Recent Advances in the Behavioral Ecology of European Plethodontid Salamanders
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
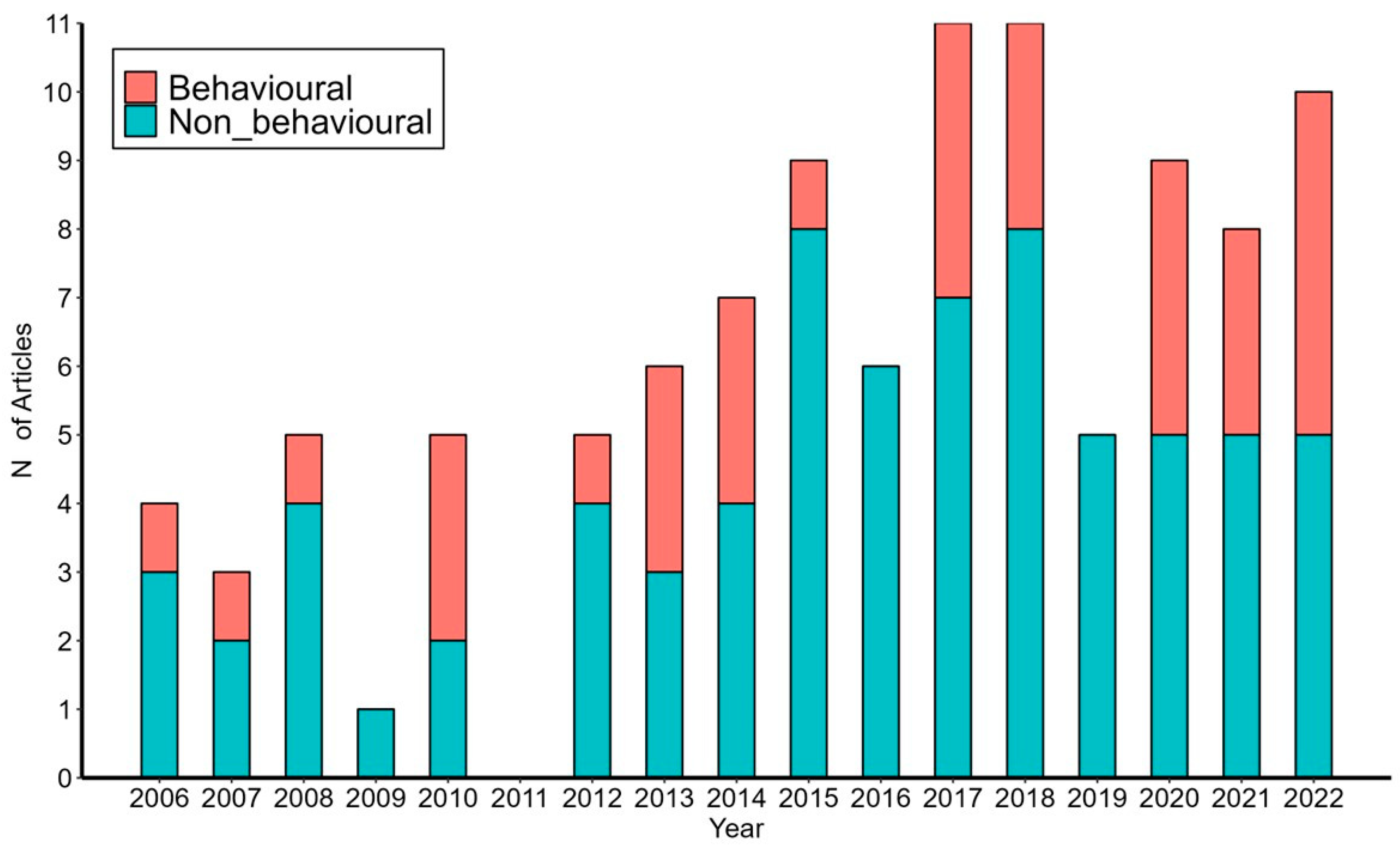
3. Results
4. Discussion
5. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Owens, I.P. Where Is Behavioural Ecology Going? Trends Ecol. Evol. 2006, 21, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.R.; Silla, A.J.; Byrne, P.G. Animal Personality and Behavioral Syndromes in Amphibians: A Review of the Evidence, Experimental Approaches, and Implications for Conservation. Behav. Ecol. Sociobiol. 2018, 72, 79. [Google Scholar] [CrossRef]
- Jaeger, R.G.; Gollmann, B.; Anthony, C.D.; Gabor, C.R.; Kohn, N.R. Behavioral Ecology of the Eastern Red-Backed Salamander: 50 Years of Research; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Wake, D.B. Lungless Salamanders (Plethodontidae). In Grzimek’s Animal Life Encyclopaedia; Hutchins, M., Duellman, W.E., Schlager, N., Eds.; Gale/Cengage Learning: Farmington Hills, MI, USA, 2003; Volume 6, pp. 389–404. [Google Scholar]
- Wake, D.B. Taxonomy of Salamanders of the Family Plethodontidae (Amphibia: Caudata). Zootaxa 2012, 3484, 75–82. [Google Scholar] [CrossRef]
- Min, M.S.; Yang, S.-Y.; Bonett, R.; Vieites, D.; Brandon, R.; Wake, D. Discovery of the First Asian Plethodontid Salamander. Nature 2005, 435, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Dawley, E.M. Recognition of Individual, Sex and Species Odours by Salamanders of the Plethodon glutinosus-P. jordani Complex. Anim. Behav. 1984, 32, 353–361. [Google Scholar] [CrossRef]
- Dawley, E.M. Comparative Morphology of Plethodontid Olfactory and Vomeronasal Organs: How Snouts Are Packed. Herpetol. Monogr. 2017, 31, 169–209. [Google Scholar] [CrossRef]
- Burton, T.M.; Likens, G.E. Salamander Populations and Biomass in the Hubbard Brook Experimental Forest, New Hampshire. Copeia 1975, 541–546. [Google Scholar] [CrossRef]
- Welsh, H.H., Jr.; Droege, S. A Case for Using Plethodontid Salamanders for Monitoring Biodiversity and Ecosystem Integrity of North American Forests. Conserv. Biol. 2001, 15, 558–569. [Google Scholar] [CrossRef]
- Petranka, J.W. Salamanders of the United States and Canada; Smithsonian Institution Press: Washington, DC, USA, 1998. [Google Scholar]
- Cody, M.L.; Smallwood, J.A. Long-Term Studies of Vertebrate Communities; Academic Press: New York, NY, USA, 1996; ISBN 0-08-053562-3. [Google Scholar]
- Lanza, B. A Review of Systematics, Taxonomy, Genetics, Biogeography and Natural History of the Genus Speleomantes Dubois, 1984 (Amphibia Caudata Plethodontidae). Trieste Mus. Civ. Stor. Nat. 2006, 52, 5–135. [Google Scholar]
- Hairston, N.G. Evolution under Interspecific Competition: Field Experiments on Terrestrial Salamanders. Evolution 1980, 34, 409–420. [Google Scholar] [CrossRef]
- Hairston, N.G. Community Ecology and Salamander Guilds; Cambridge University Press: Cambridge, UK, 1987; ISBN 0-521-32578-1. [Google Scholar]
- Hairston, N.G. The Experimental Test of an Analysis of Field Distributions: Competition in Terrestrial Salamanders. Ecology 1980, 61, 817–826. [Google Scholar] [CrossRef]
- Campbell, D.L.; Weiner, S.A.; Starks, P.T.; Hauber, M.E. Context and Control: Behavioural Ecology Experiments in the Laboratory. Ann. Zool. Fennici 2009, 46, 112–123. [Google Scholar] [CrossRef]
- Ylönen, H.; Wolff, J.O. Experiments in Behavioural Ecology and the Real World. Trends Ecol. Evol. 1999, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J. Does the Stress of Laboratory Life and Experimentation on Animals Adversely Affect Research Data? A Critical Review. Altern. Lab. Anim. 2018, 46, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Lunghi, E.; Manenti, R.; Cianferoni, F.; Ceccolini, F.; Veith, M.; Corti, C.; Ficetola, G.F.; Mancinelli, G. Interspecific and Interpopulation Variation in Individual Diet Specialization: Do Environmental Factors Have a Role? Ecology 2020, 101, e03088. [Google Scholar] [CrossRef]
- Bruni, G. Tail-Straddling Walk and Spermatophore Transfer in Hydromantes italicus: First Observations for the Genus and Insights about Courtship Behavior in Plethodontid Salamanders. Herpetol. Rev. 2020, 51, 673–680. [Google Scholar]
- Speybroeck, J.; Beukema, W.; Crochet, P.-A. A Tentative Species List of the European Herpetofauna (Amphibia and Reptilia)—An Update. Zootaxa 2010, 2492, 1–27. [Google Scholar] [CrossRef]
- Speybroeck, J.; Beukema, W.; Dufresnes, C.; Fritz, U.; Jablonski, D.; Lymberakis, P.; Martínez-Solano, I.; Razzetti, E.; Vamberger, M.; Vences, M. Species List of the European Herpetofauna–2020 Update by the Taxonomic Committee of the Societas Europaea Herpetologica. Amphib.-Reptil. 2020, 41, 139–189. [Google Scholar] [CrossRef]
- Bingham, R.E.; Papenfuss, T.J.; Lindstrand, L.; Wake, D.B. Phylogeography and Species Boundaries in the Hydromantes shastae Complex, with Description of Two New Species (Amphibia; Caudata; Plethodontidae). Bull. Mus. Comp. Zool. 2018, 161, 403–427. [Google Scholar] [CrossRef]
- Lanza, B.; Andreone, F.; Bologna, M.; Corti, C.; Razzetti, E. Amphibia—Fauna d’Italia; Edizioni Calderini: Bologna, Italy, 2007; Volume 42. [Google Scholar]
- Ficetola, G.F.; Lunghi, E.; Canedoli, C.; Padoa-Schioppa, E.; Pennati, R.; Manenti, R. Differences between Microhabitat and Broad-Scale Patterns of Niche Evolution in Terrestrial Salamanders. Sci. Rep. 2018, 8, 10575. [Google Scholar] [CrossRef]
- Lanza, B.; Caputo Barucchi, V.; Nascetti, G.; Bullini, L. Morphologic and Genetic Studies on the European Plethodontid Salamanders: Taxonomic Inferences (Genus Hydromantes); Museo Regionale Scienze Naturali: Torino, Italy, 1995; Volume 16, ISBN 88-86041-10-1. [Google Scholar]
- Serra, G.; Crnjar, R.; Lilliu, V.; Cardinale Ciccotti, F.; Spiga, M.A. Morphometric Analysis of the Retinic Photoreceptors in the Cave Salamander Hydromantes Genei (Speleomantes) (Temm. and Schl.): Functional and Anatomical Considerations. Ital. J. Anat. Embryol. 1995, 100, 99–110. [Google Scholar]
- Lombard, R.E.; Wake, D.B. Tongue Evolution in the Lungless Salamanders, Family Plethodontidae I. Introduction, Theory and a General Model of Dynamics. J. Morphol. 1976, 148, 265–286. [Google Scholar] [CrossRef] [PubMed]
- Eric Lombard, R.; Wake, D.B. Tongue Evolution in the Lungless Salamanders, Family Plethodontidae. II. Function and Evolutionary Diversity. J. Morphol. 1977, 153, 39–79. [Google Scholar] [CrossRef] [PubMed]
- Roth, G. Experimental Analysis of the Prey Catching Behavior of Hydromantes italicus Dunn (Amphibia, Plethodontidae). J. Comp. Physiol. 1976, 109, 47–58. [Google Scholar] [CrossRef]
- Roth, G. The Role of Stimulus Movement Patterns in the Prey Catching Behavior of Hydromantes genei (Amphibia, Plethodontidae). J. Comp. Physiol. 1978, 123, 261–264. [Google Scholar] [CrossRef]
- Zanetti, L.; Salvidio, S. Preliminary Data on the Territorial Behaviour of Speleomantes strinatii; Museo Civico di Zoologia: Roma, Italy, 2006; pp. 160–161. [Google Scholar]
- Jaeger, R.G.; Forester, D.C. Social Behavior of Plethodontid Salamanders. Herpetologica 1993, 49, 163–175. [Google Scholar]
- Bruce, R. Sexual Size Dimorphism in the Plethodontidae. In The Biology of Plethodontid Salamanders; Bruce, R.C., Jaeger, R.G., Houck, L.D., Eds.; Klucer Academic: New York, NY, USA, 2000; pp. 243–260. [Google Scholar]
- Lunghi, E.; Manenti, R.; Ficetola, G.F. Do Cave Features Affect Underground Habitat Exploitation by Non-Troglobite Species? Acta Oecologica 2014, 55, 29–35. [Google Scholar] [CrossRef]
- Lunghi, E.; Martinez, A.; Hesselberg, T.; Mammola, S. Behavioural Adjustments Enable the Colonization of Subterranean Environments. Zool. J. Linn. Soc. 2023. [Google Scholar] [CrossRef]
- Mammola, S. Finding Answers in the Dark: Caves as Models in Ecology Fifty Years after Poulson and White. Ecography 2019, 42, 1331–1351. [Google Scholar] [CrossRef]
- Fire, M.; Guestrin, C. Over-Optimization of Academic Publishing Metrics: Observing Goodhart’s Law in Action. GigaScience 2019, 8, giz053. [Google Scholar] [CrossRef]
- Gilbert, R. Skewed Distributions and Goodness-of-Fit Tests. In Statistical Methods for Environmental Pollution Monitoring; Wiley: Cambridge, MA, USA, 1987; pp. 152–163. [Google Scholar]
- Siegel, S. Nonparametric Statisticsfor the Behavioral Sciences; McGraw-Hill Companies: New York, NY, USA, 1956. [Google Scholar]
- Lunghi, E.; Cianferoni, F.; Ceccolini, F.; Veith, M.; Manenti, R.; Mancinelli, G.; Corti, C.; Ficetola, G.F. What Shapes the Trophic Niche of European Plethodontid Salamanders? PLoS ONE 2018, 13, e0205672. [Google Scholar] [CrossRef]
- Lunghi, E.; Cianferoni, F.; Corti, C.; Zhao, Y.; Manenti, R.; Ficetola, G.F.; Mancinelli, G. The Trophic Niche of Subterranean Populations of Speleomantes italicus. Sci. Rep. 2022, 12, 18257. [Google Scholar] [CrossRef] [PubMed]
- Salvidio, S.; Oneto, F.; Ottonello, D.; Costa, A.; Romano, A. Trophic Specialization at the Individual Level in a Terrestrial Generalist Salamander. Can. J. Zool. 2015, 93, 79–83. [Google Scholar] [CrossRef]
- Cianferoni, F.; Lunghi, E. Inferring on Speleomantes Foraging Behavior from Gut Contents Examination. Animals 2023, 13, 2782. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Romano, A.; Rosa, G.; Salvidio, S. Weighted Individual-Resource Networks in Prey–Predator Systems: The Role of Prey Availability on the Emergence of Modular Structures. Integr. Zool. 2020, 17, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Salvidio, S.; Romano, A.; Oneto, F.; Ottonello, D.; Michelon, R. Different Season, Different Strategies: Feeding Ecology of Two Syntopic Forest-Dwelling Salamanders. Acta Oecologica 2012, 43, 42–50. [Google Scholar]
- Gillespie, J.H. Application of Stable Isotope Analysis to Study Temporal Changes in Foraging Ecology in a Highly Endangered Amphibian. PLoS ONE 2013, 8, e53041. [Google Scholar] [CrossRef] [PubMed]
- Lunghi, E.; De Falco, G.; Buschettu, S.; Murgia, R.; Mulas, C.; Mulargia, M.; Canedoli, C.; Ficetola, G.F.; Manenti, R. First Data on Nesting Ecology and Behaviour in the Imperial Cave Salamander Hydromantes imperialis. North-West. J. Zool. 2015, 11, 324–330. [Google Scholar]
- Lunghi, E.; Corti, C.; Manenti, R.; Barzaghi, B.; Buschettu, S.; Canedoli, C.; Cogoni, R.; De Falco, G.; Fais, F.; Manca, A.; et al. Comparative Reproductive Biology of European Cave Salamanders (Genus Hydromantes): Nesting Selection and Multiple Annual Breeding. Salamandra 2018, 54, 101–108. [Google Scholar]
- Oneto, F.; Ottonello, D.; Pastorino, M.V.; Salvidio, S. Posthatching Parental Care in Salamanders Revealed by Infrared Video Surveillance. J. Herpetol. 2010, 44, 649–653. [Google Scholar] [CrossRef]
- Mammola, S.; Lunghi, E.; Bilandžija, H.; Cardoso, P.; Grimm, V.; Schmidt, S.I.; Hesselberg, T.; Martínez, A. Collecting Eco-evolutionary Data in the Dark: Impediments to Subterranean Research and How to Overcome Them. Ecol. Evol. 2021, 11, 5911–5926. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Lunghi, E.; Cimmaruta, R.; Manenti, R. Transgressive Niche across a Salamander Hybrid Zone Revealed by Microhabitat Analyses. J. Biogeogr. 2019, 46, 1342–1354. [Google Scholar] [CrossRef]
- Bruni, G.; Chiocchio, A.; Nascetti, G.; Cimmaruta, R. Different Patterns of Introgression in a Three Species Hybrid Zone among European Cave Salamanders. Ecol. Evol. 2023, 13, e10437. [Google Scholar] [CrossRef]
- Cimmaruta, R.; Forti, G.; Lucente, D.; Nascetti, G. Thirty Years of Artificial Syntopy between Hydromantes italicus and H. ambrosii ambrosii (Amphibia, Plethodontidae). Amphib.-Reptil. 2013, 34, 413–420. [Google Scholar] [CrossRef]
- Manenti, R.; Ficetola, G. Salamanders Breeding in Subterranean Habitats: Local Adaptations or Behavioural Plasticity? J. Zool. 2013, 289, 182–188. [Google Scholar] [CrossRef]
- Angelini, C.; Vanni, S.; Vignoli, L. Salamandrina Terdigitata (Bonnaterre, 1789) Salamandrina Perspicillata (Savi, 1821). Fauna D’italia 2007, 42, 228–237. [Google Scholar]
- Rosa, G.; Salvidio, S.; Costa, A. Disentangling Exploitative and Interference Competition on Forest Dwelling Salamanders. Animals 2023, 13, 2003. [Google Scholar] [CrossRef]
- Lunghi, E.; Corti, C.; Biaggini, M.; Zhao, Y.; Cianferoni, F. The Trophic Niche of Two Sympatric Species of Salamanders (Plethodontidae and Salamandridae) from Italy. Animals 2022, 12, 2221. [Google Scholar] [CrossRef]
- Salvidio, S. Spatial Segregation in the European Plethodontid Speleomantes strinatii in Relation to Age and Sex. Amphib.-Reptil. 2002, 23, 505–510. [Google Scholar]
- Ficetola, G.F.; Pennati, R.; Manenti, R. Spatial Segregation among Age Classes in Cave Salamanders: Habitat Selection or Social Interactions? Popul. Ecol. 2013, 55, 217–226. [Google Scholar] [CrossRef]
- Rosa, G.; Salvidio, S.; Costa, A. European Plethodontid Salamanders on the Forest Floor: Testing for Age-Class Segregation and Habitat Selection. J. Herpetol. 2022, 56, 27–33. [Google Scholar] [CrossRef]
- Bloomberg, S.P.; Garland, T.; Ives, A.R. Testing for Phylogenetic Signal in Comparative Data: Behavioral Traits Are More Labile. Evolution 2003, 57, 717–745. [Google Scholar]
- van der Meijden, A.; Chiari, Y.; Mucedda, M.; Carranza, S.; Corti, C.; Veith, M. Phylogenetic Relationships of Sardinian Cave Salamanders, Genus Hydromantes, Based on Mitochondrial and Nuclear DNA Sequence Data. Mol. Phylogenet. Evol. 2009, 51, 399–404. [Google Scholar] [CrossRef]
- Chiari, Y.; van der Meijden, A.; Mucedda, M.; Lourenco, J.M.; Hochkirch, A.; Veith, M. Phylogeography of Sardinian Cave Salamanders (Genus Hydromantes) Is Mainly Determined by Geomorphology. PLoS ONE 2012, 7, e32332. [Google Scholar] [CrossRef]
- Mammola, S.; Amorim, I.R.; Bichuette, M.E.; Borges, P.A.; Cheeptham, N.; Cooper, S.J.; Cardoso, P. Fundamental Research Questions in Subterranean Biology. Biol. Rev. 2020, 95, 1855–1872. [Google Scholar] [CrossRef]
- Howarth, F.G.; Moldovan, O.T. The Ecological Classification of Cave Animals and Their Adaptations. In Cave Ecology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2018; pp. 41–67. [Google Scholar]
- Hervant, F.; Mathieu, J.; Durand, J. Behavioural, Physiological and Metabolic Responses to Long-Term Starvation and Refeeding in a Blind Cave-Dwelling (Proteus anguinus) and a Surface-Dwelling (Euproctus asper) Salamander. J. Exp. Biol. 2001, 204, 269–281. [Google Scholar] [CrossRef]
- Beasley-Hall, P.G.; Bertozzi, T.; Bradford, T.M.; Foster, C.S.P.; Jones, K.; Tierney, S.M.; Humphreys, W.F.; Austin, A.D.; Cooper, S.J.B. Differential Transcriptomic Responses to Heat Stress in Surface and Subterranean Diving Beetles. Sci. Rep. 2022, 12, 16194. [Google Scholar] [CrossRef]
- Lunghi, E.; Bilandžija, H. Longevity in Cave Animals. Front. Ecol. Evol. 2022, 10, 874123. [Google Scholar] [CrossRef]
- Lipovšek, S.; Leitinger, G.; Janžekovič, F.; Kozel, P.; Dariš, B.; Perc, M.; Devetak, D.; Weiland, N.; Novak, T. Towards Understanding Partial Adaptation to the Subterranean Habitat in the European Cave Spider, Meta menardi: An Ecocytological Approach. Sci. Rep. 2019, 9, 9121. [Google Scholar] [CrossRef]
- Borowsky, R. Selection maintains the phenotypic divergence of cave and surface fish. Am. Nat. 2023, 202, 55–63. [Google Scholar] [CrossRef]
- Gross, J.B.; Boggs, T.E.; Rétaux, S.; Torres-Paz, J. Developmental and genetic basis of troglomorphic traits in the teleost fish Astyanax mexicanus. In Groundwater Ecology and Evolution; Malard, F., Griebler, C., Rétaux, S., Eds.; Academic Press: New York, NY, USA, 2023; pp. 351–371. [Google Scholar]
- Guillaume, O. Surface Newt Calotriton Asper Acclimation to Cave Conditions Improved Their Foraging Ability in Darkness. Front. Ecol. Evol. 2022, 10, 1057023. [Google Scholar] [CrossRef]

| Behavioral Ecology Category According to [3] | Behavioral Ecology Category Used in This Study | Most studied Speleomantes Species for Each Category |
|---|---|---|
| Interspecific territoriality | Interspecific territoriality and homing | S. strinatii |
| Foraging tactics | Foraging tactics | S. italicus |
| Pheromonal glands and pheromonal communication | Pheromonal glands and pheromonal communication | / |
| Interspecific behavioral interactions | Interspecific behavioral interactions | S. strinatii |
| Intraspecific social behavior | Intraspecific social behavior, courtship, mating, and parental care | S. strinatii |
| Interactions with predators | Interactions with predators and parasites | S. italicus |
| Cognitive ecology | Cognitive ecology and personality | S. strinatii |
| Behavioral Category | Behavioral Ecology Question | Suggested Focal Species | Suggested Experimental Approach |
|---|---|---|---|
| Intraspecific social behavior, courtship, mating and parental care; Pheromonal glands and pheromonal communication | Do individuals communicate through tactile, visual or chemical signals? | All species | Experiments/observations in controlled conditions |
| Intraspecific social behavior, courtship, mating, and parental care; Pheromonal glands and pheromonal communication | How is mate selection made? | All species | Experiments/observations in controlled conditions |
| Intraspecific social behavior, courtship, mating, and parental care | How different species/populations adjust their behavior depending on habitat features? | All species | Experiments/observations in controlled conditions or in the wild |
| Intraspecific social behavior, courtship, mating, and parental care | Is the behavior of hybrid individuals related to that of a parent? | S. ambrosii, S. italicus, S. strinatii and their hybrids | Experiments in controlled conditions of genetically identified individuals |
| Cognitive ecology and personality | Are space use, displacement patterns and overall fitness linked to individual behavioral traits? | All species | Capture-mark-recapture study in the wild |
| Cognitive ecology and personality | How individuals modify their behavior after environmental or social experiences? | All species | Controlled conditions in outdoor mesocosms |
| Interspecific territoriality | How interspecific coexistence shapes resource use or activity patterns? | S. ambrosii; S. italicus; S. strinatii | Controlled conditions as laboratory settings or outdoor mesocosms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.; Lunghi, E.; Rosa, G.; Salvidio, S. Recent Advances in the Behavioral Ecology of European Plethodontid Salamanders. Animals 2023, 13, 3667. https://doi.org/10.3390/ani13233667
Costa A, Lunghi E, Rosa G, Salvidio S. Recent Advances in the Behavioral Ecology of European Plethodontid Salamanders. Animals. 2023; 13(23):3667. https://doi.org/10.3390/ani13233667
Chicago/Turabian StyleCosta, Andrea, Enrico Lunghi, Giacomo Rosa, and Sebastiano Salvidio. 2023. "Recent Advances in the Behavioral Ecology of European Plethodontid Salamanders" Animals 13, no. 23: 3667. https://doi.org/10.3390/ani13233667
APA StyleCosta, A., Lunghi, E., Rosa, G., & Salvidio, S. (2023). Recent Advances in the Behavioral Ecology of European Plethodontid Salamanders. Animals, 13(23), 3667. https://doi.org/10.3390/ani13233667









