of osteoarthritis. Niger. J. Physiol. Sci. 2018, 33, 105–108. [Google Scholar] [PubMed]
- Asawapattanakul, T.; Pintapagung, T.; Piratae, S.; Juntautsa, S.; Chancharoen, P. Erythrocyte sedimentation rate, C-reactive protein, and interleukin-6 as inflammatory biomarkers in dogs naturally infected with Ehrlichia canis. Vet. World 2021, 14, 2325–2331. [Google Scholar] [CrossRef]
- Cavalera, M.A.; Gernone, F.; Uva, A.; Donghia, R.; Carelli, G.; Iatta, R.; Zatelli, A. Erythrocyte sedimentation rate in canine leishmaniosis diagnosis: A new resource. Front. Vet. Sci. 2022, 9, 949372. [Google Scholar] [CrossRef]
- Gori, E.; Pasquini, A.; Diamanti, D.; Carletti, C.; Marchetti, V. Effect of time and storage temperature on canine and feline erythrocyte sedimentation rate. MethodsX 2022, 24, 101934. [Google Scholar] [CrossRef]
- Jain, N.C.; Kono, C.S. Erythrocyte sedimentation rate in the dog and cat: Comparison of two methods and influence of packed cell volume, temperature and storage of blood. J. Small Anim. Pract. 1975, 16, 671–678. [Google Scholar] [CrossRef]
- Harley, L.; Langston, C. Proteinuria in dogs and cats. Can. Vet. J. 2012, 53, 631–638. [Google Scholar]
- Dorsch, R.; Teichmann-Knorrn, S.; Sjetne Lund, H. Urinary tract infection and subclinical bacteriuria in cats: A clinical update. J. Feline Med. Surg. 2019, 21, 1023–1038. [Google Scholar] [CrossRef]
- Tvedten, H. Basic Approach to Anemia Diagnosis. In Proceedings of the World Small Animal Veterinary Association (WSAVA)—Annual Congress, Mexico City, Mexico, 14 May 2005. [Google Scholar]
- Hansen, A.E.; Schaap, M.K.; Kjelgaard-Hansen, M. Evaluation of a commercially available human serum amyloid A (SAA) turbidimetric immunoassay for determination of feline SAA concentration. Vet. Res. Commun. 2006, 30, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Tamamoto, T.; Ohno, K.; Ohmi, A.; Goto-Koshino, Y.; Tsujimoto, H. Verification of measurement of the feline serum amyloid A (SAA) concentration by human SAA turbidimetric immunoassay and its clinical application. J. Vet. Med. Sci. 2008, 70, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Prompetchara, E.; Parnsamut, C.; Wangviwat, N.; Pitakpolrat, P.; Chaiwong, K.; Limpornpukdee, O.; Tanticharoenkarn, S.; Ketloy, C. Performance evaluation of alternate ESR measurement method using BC-780 automated hematology analyzer: A comparison study with the Westergren reference method. Clin. Chem. Lab. Med. 2023, 22. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M. Erythrocyte sedimentation rate and C-reactive protein. Aust. Prescr. 2015, 38, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Ceron, J.J.; Eckersall, P.D.; Martýnez-Subiela, S. Acute phase proteins in dogs and cats: Current knowledge and future perspectives. Vet. Clin. Pathol. 2005, 34, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Petersen, H.H.; Nielsen, J.P.; Heegaard, P.M. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 2004, 35, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Chikazawa, S.; Dunning, M.D. A review of anaemia of inflammatory disease in dogs and cats. J. Small Anim. Pract. 2016, 57, 348–353. [Google Scholar] [CrossRef]
- Moore, A.R.; Avery, P.R. Protein characterization using electrophoresis and immunofixation; a case-based review of dogs and cats. Vet. Clin. Pathol. 2019, 48, 29–44. [Google Scholar] [CrossRef]
- Syme, H.M.; Markwell, P.J.; Pfeiffer, D.; Elliott, J. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J. Vet. Intern. Med. 2006, 20, 528–535. [Google Scholar] [CrossRef]
- Langston, C.E.; Reine, N.J. Hyperthyroidism and the kidney. Clin. Tech. Small Anim. Pract. 2006, 21, 17–21. [Google Scholar] [CrossRef]
- Qin, Z.; Li, H.; Wang, L.; Geng, J.; Yang, Q.; Su, B.; Liao, R. Systemic immune-inflammation index is associated with increased urinary albumin excretion: A population-based study. Front. Immunol. 2022, 13, 863640. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Esposito, E.; Attley, J.; Cuzzocrea, S. Targeting inflammation: New therapeutic approaches in chronic kidney disease (CKD). Pharmacol. Res. 2014, 81, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.A.; Elliott, J.; Schmiedt, C.W.; Brown, S.A. Chronic kidney disease in aged cats: Clinical features, morphology, and proposed pathogeneses. Vet. Pathol. 2016, 53, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Giordano, A.; Spagnolo, V. The systemic reaction during inflammation: The acute-phase proteins. Protein Pept. Lett. 2002, 9, 211–223. [Google Scholar] [CrossRef]
- Sharland, D.E. Erythrocyte sedimentation rate: The normal range in the elderly. J. Am. Geriatr. Soc. 1980, 28, 346–348. [Google Scholar] [CrossRef]
| Parameter (Unit) * Reference Interval * | Total Cohort (n = 42) | Group | p ^ | |
|---|---|---|---|---|
| Control (n = 10) | CKD (n = 32) | |||
| ESR (mm/h) | 45.00 (33.00–71.00) | 30.00 (16.00–37.00) | 54.50 (36.00–88.00) | 0.0005 |
| SAA (µg/mL) | 0.10 (0.10–2.73) | 0.10 (0.10–0.10) | 0.25 (0.10–12.47) | 0.0007 |
| 0.1–0.5 | 28 (66.67) | 9 (90.00) | 19 (59.38) | 0.12 Ψ |
| HCT (%) | 32.25 (25.30–36.10) | 34.25 (33.20–39.00) | 29.90 (24.65–35.90) | 0.02 |
| 28–43 | 30 (71.42) | 9 (90.00) | 21 (65.62) | 0.47 Ψ |
| Hg (g/dL) | 10.20 (8.00–11.90) | 11.35 (9.80–12.10) | 9.95 (7.68–11.45) | 0.08 |
| 9.5–15 | 28 (66.67) | 9 (90.00) | 19 (59.38) | 0.12 Ψ |
| Urea (mg/dL) | 94.00 (57.00–178.00) | 51.00 (45.00–57.00) | 134.50 (71.00–258.00) | <0.0001 |
| 29–60 | 12 (28.57) | 9 (90.00) | 3 (9.38) | <0.001 Ψ |
| Creatinine (mg/dL) | 2.59 (1.75–4.53) | 1.12 (0.95–1.19) | 3.88 (2.12–5.28) | <0.0001 |
| 0.9–1.6 | 10 (23.8) | 10 (100.00) | 0 (0.00) | <0.001 Ψ |
| Albumin (g/dL) | 3.25 (2.92–3.79) | 3.77 (3.25–3.79) | 3.15 (2.85–3.80) | 0.20 |
| Alpha-1 Globulins (g/dL) | 0.08 (0.07–0.10) | 0.08 (0.06–0.09) | 0.09 (0.07–0.11) | 0.10 |
| Alpha-2 Globulins (g/dL) | 1.17 (0.98–1.33) | 1.11 (0.95–1.16) | 1.20 (1.02–1.37) | 0.11 |
| Beta Globulins (g/dL) | 0.86 (0.79–1.09) | 0.08 (0.72–0.85) | 0.93 (0.80–1.14) | 0.005 |
| Gamma Globulins (g/dL) | 1.39 (1.16–1.98) | 1.32 (1.12–1.64) | 1.65 (1.23–2.01) | 0.23 |
| USG | 1.021 (1.017–1.046) | 1.054 (1.046–1.066) | 1.019 (1.015–1.023) | <0.0001 |
| UPC | 0.29 (0.14–0.56) | 0.16 (0.12–0.27) | 0.38 (0.15–1.67) | 0.03 |
| Parameters * | Control (n = 10) (a) | IRIS Stages | p ^ | b vs. a Ψ | c vs. a Ψ | d vs. a Ψ | c vs. b Ψ | d vs. b Ψ | d vs. c Ψ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 (n = 11) (b) | 3 (n = 13) (c) | 4 (n = 8) (d) | |||||||||
| ESR | 30.00 (16.00–37.00) | 35.00 (19.00–71.00) | 64.00 (47.00–94.00) | 56.00 (47.00–98.50) | 0.001 | 0.07 | 0.0003 | 0.0007 | 0.02 | 0.03 | 0.45 |
| SAA | 0.10 (0.10–0.10) | 0.10 (0.10–0.30) | 0.68 (0.10–49.84) | 1.52 (0.10–42.60) | 0.006 | 0.03 | 0.0006 | 0.002 | 0.09 | 0.12 | 0.50 |
| Parameters | Total Cohort ρ (p-Value) | Control ρ (p-Value) | CKD ρ (p-Value) |
|---|---|---|---|
| SAA | 0.45 (0.003) | 0.47 (0.18) | 0.24 (0.18) |
| HCT | −0.55 (0.0002) | 0.13 (0.71) | −0.56 (0.001) |
| Hg | −0.51 (0.0006) | 0.30 (0.39) | −0.57 (0.0008) |
| Urea | 0.58 (0.0001) | 0.26 (0.46) | 0.38 (0.03) |
| Creatinine | 0.60 (<0.0001) | 0.34 (0.33) | 0.35 (0.05) |
| STP | −0.15 (0.41) | 0.10 (0.77) | −0.15 (0.41) |
| Albumin | −0.46 (0.003) | 0.30 (0.39) | −0.53 (0.003) |
| Alpha-1 Globulins | 0.03 (0.86) | −0.35 (0.31) | −0.13 (0.49) |
| Alpha-2 Globulins | 0.12 (0.45) | −0.06 (0.86) | −0.03 (0.86) |
| Beta Globulins | 0.39 (0.01) | −0.47 (0.16) | 0.20 (0.29) |
| Gamma Globulins | 0.22 (0.17) | 0.03 (0.93) | 0.13 (0.47) |
| UPC | 0.39 (0.03) | −0.11 (0.76) | 0.39 (0.03) |
| Parameters | Total Cohort ρ (p-Value) | Control ρ (p-Value) | CKD ρ (p-Value) |
|---|---|---|---|
| ESR | 0.45 (0.003) | 0.47 (0.18) | 0.24 (0.18) |
| HCT | −0.44 (0.003) | −0.29 (0.50) | −0.35 (0.05) |
| Hg | −0.45 (0.003) | −0.06 (0.90) | −0.43 (0.01) |
| Urea | 0.59 (0.0001) | −0.29 (0.50) | 0.45 (0.01) |
| Creatinine | 0.55 (0.0002) | −0.12 (0.80) | 0.32 (0.07) |
| STP | 0.09 (0.55) | −0.12 (0.80) | 0.07 (0.68) |
| Albumin | −0.50 (0.001) | −0.06 (0.90) | −0.52 (0.004) |
| Alpha-1 Globulins | 0.20 (0.21) | −0.36 (0.42) | 0.14 (0.44) |
| Alpha-2 Globulins | 0.52 (0.0007) | −0.17 (0.70) | 0.57 (0.001) |
| Beta Globulins | 0.44 (0.005) | −0.52 (0.09) | 0.38 (0.04) |
| Gamma Globulins | 0.19 (0.24) | −0.17 (0.70) | 0.14 (0.47) |
| UPC | 0.49 (0.001) | −0.06 (0.90) | 0.46 (0.009) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uva, A.; Cavalera, M.A.; Gusatoaia, O.; Donghia, R.; Gernone, F.; Silvestrino, M.; Zatelli, A. Inflammatory Status and Chronic Kidney Disease in Cats: Old and New Inflammatory Markers—A Pilot Prospective Study. Animals 2023, 13, 3674. https://doi.org/10.3390/ani13233674
Uva A, Cavalera MA, Gusatoaia O, Donghia R, Gernone F, Silvestrino M, Zatelli A. Inflammatory Status and Chronic Kidney Disease in Cats: Old and New Inflammatory Markers—A Pilot Prospective Study. Animals. 2023; 13(23):3674. https://doi.org/10.3390/ani13233674
Chicago/Turabian StyleUva, Annamaria, Maria Alfonsa Cavalera, Oana Gusatoaia, Rossella Donghia, Floriana Gernone, Marco Silvestrino, and Andrea Zatelli. 2023. "Inflammatory Status and Chronic Kidney Disease in Cats: Old and New Inflammatory Markers—A Pilot Prospective Study" Animals 13, no. 23: 3674. https://doi.org/10.3390/ani13233674
APA StyleUva, A., Cavalera, M. A., Gusatoaia, O., Donghia, R., Gernone, F., Silvestrino, M., & Zatelli, A. (2023). Inflammatory Status and Chronic Kidney Disease in Cats: Old and New Inflammatory Markers—A Pilot Prospective Study. Animals, 13(23), 3674. https://doi.org/10.3390/ani13233674







