Enterocytozoon hepatopenaei (EHP) Infection Alters the Metabolic Processes and Induces Oxidative Stress in Penaeus vannamei
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sample Collection
2.2. DNA Extraction and EHP Detection
2.3. Microstructure and Histology
2.4. RNA Extraction and Gene Expression Analysis
2.5. Determination of Total Protein, Glycogen, Glucose and ATP Content
2.6. Detection of Oxidation Products
2.7. Growth Performance
2.8. Data Analysis
3. Results
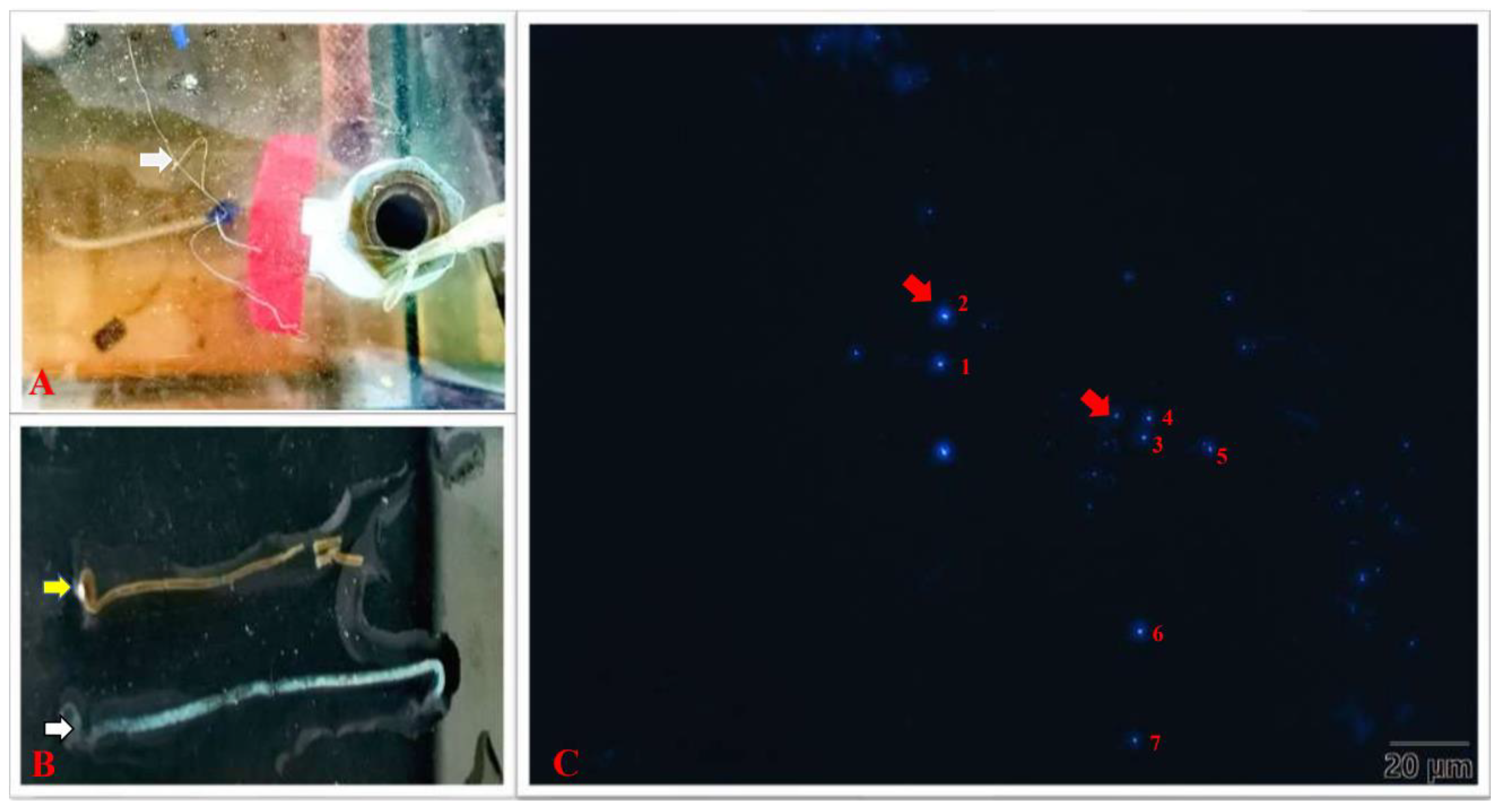
3.1. Detection of EHP Infection
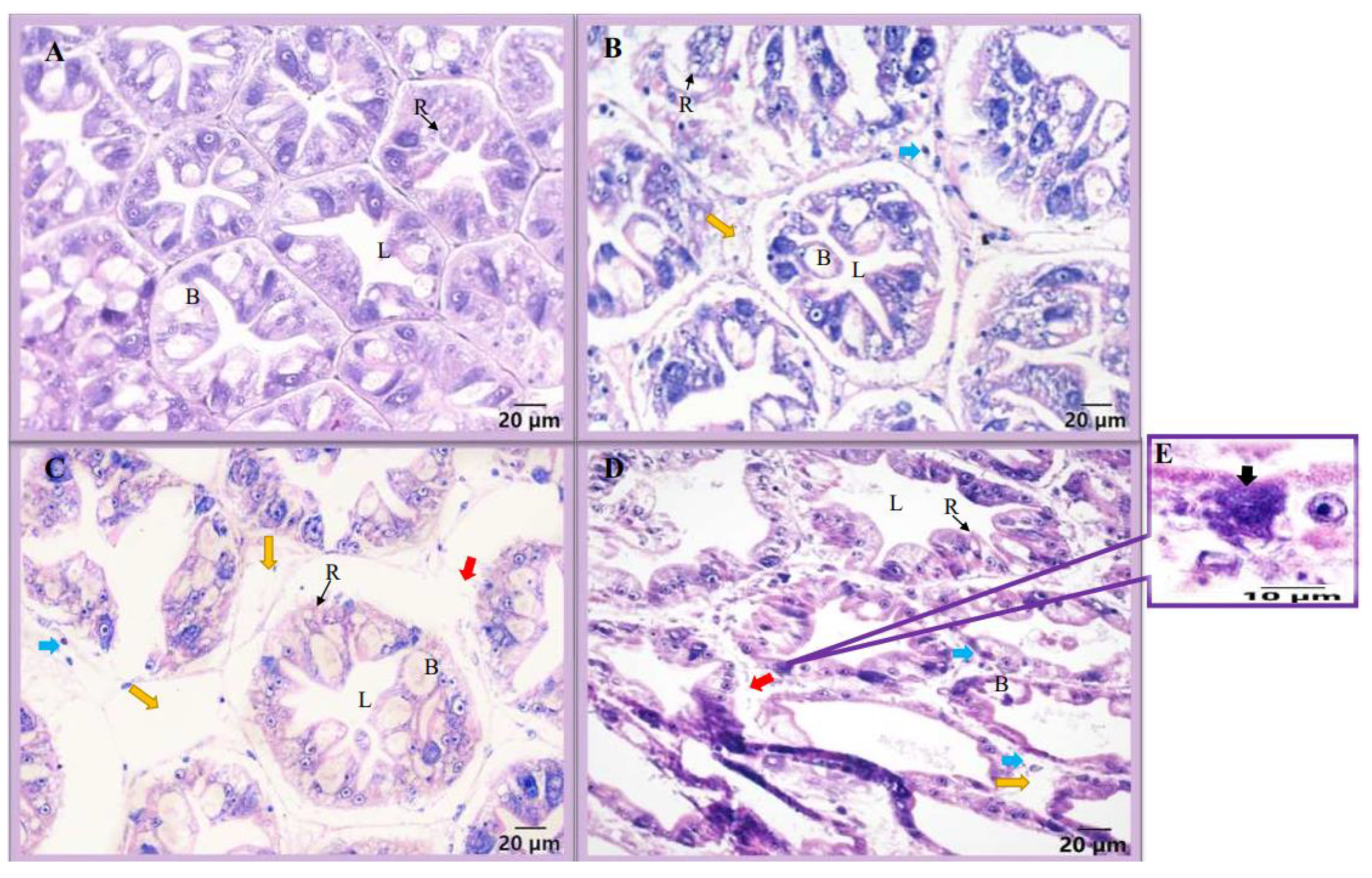
3.2. Microscopic Observation and Histology
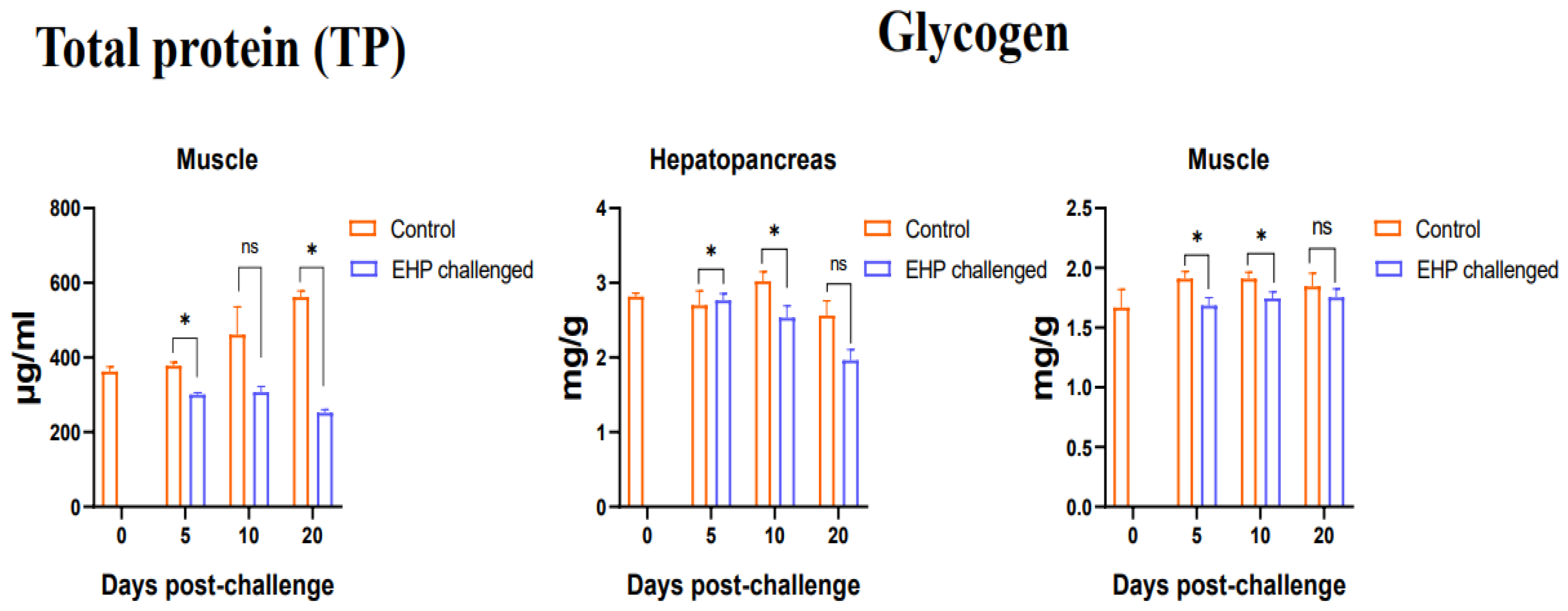
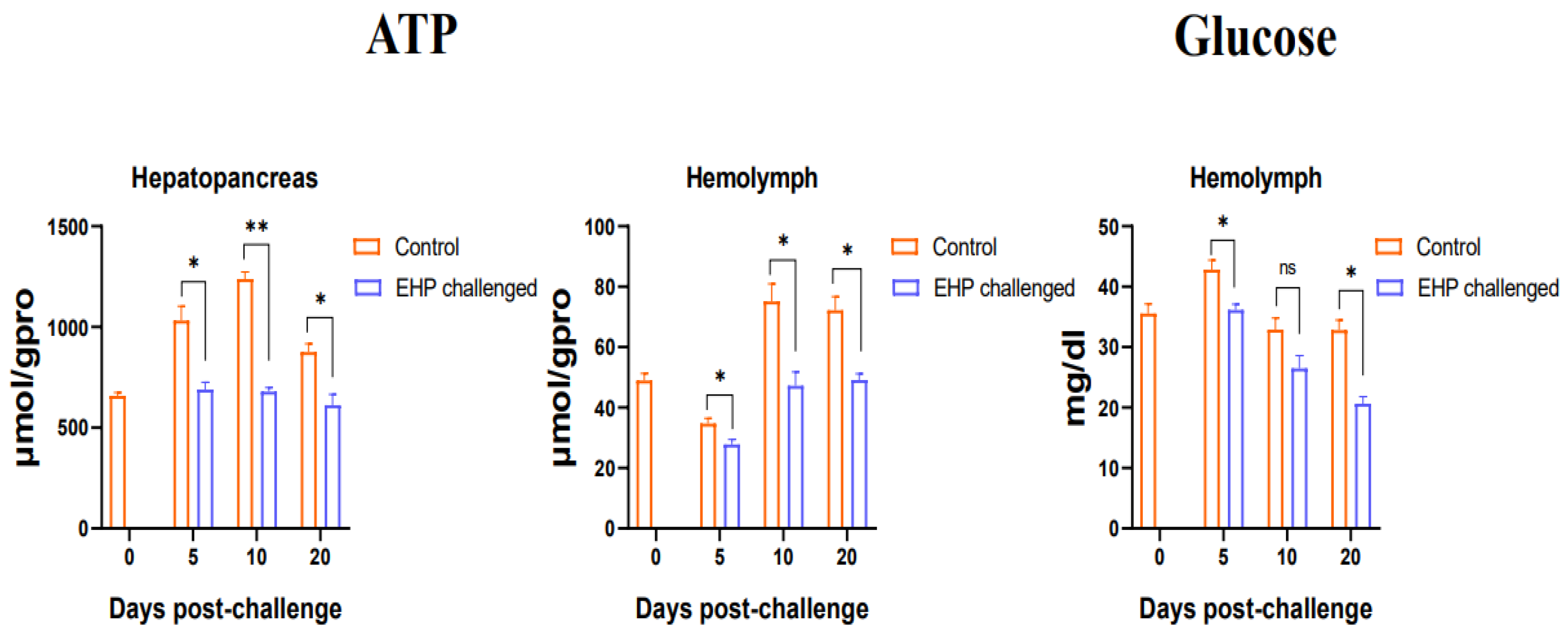
3.3. Total Protein, Glycogen, Glucose and ATP Content Assay
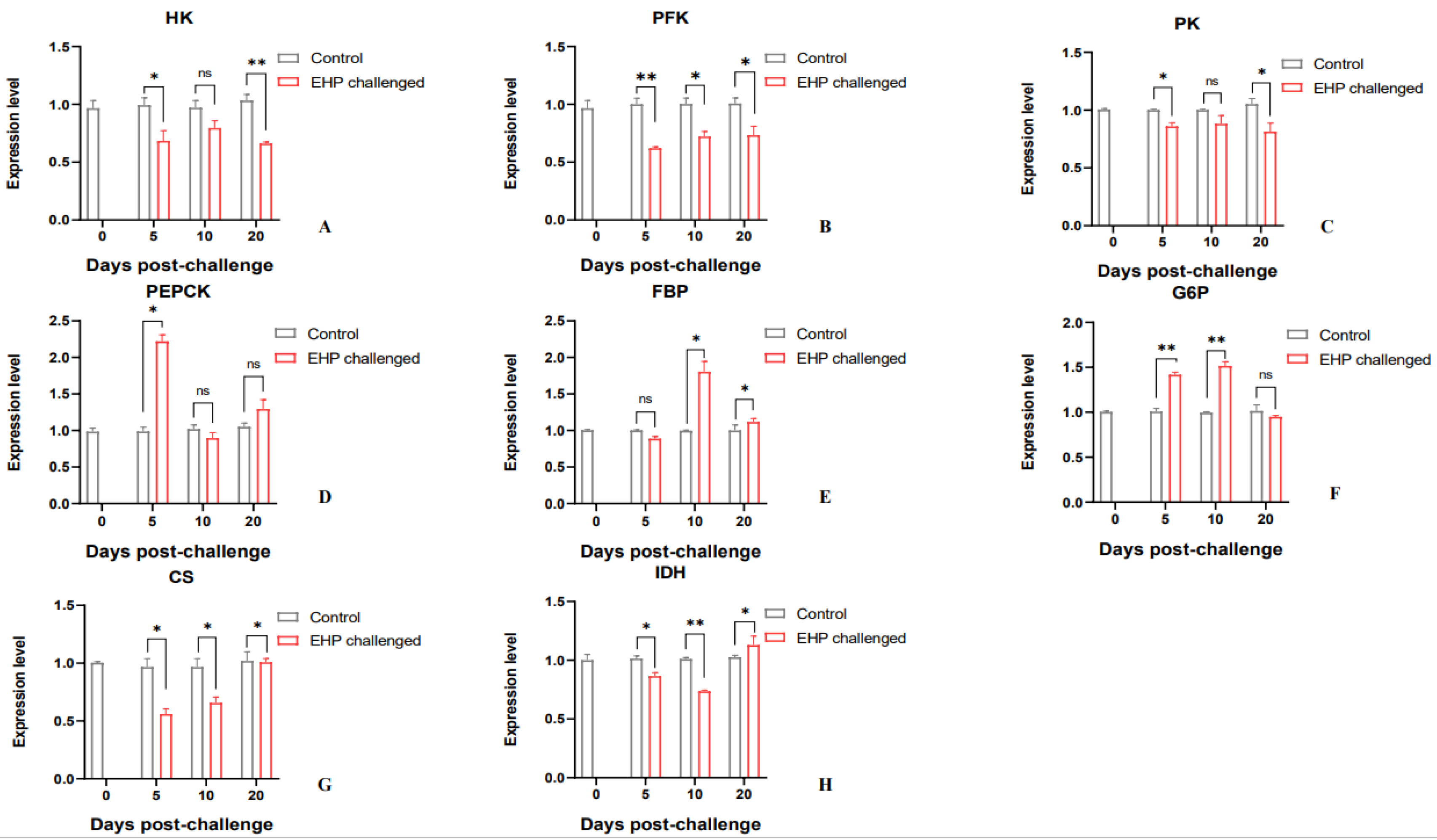
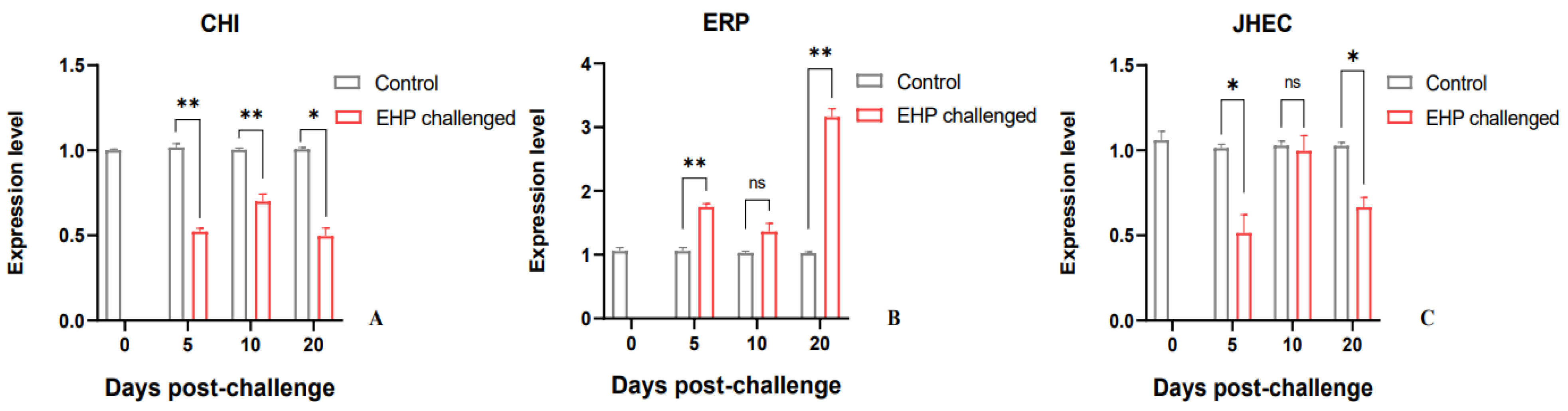
3.4. Analysis of Glucose Metabolism and Growth-Related Genes
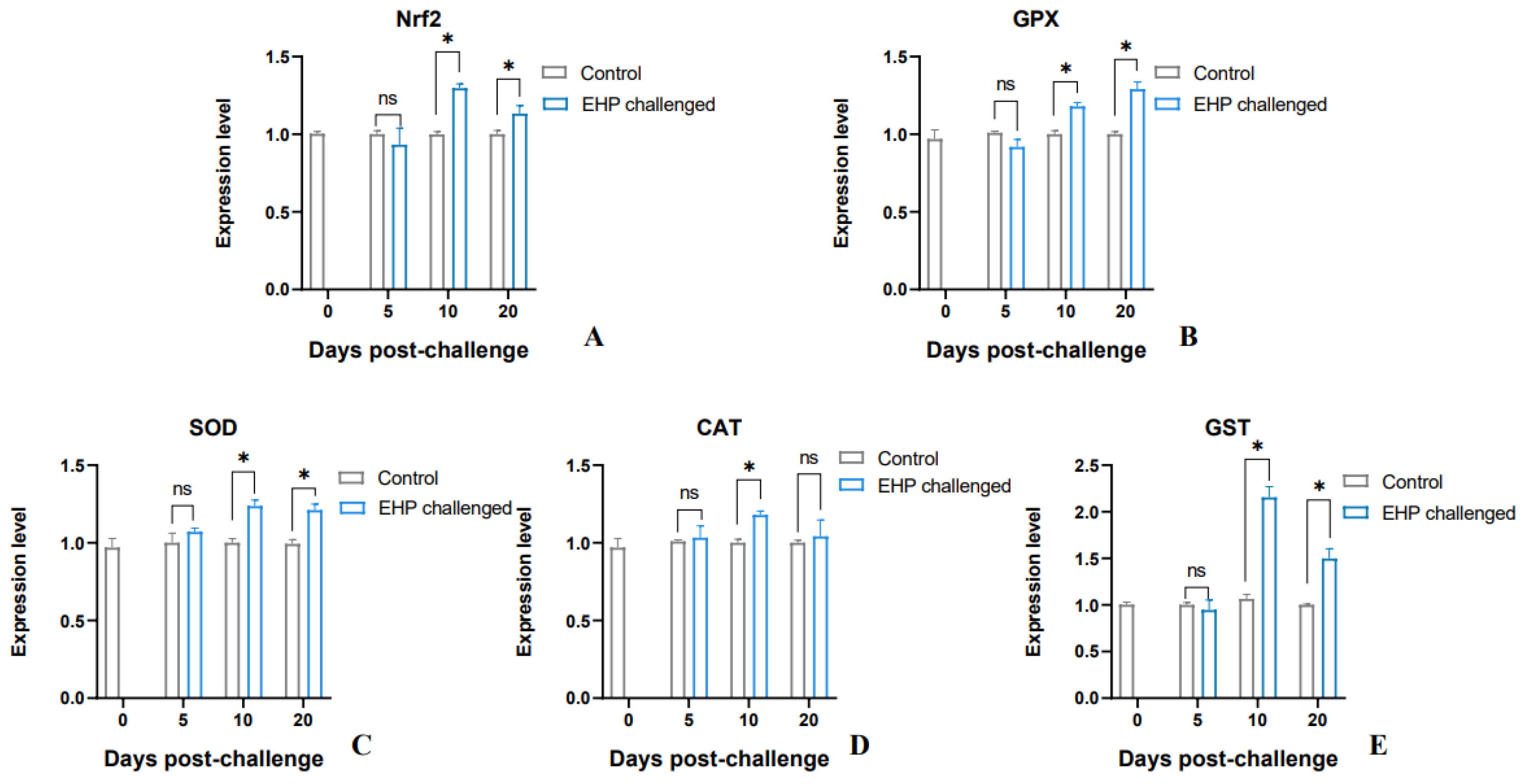
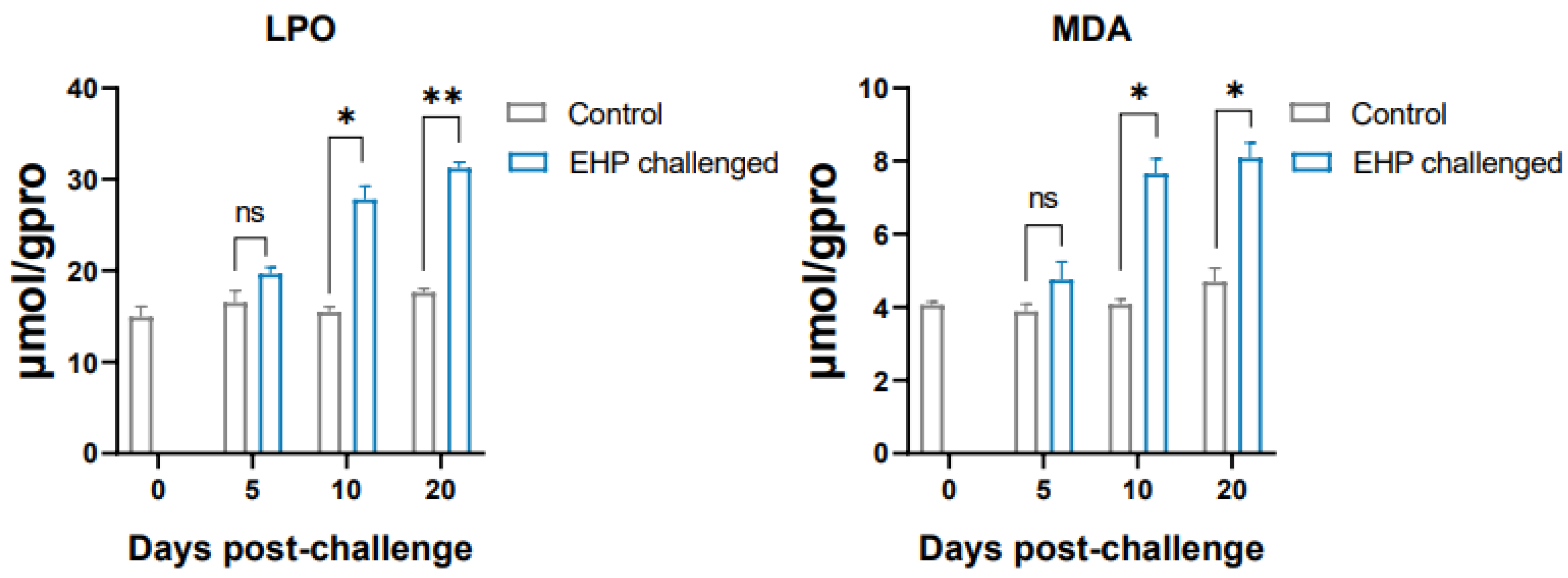
3.5. Analysis of Antioxidant Genes and Lipid Peroxidation Products
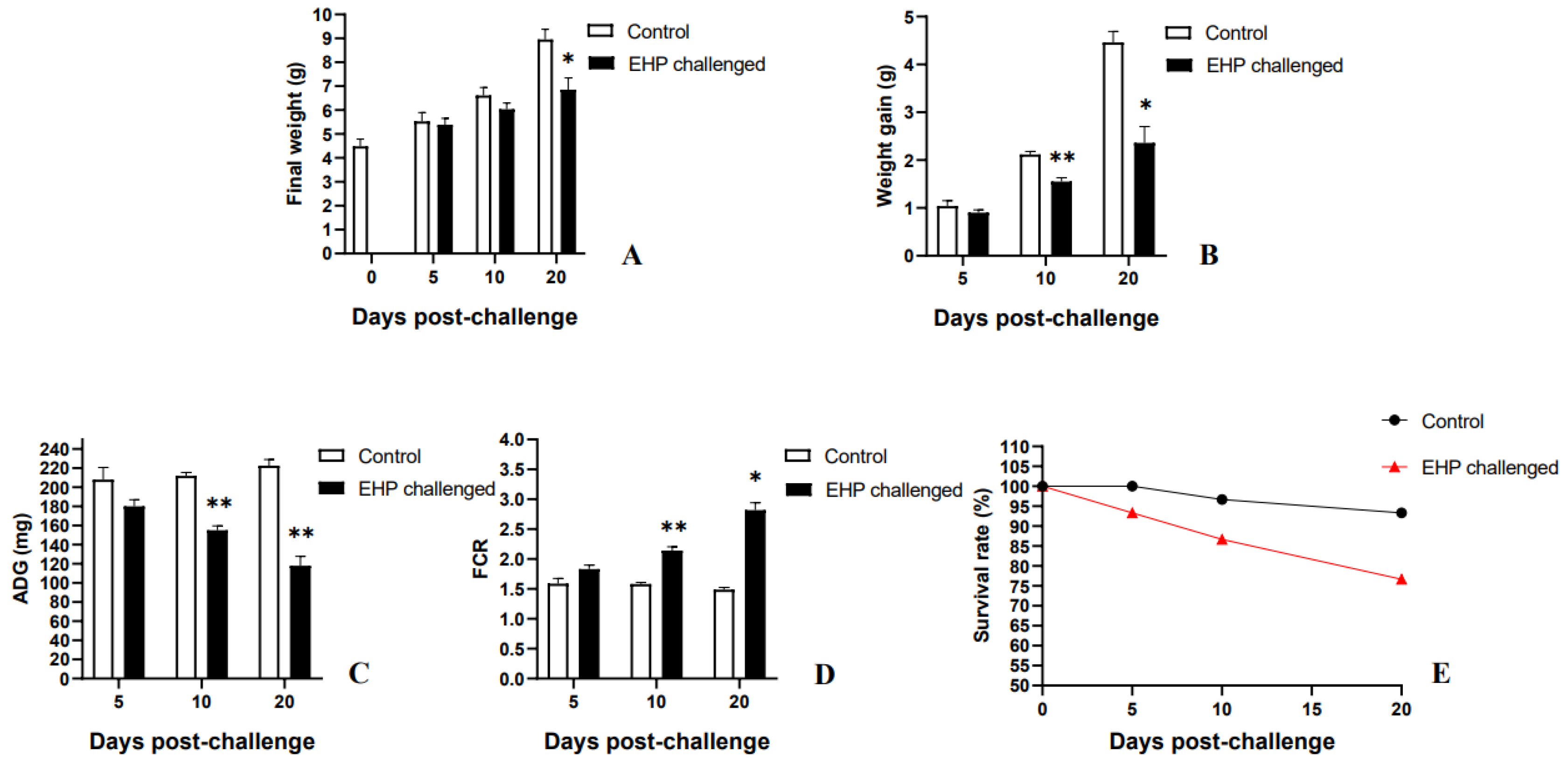
3.6. Growth Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Food and Agriculture Organization of the United Nations TUNA. GLOBEFISH Highlights April 2019, Statistics a Quarterly Update on World Seafood Markets FAO. Roma. December 2018. Available online: https://www.fao.org/in-action/globefish/publications/details-publication/es/c/1201730/ (accessed on 7 June 2022).
- National modern agricultural industry technology system white shrimp industry development report. China Fish. 2021, 5, 27–36.
- Chang, Z.Q.; Neori, A.; He, Y.Y.; Li, J.T.; Qiao, L.; Preston, S.I.; Liu, P.; Li, J. Development and current state of seawater shrimp farming, with an emphasis on integrated multi-trophic pond aquaculture farms, in China a review. Rev. Aquacult. 2020, 12, 2544–2558. [Google Scholar] [CrossRef]
- Tourtip, S.; Wongtripop, S.; Stentiford, G.D.; Bateman, K.S.; Sriurairatana, S.; Chavadej, J.; Sritunyalucksana, K.; Withyachumnarnkul, B. Enterocytozoon hepatopenaei sp. nov. (microsporida: Enterocytozoonidae), a parasite of the black tiger shrimp Penaeus monodon (decapoda: Penaeidae): Fine structure and phylogenetic relationships. J. Invertebr. Pathol. 2009, 102, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.H.; Yu, J.Y.; Wang, J.J.; Li, T.; Chang, L.R.; Fang, T.; Yan, D.C. Development of a PCR assay for the effective detection of Enterocytozoon hepatopenaei (EHP) and investigation of EHP prevalence in Shandong Province, China. J. Invertebr. Pathol. 2021, 184, 107653. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Jiang, G.; Wan, X.H.; Fan, X.P.; Qiao, Y.; Shi, W.J.; Wang, L.B. Multiple pathogens prevalent in shrimp Penaeus vannamei cultured from greenhouse ponds in Jiang Su province of China. J. Aquac. Res. Dev. 2017, 27, 675–683. [Google Scholar] [CrossRef]
- Ma, B.; Yu, H.; Fang, J.; Sun, C.; Zhang, M. Employing DNA binding dye to improve detection of Enterocytozoon hepatopenaei in real-time LAMP. Sci. Rep. 2019, 9, 15860. [Google Scholar] [CrossRef]
- Shi, H.; Xu, W.J.; Xie, J.J.; Wang, G.S. Pathogenicity of Penaeus vannamei slow growth syndrome in intensively cultured penaeid shrimp in Zhoushan. J. Fish. Sci. China 2017, 24, 387–394. [Google Scholar] [CrossRef]
- Patil, P.K.; Geetha, R.; Ravisankar, T.; Avunje, S.; Solanki, H.G.; Abraham, T.J.; Vinoth, S.P.; Jithendran, K.P.; Alavandi, S.V.; Vijayan, K.K. Economic loss due to diseases in Indian shrimp farming with special reference to Enterocytozoon hepatopenaei (EHP) and white spot syndrome virus (WSSV). Aquaculture 2021, 533, 9736231. [Google Scholar] [CrossRef]
- Shinn, A.P.; Pratoomyot, J.; Griffiths, D.; Trong, T.Q.; Vu, N.T.; Jiravanichpaisal, P.; Briggs, M. Asian shrimp production and the economic costs of disease. Special Acute Hepatopancreatic Necrosis Disease (AHPND). Asian Fish. Sci. 2018, 31, 29–58. [Google Scholar] [CrossRef]
- Chaijarasphong, T.; Munkongwongsiri, N.; Stentiford, G.D.; Aldama-Cano, D.J.; Thansa, K.; Flegel, T.W.; Sritunyalucksana, K.; Itsathitphaisarn, O. The shrimp microsporidian Enterocytozoon hepatopenaei (EHP): Biology, pathology, diagnostics and control. J. Invertebr. Pathol. 2021, 186, 107458. [Google Scholar] [CrossRef]
- Aranguren, L.F.; Han, J.E.; Tang, K.F.J. Enterocytozoon hepatopenaei (EHP) is a risk factor for acute hepatopancreatic necrosis disease (AHPND) and septic hepatopancreatic necrosis (SHPN) in the Pacific white shrimp Penaeus vannamei. Aquaculture 2017, 471, 37–42. [Google Scholar] [CrossRef]
- Munkongwongsiri, N.; Prachumwat, A.; Eamsaard, W.; Lertsiri, K.; Flegel, T.W.; Stentiford, G.D.; Sritunyalucksana, K. Propionigenium and Vibrio species identified as possible component causes of shrimp white feces syndrome (WFS) associated with the microsporidian Enterocytozoon hepatopenaei. J. Invertebr. Pathol. 2022, 192, 107784. [Google Scholar] [CrossRef] [PubMed]
- Sathish Kumar, T.; Makesh, M.; Alavandi, S.V.; Vijayan, K.K. Clinical manifestations of White feces syndrome (WFS), and its association with Enterocytozoon hepatopenaei in Penaeus vannamei grow-out farms: A pathobiological investigation. Aquaculture 2022, 547, 737463. [Google Scholar] [CrossRef]
- Duan, Y.F.; Chen, H.G.; Wang, J.L.; Zeng, S.M.; Wang, Y.; Mo, Z.Q.; Dan, X.M.; Li, Y.W. Response signatures of Litopenaeus vannamei to natural Enterocytozoon hepatopenaei infection revealed by the integration of the microbiome and transcriptome. Aquaculture 2021, 542, 736885. [Google Scholar] [CrossRef]
- Vogt, G. Life-cycle and functional cytology of the hepatopancreatic cells of Astacus astacus (Crustacea, Decapoda). Zoomorphology 1994, 114, 83–101. [Google Scholar] [CrossRef]
- Wang, W.; Wu, X.; Liu, Z.; Zheng, H.; Cheng, Y. Insights into hepatopancreatic functions for nutrition metabolism and ovarian development in the crab Portunus trituberculatus: Gene discovery in the comparative transcriptome of different hepatopancreas stages. PLoS ONE 2014, 9, e84921. [Google Scholar] [CrossRef]
- Ning, M.; Wei, P.; Shen, H.; Wan, X.; Jin, M.; Li, X.; Shi, H.; Qiao, Y.; Jiang, G.; Gu, W.; et al. Proteomic and metabolomic responses in hepatopancreas of whiteleg shrimp Litopenaeus vannamei infected by microsporidian Enterocytozoon hepatopenaei. Fish Shellfish Immun. 2019, 87, 534–545. [Google Scholar] [CrossRef]
- Wiredu Boakye, D.; Jaroenlak, P.; Prachumwat, A.; Williams, T.A.; Bateman, K.S.; Itsathitphaisarn, O.; Sritunyalucksana, K.; Paszkiewicz, K.H.; Moore, K.A.; Stentiford, G.D.; et al. Decay of the glycolytic pathway and adaptation to intranuclear parasitism within Enterocytozoonidae microsporidia. Environ. Microbiol. 2017, 19, 2077–2089. [Google Scholar] [CrossRef]
- Cao, Z.; Gao, Y.; Xu, J.H.; Yang, N.; Li, T.; Chang, L.R.; Si, L.J.; Yan, D.C. Transcriptome analysis of the hepatopancreas in Penaeus vannamei under experimental infection with Enterocytozoon hepatopenaei (EHP). Fish Shellfish Immun. 2023, 134, 108605. [Google Scholar] [CrossRef]
- Oakes, K.D.; Van Der Kraak, G.J. Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat. Toxicol. 2003, 29, 447–463. [Google Scholar] [CrossRef]
- Lightner, D.V. A Handbook of Shrimp Pathology and Diagnostic Procedures for Diseases of Cultured Penaeid Shrimp; World Aquatic Society: Baton Rouge, LA, USA, 1996. [Google Scholar]
- Ji, P.F.; Yao, C.L.; Wang, Z.Y. Reactive oxygen system plays an important role in shrimp Litopenaeus vannamei defense against Vibrio parahaemolyticus and WSSV infection. Dis. Aquat. Organ. 2011, 29, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, A.; Monserrat, J.M. Effects of methyl parathion on Chasmagnathus granulatus hepatopancreas: Protective role of sesamol. Ecotoxicol. Environ. Saf. 2007, 67, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Subash, P.; Chrisolite, B.; Sivasankar, P.; Rosalind George, M.; Vijay Amirtharaj, K.S.; Padmavathy, P.; Rani, V.; Sankar Sri Balaje, R.; Gowtham, S.; Mageshkumar, P. White feces syndrome in Penaeus vannamei is potentially an Enterocytozoon hepatopenaei (EHP) associated pathobiome origin of Vibrio spp. J. Invertebr. Pathol. 2023, 198, 107932. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Guo, X.M.; Wang, H.L.; Zhao, R.H.; Xie, G.S.; Li, C.; Huang, J. A double staining method using calcofluor white and acridine orange to differentiate life stages of Enterocytozoon hepatopenaei (EHP) on hepatopancreatic sections. Aquaculture 2020, 528, 735628. [Google Scholar] [CrossRef]
- Li, G.; Cong, F.; Cai, W.Y.; Li, J.H.; Wu, M.L.; Xiao, L.; Hu, X.L.; Zeng, W.W.; He, D.S. Development of a recombinase polymerase amplification (RPA) fluorescence assay for the detection of Enterocytozoon hepatopenaei (EHP). Aquacult. Rep. 2021, 19, 100584. [Google Scholar] [CrossRef]
- Tang, K.F.J.; Pantoja, C.R.; Redman, R.M.; Han, J.E.; Tran, L.H.; Lightner, D.V. Development of in situ hybridization and PCR assays for the detection of Enterocytozoon hepatopenaei (EHP), a microsporidian parasite infecting penaeid shrimp. J. Invertebr. Pathol. 2015, 130, 37–41. [Google Scholar] [CrossRef]
- Otta, S.K.; Karunasagar, I.; Karunasagar, I. Detection of monodon baculovirus and white spot syndrome virus in apparently healthy Penaeus monodon postlarvae from India by polymerase chain reaction. Aquaculture 2003, 220, 59–67. [Google Scholar] [CrossRef]
- OIE. Manual of Diagnostic Tests for Aquatic Animals. World Organisation for Animal Health. 2019. Available online: http://www.oie.int/en/international-standard-setting/aquatic-manual (accessed on 13 June 2022).
- Söderhäll, K.; Smith, V.J. Separation of the haemocyte populations of Carcinus maenas and othermarine decapods, and prophenoloxidase distribution. Dev. Comp. Immunol. 1983, 7, 229–233. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Q.L.; Wan, X.Y.; Ma, F.; Huang, J. Development of real-time PCR assay for detecting microsporidian Enterocytozoon hepatorenal and the application in shrimp samples with different growth rates. Progress. Fish. Sci. 2016, 37, 119–126. [Google Scholar] [CrossRef]
- Bell, T.A.; Lightner, D.V. A Handbook of Normal Penaeid Shrimp Histology; World Aquaculture Society: Sorrento, LA, USA, 1988. [Google Scholar]
- Zhao, R.H.; Gao, W.; Qiu, L.; Chen, X.; Dong, X.; Li, C.; Huang, J. A staining method for detection of Enterocytozoon hepatopenaei (EHP) spores with calcofluor white. Aquaculture 2020, 172, 107347. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zarain-Herzberg, M.; Fraga, I.; Hernandez-Llamas, A. Advances in intensifying the cultivation of the shrimp Litopenaeus vannamei in floating cages. Aquaculture 2010, 300, 87–92. [Google Scholar] [CrossRef]
- Sathish Kumar, T.; Ezhil Praveena, P.; Sivaramakrishnan, T.; Joseph Sahaya Rajan, J.; Makesh, M.; Jithendran, K.P. Effect of Enterocytozoon hepatopenaei (EHP) infection on physiology, metabolism, immunity, and growth of Penaeus vannamei. Aquaculture 2022, 553, 738105. [Google Scholar] [CrossRef]
- Zhu, B.; Lu, X.D.; Liu, Y.H.; Wu, Z.N.; Cai, H.F.; Jin, S.; Li, Z.; Xie, J.S.; Li, X.B.; Sun, F.Y.; et al. Effects of Enterocytozoon hepatopenaei single-infection or co-infection with Vibrio parahaemolyticus on the hepatopancreas of Penaeus vannamei. Aquaculture 2022, 549, 737726. [Google Scholar] [CrossRef]
- de Oliveira Cesar, J.R.; Zhao, P.B.; Malecha, S.; Ako, H.; Yang, J.Z. Morphological and biochemical changes in the muscle of the marine shrimp Litopenaeus vannamei during the molt cycle. Aquaculture 2006, 261, 688–694. [Google Scholar] [CrossRef]
- Yoganandhan, K.; Thirupathi, S.; Sahul Hameed, A.S. Biochemical, physiological and hematological changes in white spot syndrome virus-infected shrimp, Penaeus indicus. Aquaculture 2003, 221, 1–11. [Google Scholar] [CrossRef]
- Chen, I.T.; Aoki, T.; Huang, Y.T.; Hirono, I.; Chen, T.C.; Huang, J.Y.; Chang, G.D.; Lo, C.F.; Wang, H.C. White spot syndrome virus induces metabolic changes resembling the warburg effect in shrimp hemocytes in the early stage of infection. J. Virol. 2011, 85, 19–28. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Li, F.X.; Yao, C.L. Glycogen phosphorylase of shrimp (Litopenaeus vannamei): Structure, expression and anti-WSSV function. Fish Shellfish Immun. 2019, 91, 275–283. [Google Scholar] [CrossRef]
- Lin, L.J.; Chen, Y.J.; Chang, Y.S.; Lee, C.Y. Neuroendocrine responses of a crustacean host to viral infection: Effects of infection of white spot syndrome virus on the expression and release of crustacean hyperglycemic hormone in the crayfish Procambarus clarkii. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 164, 327–332. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Chang, E.S.; Chang, S.A.; Neil, D.M. Carbohydrate dynamics and the crustacean hyperglycemic hormone (CHH): Effects of parasitic infection in Norway lobsters (Nephrops norvegicus). Gen. Comp. Endocrinol. 2001, 121, 13–22. [Google Scholar] [CrossRef]
- Aranguren Caro, L.F.; Mai, H.N.; Cruz-Florez, R.; Marcos, F.L.A.; Alenton, R.R.R.; Dhar, A.K. Experimental reproduction of White Feces Syndrome in whiteleg shrimp, Penaeus vannamei. PLoS ONE 2021, 16, e0261289. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, C.A.; Desjardins, C.A.; Bakowski, M.A.; Goldberg, J.; Ma, A.T.; Becnel, J.J.; Didier, E.S.; Fan, L.; Heiman, D.I.; Levin, J.Z.; et al. Microsporidian genome analysis reveals evolutionary strategies for obligate intracellular growth. Genome. Res. 2012, 22, 2478–2488. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhang, Y.L.; Yao, D.F.; Zhao, Y.Z.; Tuan, T.N.; Li, S.K.; Ma, H.Y. Aweya Jude Juventus, Metabolic reprogramming in crustaceans: A vital immune and environmental response strategy. Rev. Aquacult. 2022, 14, 1094–1119. [Google Scholar] [CrossRef]
- Xu, C.; Li, E.; Liu, Y.; Wang, S.; Wang, X.; Chen, K.; Qin, G.J.; Chen, L. Effect of dietary lipid level on growth, lipid metabolism and health status of the Pacific white shrimp Litopenaeus vannamei at two salinities. Aquacult. Nutr. 2018, 24, 204–214. [Google Scholar] [CrossRef]
- Zhan, Q.Y.; Han, T.; Li, X.Y.; Wang, J.T.; Yang, Y.X.; Yu, X.J.; Zheng, P.Q.; Liu, T.; Xu, X.Y.; Wang, C.L. Effects of dietary carbohydrate levels on growth, body composition, and gene expression of key enzymes involved in hepatopancreas metabolism in mud crab Scylla paramamosain. Aquaculture 2020, 529, 735638. [Google Scholar] [CrossRef]
- Wu, Y.J.; Chen, J.; Liao, G.L.; Hu, M.J.; Zhang, Q.; Meng, X.Z.; Li, T.; Long, M.X.; Fan, X.D.; Yu, Q.; et al. Down-Regulation of Lipid Metabolism in the Hepatopancreas of Shrimp Litopenaeus vannamei upon Light and Heavy Infection of Enterocytozoon hepatopenaei: A Comparative Proteomic Study. Int. J. Mol. Sci. 2022, 23, 11574. [Google Scholar] [CrossRef]
- Bian, X.L.; Jiang, H.F.; Meng, Y.; Li, Y.P.; Fang, J.; Lu, Z.M. Regulation of gene expression by glycolytic and gluconeogenic enzymes Trends. Cell. Biol. 2022, 32, 786–799. [Google Scholar] [CrossRef]
- Cota-Ruiz, K.; Peregrino-Uriarte, A.B.; Felix-Portillo, M.; Martínez-Quintana, J.A.; Yepiz-Plascencia, G. Expression of fructose 1,6-bisphosphatase and phosphofructokinase is induced in hepatopancreas of the white shrimp Litopenaeus vannamei by hypoxia. Mar. Environ. Res. 2015, 106, 1–9. [Google Scholar] [CrossRef]
- Jenkins, C.M.; Yang, J.Y.; Sims, H.F.; Gross Reversible, R.W. high affinity inhibition of phosphofructokinase-1 by acyl-CoA: A mechanism integrating glycolytic flux with lipid metabolism. J. Biol. Chem. 2011, 286, 11937–11950. [Google Scholar] [CrossRef]
- Ulaje, S.A.; Rojo-Arreola, L.; Lluch-Cota, S.E.; Ascencio, F.; Cruz-Hernández, P.; Sicard, M.T. Gene expression and energetic metabolism changes in the whiteleg shrimp (Litopenaeus vannamei) in response to short-term hypoxia. Aquac. Res. 2019, 50, 994–1004. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, X.B. The Wnt signaling pathway is involved in the regulation of phagocytosis of virus in Drosophila. Sci. Rep. 2013, 3, 2069. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Mayack, C.; Naug, D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.W.; Ye, Y.F.; Chen, Z.; Shao, Y.N.; Xie, X.L.; Zhang, W.W.; Liu, H.P.; Li, C.H. Metabolic product response profiles of Cherax quadricarinatus towards white spot syndrome virus infection. Dev. Comp. Immunol. 2016, 61, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Alfaro, A.; Arroyo, B.B.; Leon, J.A.R.; Sonnenholzner, S. Metabolic responses of penaeid shrimp to acute hepatopancreatic necrosis disease caused by Vibrio parahaemolyticus. Aquaculture 2021, 533, 736174. [Google Scholar] [CrossRef]
- Chen, I.T.; Lee, D.Y.; Huang, Y.T.; Kou, G.H.; Wang, H.C.; Chang, G.D.; Lo, C.F. Six Hours after Infection, the Metabolic Changes Induced by WSSV Neutralize the Host's Oxidative Stress Defenses. Sci. Rep. 2016, 6, 27732. [Google Scholar] [CrossRef]
- Wang, H.; Pan, L.; Xu, R.; Si, L.; Zhang, X. The molecular mechanism of Nrf2-Keap1 signaling pathway in the antioxidant defense response induced by BaP in the scallop Chlamys farreri. Fish Shellfish Immun. 2019, 92, 489–499. [Google Scholar] [CrossRef]
- Parrilla Taylor, D.P.; Zenteno Savín, V.T.; Magallón Barajas, F.J. Antioxidant enzyme activity in pacific white leg shrimp (Litopenaeus vannamei) in response to infection with white spot syndrome virus. Aquaculture 2013, 380, 41–46. [Google Scholar] [CrossRef]
- Sookruksawong, S.; Pongsomboon, S.; Tassanakajon, A. Genomic organization of the cytosolic manganese superoxide dismutase gene from the Pacific white shrimp, Litopenaeus vannamei, and its response to thermal stress. Fish Shellfish Immun. 2013, 35, 1395–1405. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Kang, S.W.; Rhee, S.G.; Chang, T.S.; Jeong, W.; Choi, M.H. 2-Cys peroxiredoxin function in intracellular signal transduction: Therapeutic implications. Trends Mol. Med. 2005, 11, 571–578. [Google Scholar] [CrossRef]
- Guo, H.; Xian, J.A.; Li, B.; Ye, C.X.; Wang, A.L.; Miao, Y.T.; Liao, S.A. Gene expression of apoptosis-related genes, stress protein and antioxidant enzymes in hemocytes of white shrimp Litopenaeus vannamei under nitrite stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 157, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Tseng, M.C.; Cheng, W. Identification and cloning of the antioxidant enzyme, glutathione peroxidase, of white shrimp, Litopenaeus vannamei, and its expression following Vibrio alginolyticus infection. Fish Shellfish Immun. 2007, 23, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.N.; Wang, A.L.; Wang, W.Y.; He, Q.T.; Zhou, Y.; Xu, J. Glutathione S-transferase in the white shrimp Litopenaeus vannamei: Characterization and regulation under pH stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 150, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pan, L.; Si, L.; Ji, R.; Cao, Y. Effects of Nrf2-Keap1 signaling pathway on antioxidant defense system and oxidative damage in the clams Ruditapes philippinarum exposure to PAHs. Environ. Sci. Pollut. Res. 2021, 26, 33060–33071. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Q.; Yuan, Y.; Zhang, Z.; Jiang, B.; Yang, S.; Jian, J. Silencing of Nrf2 in Litopenaeus vannamei, decreased the antioxidant capacity, and increased apoptosis and autophagy. Fish Shellfish Immun. 2022, 122, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Guan, H.; Huang, Y.; Luo, J.; Jian, J.; Cai, S.; Yang, S. Involvement of Nrf2 in the immune regulation of Litopenaeus vannamei against Vibrio harveyi infection. Fish Shellfish Immun. 2023, 133, 108547. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Zhang, J.; Liu, Q.; Ding, X. Morphologic, digestive enzymes and immunological responses of intestine from Litopenaeus vannamei after lipopolysaccharide injection. J. Invertebr. Pathol. 2018, 153, 186–194. [Google Scholar] [CrossRef]
- Lee, S.O.; Jeon, J.M.; Oh, C.W.; Kim, Y.M.; Kang, C.K.; Lee, D.S.; Mykles, D.L.; Kim, H.W. Two juvenile hormone esterase-like carboxylesterase cDNAs from a Pandalus shrimp (Pandalopsis japonica): Cloning, tissue expression, and effects of eyestalk ablation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011, 159, 148–156. [Google Scholar] [CrossRef]
- He, C.B.; Chen, P.H.; Gao, X.G.; Gao, L.; Li, L. Expression and purification of ecdysteroid-regulated protein from Chinese mitten crab Eriocheir sinensis in E. coli. Mol. Biol. Rep. 2013, 40, 6987–6995. [Google Scholar] [CrossRef]
- Ning, N.X.; Bi, J.X.; Sun, W.; Xie, X.J.; Huang, Y.L.; Gu, W.; Wang, W.; Qiao, Y.; Jiang, G.; Shen, H.; et al. Linolenic acid improves growth performance and immune status of Penaeus vannamei infected by Enterocytozoon hepatopenaei. Aquaculture 2021, 535, 736397. [Google Scholar] [CrossRef]
- Priya, T.A.; Li, F.H.; Zhang, J.Q.; Wang, B.; Zhao, C.; Xiang, J.H. Molecular characterization and effect of RNA interference of retinoid X receptor (RXR) on E75 and chitinase gene expression in Chinese shrimp Fenneropenaeus chinensis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 153, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Proespraiwong, P.; Tassanakajon, A.; Rimphanitchayakit, V. Chitinases from the black tiger shrimp Penaeus monodon: Phylogenetics, expression and activities. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 156, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Qiu, L.; Cheng, D.Y.; Zhang, Q.L.; Wan, X.Y.; Huang, J. The Relationship of Body Length and Weight in the Litopenaeus vannamei Populations Detected Enterocytozoon hepatopenaei. Progress. Fish. Sci. 2017, 38, 96–103. [Google Scholar] [CrossRef]
- Kongplong, S.; Kanjanasopa, D.; Pongtippatee, P.; Vanichviriyakit, R.; Withyachumnarnkul, B. 5-Aminolaevulinic acid reduced the mortality of the Pacific white shrimp Litopenaeus vannamei infected with Enterocytozoon hepatopenaei. Aquaculture 2023, 568, 739322. [Google Scholar] [CrossRef]
- Tang, K.F.J.; Aranguren, L.F.; Piamsomboon, P.; Han, J.E.; Maskaykina, I.Y.; Schmidt, M.M. Detection of the microsporidian Enterocytozoon hepatopenaei (EHP) and Taura syndrome virus in Penaeus vannamei cultured in Venezuela. Aquaculture 2017, 480, 17–21. [Google Scholar] [CrossRef]
- Wang, H.L.; Wan, X.Y.; Xie, G.S.; Dong, X.; Wang, X.H.; Huang, J. Insights into the histopathology and microbiome of Pacific white shrimp, Penaeus vannamei, suffering from white feces syndrome. Aquaculture 2020, 527, 735447. [Google Scholar] [CrossRef]
| Gene | Primers (5′-3′) | GenBank Accession No. |
|---|---|---|
| Growth and metabolic genes | ||
| ERP | F: CCTACAGCGTCAACATCCA R: GCATCCGTCGGTGTCTATT | JQ009182.1 |
| JHEC1 | F: GGCGGAGCAGAGGACTAT R: CGAGGTCACGGATGTTGTC | APO14259.1 |
| CHI | F: GAAGGCGTCGTATATTGTGTCAC R: AGCACGGTCCTGATGGTAGT | ACR23315 |
| G6P | F: CTTATGAATCGTGGGTGGTGC R: CAATAGGGTCGGTCTCCTCTGA | PRJNA660490 |
| FBP | F: TGTGTCGGAAGAAAACAAAACT R: CTATGGAGACGAGGCAATCAAT | KP057246 |
| PEPCK | F: AAGACCAGTGATGGAGGAGTG R: GGGAGTTGGGATGAGCAG | FJ441189 |
| PK | F: ATCCTTGATGGTGCTGAC R: TGAAGAGTTGCTTGTGCC | EF102105 |
| PFK | F: AGAGGACGGGGAAGTTTTACAG R: GTTCTTGCCTGGGTTCAAATAG | PRJNA660490 |
| HK | F: ATCGGCAAGTTAGATACGC R: AGGACACCACGGTAGGAA | EF102106 |
| CS | F: AAAGAATACGGTAACACCAAAGT R: ATGGAGTAACCTCGGAACCTGAT | XM027367291 |
| IDH | F: GCCTCTGCCTCAAGGGTAT R: CCTCAGTCTGCTCACGGAT | XM027354101.1 |
| Antioxidant genes | ||
| CAT | F: TCAGCGTTTGGTGGAGAA R: GCCTGGCTCATCTTTATC | AY518322 |
| MnSOD | F: CGTAGAGGGTATTGTCGT R: TTGAAATCATACTTGAGGG | DQ005531 |
| GPX | F: AGGGACTTCCACCAGATG R: CAACAACTCCCCTTCGGTA | AY973252 |
| GST | F: AAGATAACGCAGAGCAAGG R: TCGTAGGTGACGGTAAAGA | AY573381 |
| Nrf2 | F: GATGAAGCGAGCCAGAGCG R: GCCGTCGGATGTCTCGGATAA | XM_027367068.1 |
| β-actin | F: TGGACTTCGAGCAGGAGATG R: GGAATGAGGGCTGGAACAGG | AF300705 |
| 18s | F: TATACGCTAGTGGAGCTGGAA R: GGGGAGGTAGTGACGAAAAAT | EU920969 |
| Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Size (μm) | 1.102 | 1.193 | 0.928 | 0.717 | 1.009 | 1.055 | 0.820 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Chen, C.; Wang, C.; Li, T.; Chang, L.; Si, L.; Yan, D. Enterocytozoon hepatopenaei (EHP) Infection Alters the Metabolic Processes and Induces Oxidative Stress in Penaeus vannamei. Animals 2023, 13, 3661. https://doi.org/10.3390/ani13233661
Cao Z, Chen C, Wang C, Li T, Chang L, Si L, Yan D. Enterocytozoon hepatopenaei (EHP) Infection Alters the Metabolic Processes and Induces Oxidative Stress in Penaeus vannamei. Animals. 2023; 13(23):3661. https://doi.org/10.3390/ani13233661
Chicago/Turabian StyleCao, Zheng, Caiyi Chen, Cuixia Wang, Ting Li, Linrui Chang, Lingjun Si, and Dongchun Yan. 2023. "Enterocytozoon hepatopenaei (EHP) Infection Alters the Metabolic Processes and Induces Oxidative Stress in Penaeus vannamei" Animals 13, no. 23: 3661. https://doi.org/10.3390/ani13233661
APA StyleCao, Z., Chen, C., Wang, C., Li, T., Chang, L., Si, L., & Yan, D. (2023). Enterocytozoon hepatopenaei (EHP) Infection Alters the Metabolic Processes and Induces Oxidative Stress in Penaeus vannamei. Animals, 13(23), 3661. https://doi.org/10.3390/ani13233661






