Factors Influencing Milk Quality and Subclinical Mastitis in Dairy Herds Housed in Compost-Bedded Pack Barn System
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Herd Selection and Study Protocols
2.2. Fans Wind Speed and Meteorological Conditions Data
2.3. Bedding Sampling and Milk Data Collection
2.4. Bedding Analysis
2.5. Data Analysis
3. Results
3.1. Milk, Bedding, and Environmental Characteristics
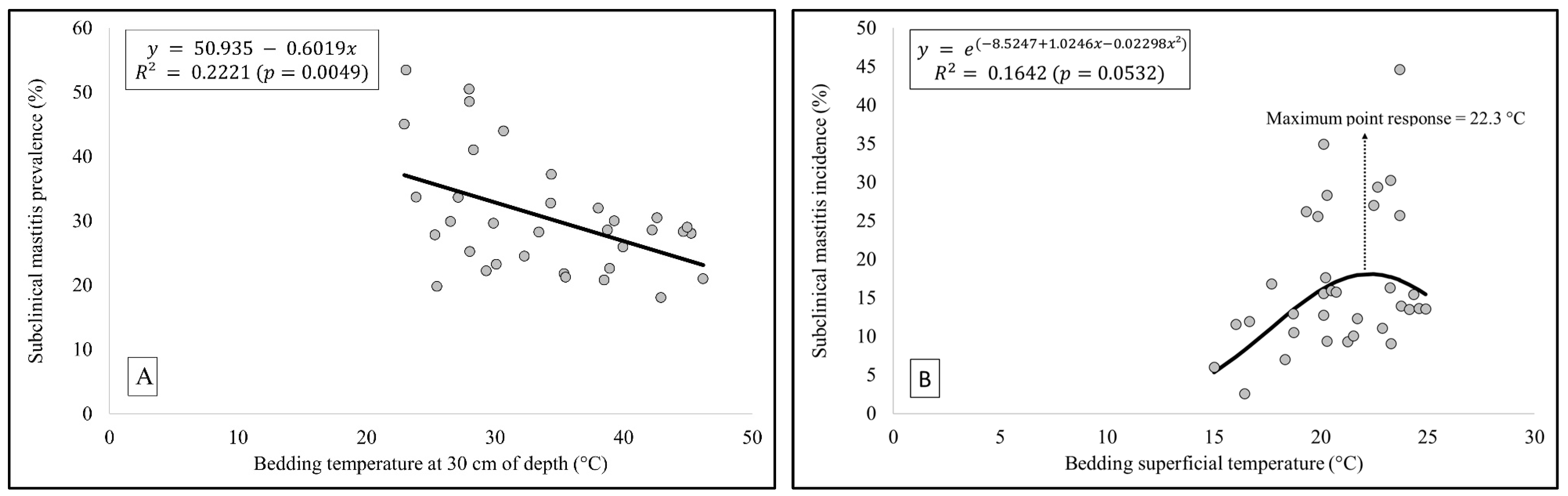
3.2. Subclinical Mastitis Prevalence and Incidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piovesan, S.M.; Oliveira, D.S. Fatores que influenciam a sanidade e conforto térmico de bovinos em sistemas compost barn. Rev. Vivências. 2020, 16, 247–258. [Google Scholar] [CrossRef]
- Marcondes, M.I.; Mariano, W.H.; De Vries, A. Production, economic viability and risks associated with switching dairy cows from drylots to compost bedded pack systems. Animal 2019, 14, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Barberg, A.E.; Endres, M.I.; Janni, K.A. Compost dairy barns in Minnesota: A descriptive study. Appl. Eng. Agric. 2007, 23, 231–238. [Google Scholar] [CrossRef]
- Klopčič, M.; Erjavec, K.; Waldrop, M.; Roosen, J.; Engel, P.; Galama, P.; Kuipers, A. Consumers’ and farmers’ perceptions in Europe regarding the use of composted bedding material from cattle. Sustainability 2020, 13, 5128. [Google Scholar] [CrossRef]
- Silva, K.H.; Teixeira, M.C.; Stracke, M.P.; Girardello, V.C.; Knob, D.; Lenz, R.L.S.; Huber, E.; Backes, T.R. Evaluation of the compost barn system of milk producers from cooperatives in the mission region. Braz. J. Dev. 2021, 7, 27227–27241. [Google Scholar] [CrossRef]
- Janni, K.A.; Endres, M.I.; Reneau, J.K.; Schoper, W.W. Compost dairy barn layout and management recommendations. Appl. Eng. Agric. 2007, 23, 97–102. [Google Scholar] [CrossRef]
- Leso, L.; Barbari, M.; Lopes, M.A.; Damasceno, F.A.; Galama, P.; Taraba, J.L.; Kuipers, A. Invited review: Compost-bedded pack barns for dairy cows. J. Dairy Sci. 2020, 103, 1072–1099. [Google Scholar] [CrossRef]
- Fávero, S.; Portilho, F.V.R.; Oliveira, A.C.R.; Langoni, H.; Pantoja, J.C.F. Factors associated with mastitis epidemiologic indexes, animal hygiene, and bulk milk bacterial concentrations in dairy herds housed on compost bedding. Livest. Sci. 2015, 181, 220–230. [Google Scholar] [CrossRef]
- Freu, G.; Garcia, B.L.N.; Tomazi, T.; Di Leo, G.S.; Gheller, L.S.; Bronzo, V.; Moroni, P.; Dos Santos, M.V. Association between mastitis occurrence in dairy cows and bedding characteristics of compost-bedded pack barns. Pathogens 2023, 12, 583. [Google Scholar] [CrossRef]
- Lobeck, K.M.; Endres, M.I.; Janni, K.A.; Godden, S.M.; Fetrow, J. Environmental characteristics and bacterial counts in bedding and milk bulk tank of low profile cross-ventilated, naturally ventilated, and compost bedded pack dairy barns. Appl. Eng. Agric. 2012, 28, 117–128. [Google Scholar] [CrossRef]
- Tomazi, T.; Freu, G.; Alves, B.G.; De Souza Filho, A.F.; Heinemann, M.B.; Dos Santos, M.V. Genotyping and antimicrobial resistance of Streptococcus uberis isolated from bovine clinical mastitis. PLoS ONE 2019, 14, e0223719. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.H.A.; Ribeiro, L.F. Mastites causadas por Escherichia coli, Klebsiella spp. e Streptococcus uberis relacionadas ao sistema de produção Compost Barn e o impacto na qualidade do leite. Rev. GeTeC 2022, 11, 1–18. [Google Scholar]
- Oliveira, C.E.A.; Tinôco, I.d.F.F.; Souza, C.d.F.; Baêta, F.d.C.; Andrade, R.R.; Vieira, F.M.C.; Barbari, M.; Bambi, G. Physicochemical bedding quality in compost-bedded pack barn systems for dairy cows: A systematic review. Appl. Sci. 2023, 13, 9832. [Google Scholar] [CrossRef]
- Mader, T.L.; Davis, M.S.; Brown-Brandl, T. Environmental factors influencing heat stress in feedlot cattle. J. Anim. Sci. 2006, 84, 712–719. [Google Scholar] [CrossRef]
- Minuzzi, R.B.; Caramori, P.H.; Borrozino, E. Tendências na variabilidade climática sazonal e anual das temperaturas máxima e mínima do ar no Estado do Paraná. Bragantia 2011, 70, 471–479. [Google Scholar] [CrossRef]
- Albino, R.L.; Taraba, J.L.; Marcondes, M.I.; Eckelkamp, E.A.; Bewley, J.M. Comparison of bacterial populations in bedding material, on teat ends, and in milk of cows housed in compost bedded pack barns. Anim. Prod. Sci. 2018, 58, 1686. [Google Scholar] [CrossRef]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; Agricultural Chemical; Contaminants; Drugs; AOAC: Arlington, TX, USA, 1990; p. 768. [Google Scholar]
- Marques, R. Caracterização química da fertilidade do solo. In Diagnóstico e Recomendações de Manejo do Solo: Aspectos Teóricos e Metodológicos, 1st ed.; De Lima, M.R., Ed.; UFPR: Curitiba, Brazil, 2004; pp. 99–123. [Google Scholar]
- Dieckow, J.; Mielniczuk, J.; Knicker, H.; Bayer, C.; Dick, D.P.; Kögel-Knabner, I. Comparison of carbon and nitrogen determination methods for samples of a paleudult subjected to no-till cropping systems. Sci. Agric. 2007, 64, 532–540. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Shelford, J.A.; Tucker, C.B.; Weary, D.M.; Von Keyserlingk, M.A. Bacterial populations on teat ends of dairy cows housed in free stalls and bedded with either sand or sawdust. J. Dairy Sci. 2004, 87, 1694–1701. [Google Scholar] [CrossRef]
- Capurro, A.; Aspán, A.; Unnerstad, H.E.; Waller, K.P.; Artursson, K. Identification of potential sources of Staphylococcus aureus in herds with mastitis problems. J. Dairy Sci. 2010, 93, 180–191. [Google Scholar] [CrossRef]
- Godden, S.; Royster, E.; Crooker, B.; Timmerman, J.; Mosca, F.P. Methods of processing recycled manure solids bedding on Midwest dairy farms I: Associations with bedding bacteria counts, milk quality, udder health and milk production. Bov. Pract. 2023, 57, 10–20. [Google Scholar]
- Johnson, R.A.; Wichern, D.V. Applied Multivariate Analysis, 4th ed.; Pearson plc: London, UK, 1998; 799p. [Google Scholar]
- Silveira, R.M.F.; Ferreira, J.; Busanello, M.; De Vasconcelos, A.M.; Valente, F.L.J.; Façanha, D.A.E. Relationship between thermal environment and morphophysiological, performance and carcass traits of Brahman bulls raised on tropical pasture: A canonical approach to a set of indicators. J. Therm. Biol. 2021, 96, 102814. [Google Scholar] [CrossRef]
- De Menezes, J.P.C.; Bertossi, A.P.A.; Santos, A.R.; Neves, M.A. Correlation between land use and groundwater quality. Eng. Sanit. Ambient. 2014, 19, 173–186. [Google Scholar] [CrossRef]
- Dohoo, I.R.; Leslie, K.E. Evaluation of changes in somatic cell counts as indicators of new intramammary infections. Prev. Vet. Med. 1991, 10, 225–237. [Google Scholar] [CrossRef]
- Busanello, M.; Rossi, R.S.; Cassoli, L.D.; Pantoja, J.C.F.; Machado, P.F. Estimation of prevalence and incidence of subclinical mastitis in a large population of Brazilian dairy herds. J. Dairy Sci. 2017, 100, 6545–6553. [Google Scholar] [CrossRef]
- SAS Institute, Inc. SAS OnDemand for Academics. Release 9.04.01M5P09132017; SAS Institute, Inc.: Cary, NC, USA, 2012. [Google Scholar]
- Leso, L.; Ferraz, P.F.P.; Ferraz, G.A.S.; Rossi, G.; Barbari, M. Factors affecting evaporation of water from cattle bedding materials. Biosyst. Eng. 2021, 205, 164–173. [Google Scholar] [CrossRef]
- Bewley, J.; Taraba, J.; Day, G.; Black, R.; Damasceno, F. Compost Bedded Pack Barn Design: Features and Management Considerations; Cooperative Extension Service; University of Kentucky College of Agriculture: Lexington, KY, USA, 2012; p. 32. [Google Scholar]
- Shogor, A.L.B.; Danieli, B.; Savio, R.L. Conhecendo o Compost Barn: Desafios e virtudes. RCPA 2018, 20, 99–104. [Google Scholar]
- Zhang, Q.; Liu, J.; Guo, H.; Li, E.; Yan, Y. Characteristics and optimization of dairy manure composting for reuse as a dairy mattress in areas with large temperature differences. J. Clean Prod. 2019, 232, 1053–1061. [Google Scholar] [CrossRef]
- Oliveira, C.E.A.; Damasceno, F.A.; Ferraz, G.A.S.; Do Nascimento, J.A.C.; Vega, F.A.O.; Tinôco, I.F.F.; Andrade, R.R. Assessment of spatial variability of bedding variables in compost bedded pack barns with climate control system. An. Acad. Bras. Ciências 2021, 93, e20200384. [Google Scholar] [CrossRef]
- Neher, D.A.; Andrews, T.D.; Weicht, T.R.; Hurd, A.; Barlow, J.W. Organic farm bedded pack system microbiomes: A case study with comparisons to similar and different bedded packs. Dairy 2022, 3, 587–607. [Google Scholar] [CrossRef]
- Black, R.A.; Taraba, J.L.; Day, G.B.; Damasceno, F.A.; Bewley, J.M. Compost bedded pack dairy barn management, performance, and producer satisfaction. J. Dairy Sci. 2013, 96, 8060–8074. [Google Scholar] [CrossRef]
- Eckelkamp, E.A.; Taraba, J.L.; Akers, K.A.; Harmon, R.J.; Bewley, J.M. Understanding compost bedded pack barns: Interactions among environmental factors, bedding characteristics, and udder health. Livest. Sci. 2016, 190, 35–42. [Google Scholar] [CrossRef]
- Ferraz, P.F.P.; Ferraz, G.A.S.; Leso, L.; Klopčič, M.; Barbari, M.; Rossi, G. Properties of conventional and alternative bedding materials for dairy cattle. J. Dairy Sci. 2020, 103, 8661–8674. [Google Scholar] [CrossRef] [PubMed]
- Fávero, S.; Portilho, F.V.R.; Oliveira, A.C.R.; Langoni, H.; Pantoja, J.C.F. Longitudinal trends and associations between compost bedding characteristics and bedding bacterial concentrations. J. Agric. Sci. 2015, 7, 58–70. [Google Scholar] [CrossRef]
- Llonch, L.; Castillejos, L.; Mainau, E.; Manteca, X.; Ferret, A. Effect of forest biomass as bedding material on compost-bedded pack performance, microbial content, and behavior of nonlactating dairy cows. J. Dairy Sci. 2020, 103, 10676–10688. [Google Scholar] [CrossRef] [PubMed]
- Kappes, R.; Knob, D.A.; Thaler Neto, A.; Alessio, D.R.M.; Rodrigues, W.B.; Scholz, A.M.; Bonotto, R. Cow’s functional traits and physiological status and their relation with milk yield and milk quality in a compost bedded pack barn system. Rev. Bras. Zootec. 2020, 49, e20190213. [Google Scholar] [CrossRef]
- Weber, C.T.; Schneider, C.L.C.; Busanello, M.; Calgaro, J.L.B.; Fioresi, J.; Gehrke, C.R.; Da Conceição, J.M.; Haygert-Velho, I.M.P. Season effects on the composition of milk produced by a Holstein herd managed under semi-confinement followed by compost bedded dairy barn management. Semin. Cienc. Agrar. 2020, 42, 1667–16782. [Google Scholar] [CrossRef]
- Nogara, K.F.; Busanello, M.; Haygert-Velho, I.M.P.; Zopollatto, M.; Frigeri, K.D.M.; Almeida, P.S.G. Characterization and relationship between bulk tank milk composition and compost bedded variables from dairy barns in Rio Grande do Sul state, Brazil. Turkish J. Vet. Anim. Sci. 2021, 45, 890–900. [Google Scholar] [CrossRef]
- Doska, M.C.; Da Silva, D.F.F.; Horst, J.A.; Valloto, A.A.; Rossi Junior, P.; De Almeida, R. Sources of variation in milk urea nitrogen in Paraná dairy cows. Rev. Bras. Zootec. 2012, 41, 692–697. [Google Scholar] [CrossRef]
- Peixoto, L.A.O.; Brondani, I.L.; Nörnberg, J.L.; Restle, J.; Alves Filho, D.C.; Pazini, M.; Coradini, M.T.; Dos Santos, C.V.M. Perfil metabólico proteico e taxas de concepção de vacas de corte mantidas em pastagem natural ou suplementadas com farelo de trigo com ou sem ureia. Cienc. Rural 2006, 36, 1873–1877. [Google Scholar] [CrossRef]
- Dos Santos, M.V.; Fonseca, L.F.L. Controle de Mastite e Qualidade do Leite—Desafios e Soluções, 1st ed.; Edição Independente dos Autores: Pirassununga, Brazil, 2019; p. 301. [Google Scholar]
- Hutjens, M. Guia de Alimentação de Vacas Leiteiras, 4th ed.; Hoards Dairyman: Atkinson, WI, USA, 2018; p. 101. [Google Scholar]
- Nousiainen, J.; Shingfield, K.J.; Huhtanen, P. Evaluation of milk urea nitrogen as a diagnostic of protein feeding. J. Dairy Sci. 2004, 87, 386–398. [Google Scholar] [CrossRef]
- Meyer, P.M.; Machado, P.F.; Coldebella, A.; Cassoli, L.D.; Coelho, K.O.; Rodrigues, P.H.M. Fatores não-nutricionais e concentração de nitrogênio uréico no leite de vacas da raça Holandesa. Rev. Bras. Zootec. 2006, 35, 1114–1121. [Google Scholar] [CrossRef]
- Rodriguez-Venegas, R.; Meza-Herrera, C.A.; Robles-Trillo, P.A.; Angel-Garcia, O.; Rivas-Madero, J.S.; Rodriguez-Martínez, R. Heat Stress Characterization in a dairy cattle intensive production cluster under arid land conditions: An annual, seasonal, daily, and minute-to-minute, big data approach. Agriculture 2022, 12, 760. [Google Scholar] [CrossRef]
- Collier, R.J.; Zimbelman, R.B.; Rhoads, R.P.; Rhoads, M.L.; Baumgard, L.H. A re-evaluation of the impact of temperature humidity index (THI) and black globe humidity index (BGHI) on milk production in high producing dairy cows. In Proceedings of the Western Dairy Management Conference, Reno, NV, USA, 9–11 March 2011. [Google Scholar]
- Bohmanova, J.; Misztal, I.; Cole, J.B. Temperature-humidity indices as indicators of milk production losses due to heat stress. J. Dairy Sci. 2007, 90, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- De Vliegher, S.; Ohnstad, I.; Piepers, S. Management and prevention of mastitis: A multifactorial approach with a focus on milking, bedding and data-management. J. Integr. Agric. 2018, 17, 1214–1233. [Google Scholar] [CrossRef]
- Borges, E.C.; Da Silva, L.C.; De Alencar, S.M.; De Aguiar, C.L. Caracterização química de extratos etanólicos de própolis com atividade inibitória do crescimento de estafilococos isolados de mastite bovina. Rev. Bras. Tecnol. Agroind. 2014, 8, 1–14. [Google Scholar] [CrossRef]
- Alves, T.; Moreira, M.A.S. Mastite bovina: Tratamento convencional e ação de compostos extraídos de plantas. Uniciências 2021, 25, 20–25. [Google Scholar] [CrossRef]
- Zadoks, R.N.; Tikofsky, L.L.; Boor, K.J. Ribotyping of Streptococcus uberis from a dairy’s environment, bovine feces and milk. Vet. Microbiol. 2005, 109, 257–265. [Google Scholar] [CrossRef]
- Picoli, T.; Zani, J.L.; Bandeira, F.d.S.; Roll, V.F.B.; Ribeiro, M.E.R.; Vargas, G.D.; Hübner, S.d.O.; De Lima, M.; Meireles, M.C.A.; Fischer, G. Handling milking as a risk factor in the occurrence of microorganisms in raw milk. Semin. Cienc. Agrar. 2014, 35, 2471–2480. [Google Scholar] [CrossRef]
- Sherwin, V.E.; Green, M.J.; Leigh, J.A.; Egan, S.A. Assessment of the prevalence of Streptococcus uberis in dairy cow feces and implications for herd health. J. Dairy Sci. 2021, 104, 12042–12052. [Google Scholar] [CrossRef]

| Farm | Lactating Cows (n) | Breed | Stocking Density (m2/cow) | Bedding Type | Tilling Frequency (Times/Day) | Tillage Equipment | Fan Type |
|---|---|---|---|---|---|---|---|
| A | 200 ± 17.09 | Holstein | 9.04 | Sawdust and shavings | 2 | Rotating hoe | HVLS 1 |
| B | 123.80 ± 3.56 | Holstein | 11.26 | Sawdust and shavings | 2 | Rotating hoe and Cultivator | LVHS 2 |
| C | 237 ± 15.29 | Holstein | 16.13 | Sawdust and shavings | 2 | Rotating hoe | LVHS |
| D | 125.86 ± 4.49 | Jersey | 8.47 | Sawdust and shavings | 2 | Cultivator | - |
| E | 69.80 ± 8.58 | Holstein | 12.60 | Sawdust and shavings | 2 | Cultivator | LVHS |
| F | 174.57 ± 10.47 | Holstein | 13.10 | Sawdust and shavings | 2 | Rotating hoe and Cultivator | HVLS |
| G | 92.75 ± 7.80 | Holstein | 14.40 | Sawdust and shavings | 2 | Rotating hoe | LVHS |
| H | 108 ± 9.84 | Jersey | 13.81 | Sawdust and shavings | 2 | Rotating hoe | LVHS |
| Mean | 141.67 ± 57.35 | - | 12.35 | - | 2 | - | - |
| Variable | Category 1 | N 2 | Minimum | Mean | Median | Maximum |
|---|---|---|---|---|---|---|
| SCC 3 (×103 cells/mL) | Milk | 48 | 129.67 | 281.79 | 232.67 | 599.67 |
| SPC 4 (×103 cfu/mL) | 48 | 3.40 | 10.54 | 7.23 | 48.29 | |
| Fat (%) | 48 | 3.34 | 3.97 | 3.86 | 4.74 | |
| Protein (%) | 48 | 3.03 | 3.36 | 3.30 | 3.75 | |
| Total solids (%) | 48 | 12.12 | 12.91 | 12.79 | 14.04 | |
| MUN 5 (mg/dL) | 48 | 8.63 | 13.57 | 12.59 | 20.10 | |
| Moisture (%) | Bedding | 58 | 29.91 | 56.42 | 59.45 | 71.92 |
| ST 6 (°C) | 57 | 15.00 | 20.91 | 20.55 | 25.02 | |
| T10 7 (°C) | 59 | 18.10 | 30.35 | 29.30 | 46.92 | |
| T20 8 (°C) | 59 | 19.43 | 33.45 | 31.03 | 54.77 | |
| T30 9 (°C) | 58 | 21.98 | 35.08 | 32.84 | 58.86 | |
| pH | 53 | 9.25 | 9.87 | 10.01 | 10.58 | |
| C 10 (%) | 53 | 18.24 | 29.31 | 30.37 | 35.52 | |
| N 11 (%) | 53 | 1.05 | 1.95 | 1.90 | 3.95 | |
| C/N 12 | 53 | 6.92 | 15.41 | 15.71 | 19.40 | |
| Stocking density (m²/cow) | 14 | 5.14 | 12.50 | 13.46 | 16.99 | |
| Presumptive staphylococci (log10 cfu/mL) | Bedding microbiology counting | 58 | 4.60 | 6.33 | 6.28 | 9.38 |
| SSLOs 13 (log10 cfu/mL) | 58 | 4.95 | 6.33 | 6.30 | 8.84 | |
| Gram-negative bacteria (log10 cfu/mL) | 58 | 1.49 | 5.09 | 5.20 | 7.15 | |
| Total aerobic bacteria (log10 cfu/mL) | 58 | 6.00 | 7.58 | 7.53 | 9.86 | |
| Temperature (°C) | Microclimate | 48 | 16.32 | 19.71 | 20.46 | 22.30 |
| Relative humidity (%) | 48 | 69.54 | 80.04 | 80.44 | 89.30 | |
| Wind speed (m/s) | 29 | 1.10 | 3.25 | 2.60 | 6.50 |
| Canonical Correlation | (rc) 1 | 2 | Proportion | p-Value |
|---|---|---|---|---|
| Milk β 3 × Bedding € 4 | 0.8236 | 0.6787 | 0.6271 | 0.0004 |
| Milk β × Microbiology € 5 | 0.5131 | 0.2633 | 0.5459 | 0.2606 |
| Milk β × Microclimate € 6 | 0.5487 | 0.3011 | 0.7691 | 0.1055 |
| Bedding β × Microbiology € | 0.5377 | 0.2887 | 0.5496 | 0.4779 |
| Bedding β × Microclimate € | 0.6697 | 0.4486 | 0.7750 | 0.0222 |
| Microbiology β × Microclimate € | 0.3249 | 0.1056 | 0.8159 | 0.6788 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogara, K.F.; Busanello, M.; Tavares, Q.G.; De Assis, J.A.; Freu, G.; Dos Santos, M.V.; Vieira, F.M.C.; Zopollatto, M. Factors Influencing Milk Quality and Subclinical Mastitis in Dairy Herds Housed in Compost-Bedded Pack Barn System. Animals 2023, 13, 3638. https://doi.org/10.3390/ani13233638
Nogara KF, Busanello M, Tavares QG, De Assis JA, Freu G, Dos Santos MV, Vieira FMC, Zopollatto M. Factors Influencing Milk Quality and Subclinical Mastitis in Dairy Herds Housed in Compost-Bedded Pack Barn System. Animals. 2023; 13(23):3638. https://doi.org/10.3390/ani13233638
Chicago/Turabian StyleNogara, Karise Fernanda, Marcos Busanello, Queila Gouveia Tavares, Juliana Aparecida De Assis, Gustavo Freu, Marcos Veiga Dos Santos, Frederico Márcio Corrêa Vieira, and Maity Zopollatto. 2023. "Factors Influencing Milk Quality and Subclinical Mastitis in Dairy Herds Housed in Compost-Bedded Pack Barn System" Animals 13, no. 23: 3638. https://doi.org/10.3390/ani13233638
APA StyleNogara, K. F., Busanello, M., Tavares, Q. G., De Assis, J. A., Freu, G., Dos Santos, M. V., Vieira, F. M. C., & Zopollatto, M. (2023). Factors Influencing Milk Quality and Subclinical Mastitis in Dairy Herds Housed in Compost-Bedded Pack Barn System. Animals, 13(23), 3638. https://doi.org/10.3390/ani13233638








