Cytological Analysis of Crossover Frequency and Distribution in Male Meiosis of Cardueline Finches (Fringillidae, Aves)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
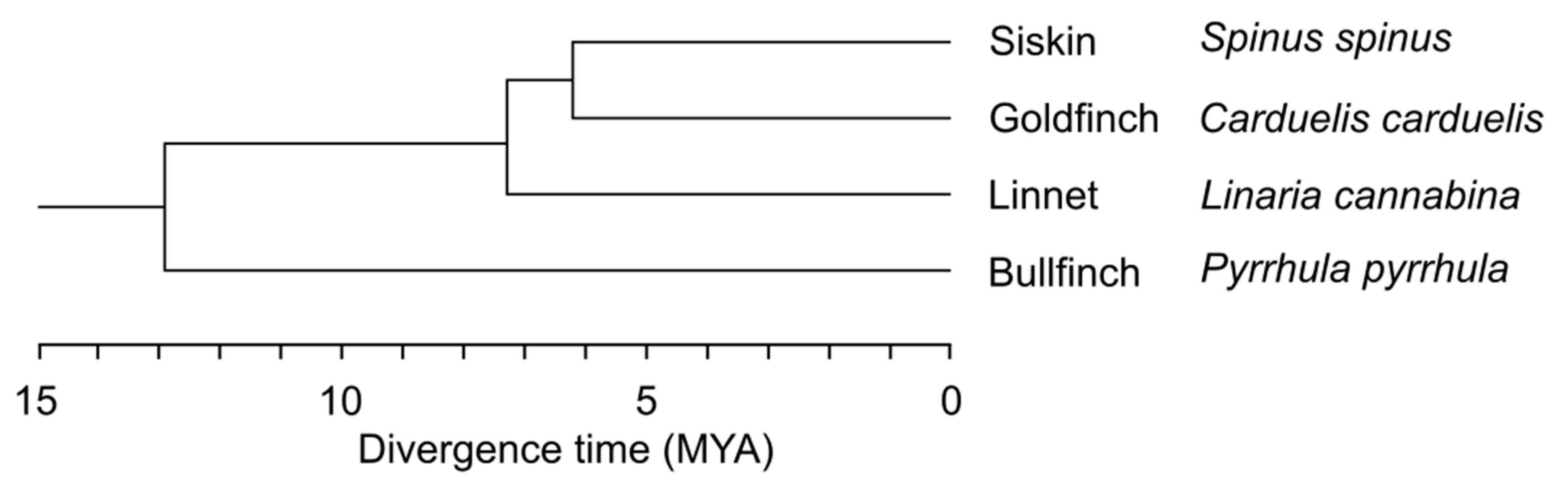
2.1. Specimens
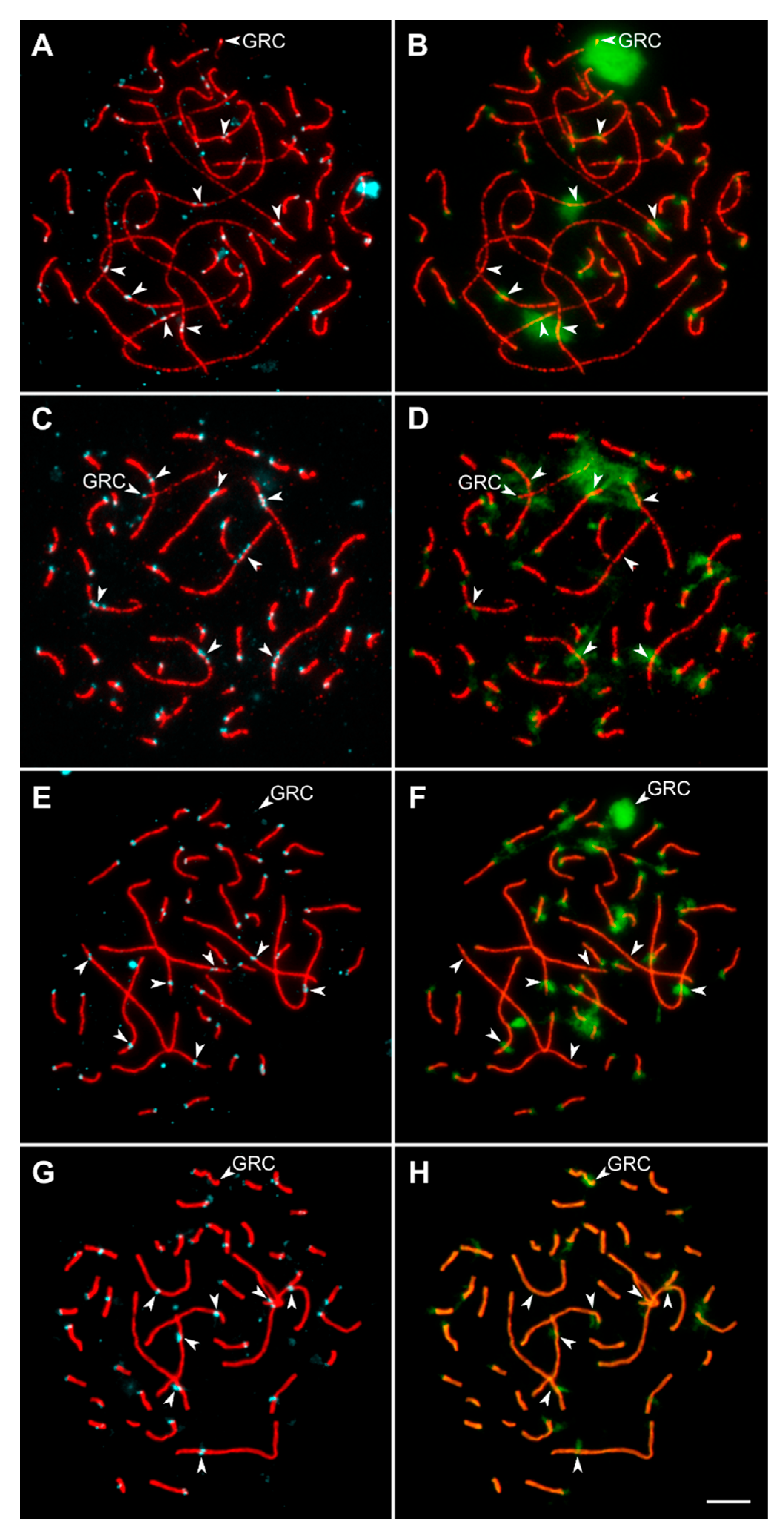
2.2. Synaptonemal Complex Spreading and Immunostaining
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
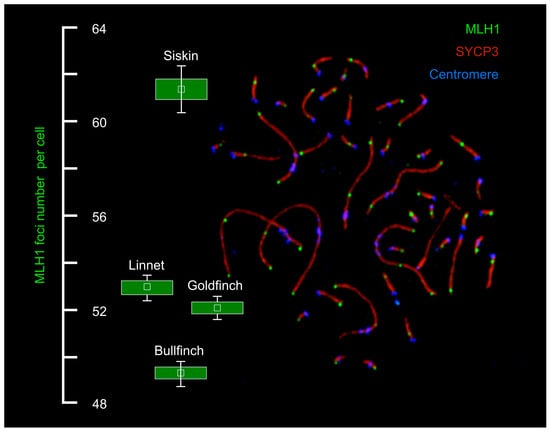
3.1. Total Recombination Rate per Cell
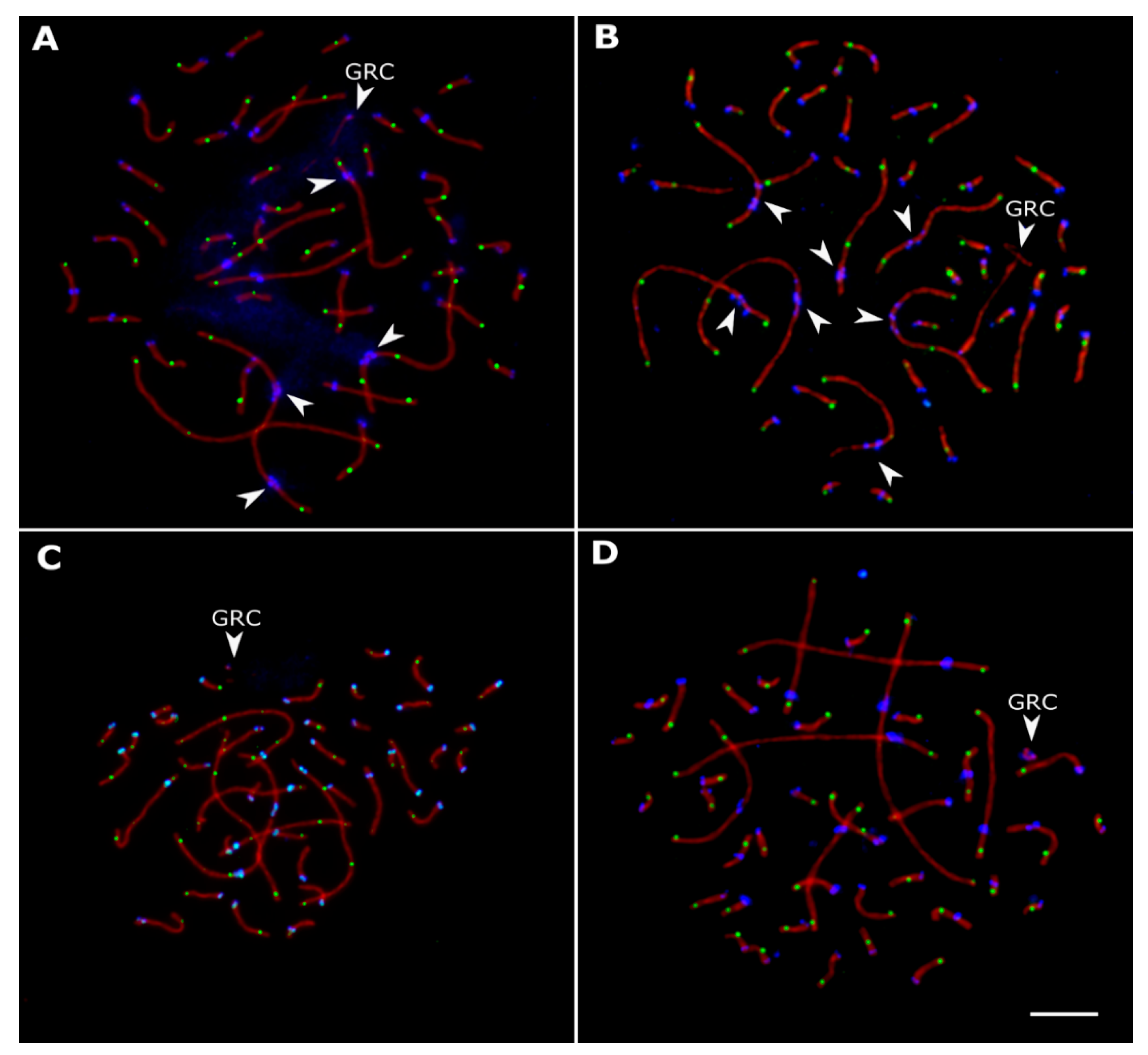
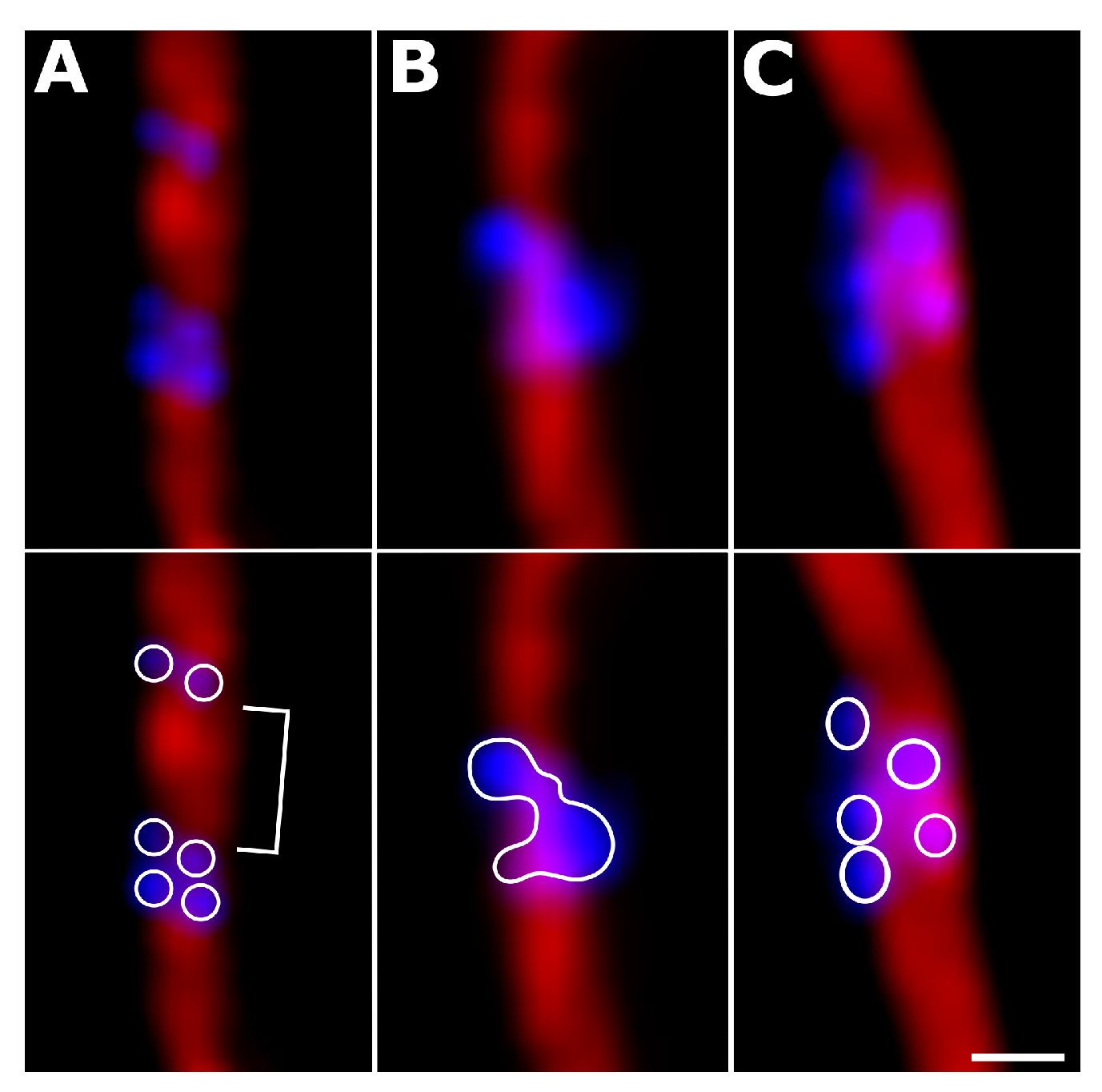
3.2. Variations in Centromere Structure
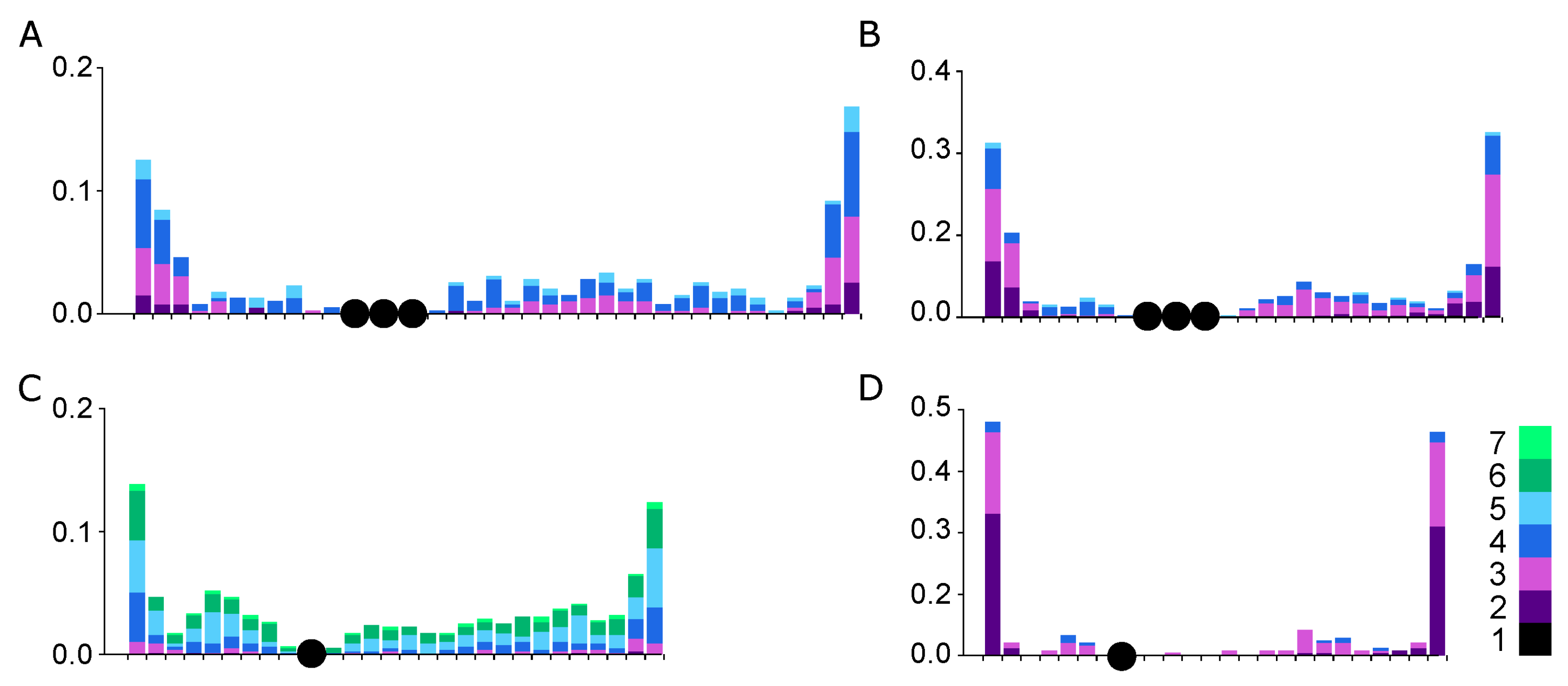
3.3. Recombination Distribution along SC1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coop, G.; Przeworski, M. An evolutionary view of human recombination. Nat. Rev. Genet. 2006, 8, 23–34. [Google Scholar] [CrossRef]
- Ritz, K.R.; Noor, M.A.F.; Singh, N.D. Variation in recombination rate: Adaptive or not? Trends Genet. 2017, 33, 364–374. [Google Scholar] [CrossRef]
- Stapley, J.; Feulner, P.G.D.D.; Johnston, S.E.; Santure, A.W.; Smadja, C.M. Variation in recombination frequency and distribution across eukaryotes: Patterns and processes. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160455. [Google Scholar] [CrossRef]
- Kumar, R.; de Massy, B. Initiation of meiotic recombination in mammals. Genes 2010, 1, 521–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, B.; Jiang, J.; Li, J.; Ma, L.; Lamb, N.E.; Sherman, S.L.; Hassold, T.J.; Tempest, H.G.; Ma, S.; et al. Effect of sex, age and genetics on crossover interference in cattle. Sci. Rep. 2016, 6, 37698. [Google Scholar] [CrossRef] [PubMed]
- Zickler, D.; Kleckner, N. Recombination, pairing, and synapsis of homologs during meiosis. Cold Spring Harb. Perspect. Biol. 2015, 7, a016626. [Google Scholar] [CrossRef] [PubMed]
- Grey, C.; Baudat, F.; de Massy, B. Genome-wide control of the distribution of meiotic recombination. PLoS Biol. 2009, 7, 327–339. [Google Scholar] [CrossRef]
- Singhal, S.; Leffler, E.M.; Sannareddy, K.; Turner, I.; Venn, O.; Hooper, D.M.; Strand, A.I.; Li, Q.Y.; Raney, B.; Balakrishnan, C.N.; et al. Stable recombination hotspots in birds. Science 2015, 350, 928–932. [Google Scholar] [CrossRef]
- Paul, P.; Nag, D.; Chakraborty, S. Recombination hotspots: Models and tools for detection. DNA Repair (Amst.) 2016, 40, 47–56. [Google Scholar] [CrossRef]
- Phadnis, N.; Hyppa, R.W.; Smith, G.R. New and old ways to control meiotic recombination. Trends Genet. 2011, 27, 411–421. [Google Scholar] [CrossRef]
- Storey, A.J.; Wang, H.-P.; Protacio, R.U.; Davidson, M.K.; Tackett, A.J.; Wahls, W.P. Chromatin-mediated regulators of meiotic recombination revealed by proteomics of a recombination hotspot. Epigenetics Chromatin 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Sardell, J.M.; Kirkpatrick, M. Sex differences in the recombination landscape. Am. Nat. 2020, 195, 361–379. [Google Scholar] [CrossRef]
- Pazhayam, N.M.; Turcotte, C.A.; Sekelsky, J. Meiotic crossover patterning. Front. Cell Dev. Biol. 2021, 9, 681123. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.R.G. A possible interpretation of the apparent interference across the centromere found by Callan and Montalenti in Culex pipiens. Heredity 1949, 3, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Berchowitz, L.E.; Copenhaver, G.P. Genetic interference: Don’t stand so close to me. Curr. Genomics 2010, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, M.; Smith, G.R. Repression of harmful meiotic recombination in centromeric regions. Semin. Cell Dev. Biol. 2016, 54, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Dumont, B.L.; Payseur, B.A. Evolution of the genomic rate of recombination in mammals. Evolution 2008, 62, 276–294. [Google Scholar] [CrossRef]
- Segura, J.; Ferretti, L.; Ramos-Onsins, S.; Capilla, L.; Farré, M.; Reis, F.; Oliver-Bonet, M.; Fernández-Bellón, H.; Garcia, F.; Garcia-Caldés, M.; et al. Evolution of recombination in eutherian mammals: Insights into mechanisms that affect recombination rates and crossover interference. Proc. R. Soc. B Biol. Sci. 2013, 280, 1945. [Google Scholar] [CrossRef]
- Capilla, L.; Garcia Caldés, M.; Ruiz-Herrera, A. Mammalian meiotic recombination: A toolbox for genome evolution. Cytogenet. Genome Res. 2016, 150, 1–6. [Google Scholar] [CrossRef]
- Dapper, A.L.; Payseur, B.A. Molecular evolution of the meiotic recombination pathway in mammals. Evolution 2019, 73, 2368–2389. [Google Scholar] [CrossRef]
- Pigozzi, M.I. The chromosomes of birds during meiosis. Cytogenet. Genome Res. 2016, 150, 128–138. [Google Scholar] [CrossRef]
- Semenov, G.A.; Basheva, E.A.; Borodin, P.M.; Torgasheva, A.A. High rate of meiotic recombination and its implications for intricate speciation patterns in the white wagtail (Motacilla alba). Biol. J. Linn. Soc. 2018, 125, 600–612. [Google Scholar] [CrossRef]
- Malinovskaya, L.P.; Tishakova, K.; Shnaider, E.P.; Borodin, P.M.; Torgasheva, A.A. Heterochiasmy and sexual dimorphism: The case of the barn swallow (Hirundo rustica, Hirundinidae, Aves). Genes 2020, 11, 1119. [Google Scholar] [CrossRef]
- Griffin, D.; Burt, D.W. All chromosomes great and small: 10 years on. Chromosom. Res. 2014, 22, 1–6. [Google Scholar] [CrossRef]
- Damas, J.; Kim, J.; Farré, M.; Griffin, D.K.; Larkin, D.M. Reconstruction of avian ancestral karyotypes reveals differences in the evolutionary history of macro- and microchromosomes. Genome Biol. 2018, 19, 155. [Google Scholar] [CrossRef]
- Kiazim, L.G.; O’Connor, R.E.; Larkin, D.M.; Romanov, M.N.; Narushin, V.G.; Brazhnik, E.A.; Griffin, D.K. Comparative mapping of the macrochromosomes of eight avian species provides further insight into their phylogenetic relationships and avian karyotype evolution. Cells 2021, 10, 362. [Google Scholar] [CrossRef]
- Bravo, G.A.; Schmitt, C.J.; Edwards, S.V. What have we learned from the first 500 avian genomes? Annu. Rev. Ecol. Evol. Syst. 2021, 52, 611–639. [Google Scholar] [CrossRef]
- Zhang, G.; Li, C.; Li, Q.; Li, B.; Larkin, D.M.; Lee, C.; Storz, J.F.; Antunes, A.; Greenwold, M.J.; Meredith, R.W.; et al. Comparative genomics reveals insights into avian genome evolution and adaptation. Science 2014, 346, 1311–1320. [Google Scholar] [CrossRef]
- Malinovskaya, L.P.; Slobodchikova, A.Y.; Grishko, E.O.; Pristyazhnyuk, I.E.; Torgasheva, A.A.; Borodin, P.M. Germline-restricted chromosomes and autosomal variants revealed by pachytene karyotyping of 17 avian species. Cytogenet. Genome Res. 2022, 162, 148–160. [Google Scholar] [CrossRef]
- Neumann, P.; Navrátilová, A.; Schroeder-Reiter, E.; Koblížková, A.; Steinbauerová, V.; Chocholová, E.; Novák, P.; Wanner, G.; Macas, J. Stretching the rules: Monocentric chromosomes with multiple centromere domains. PLoS Genet. 2012, 8, e1002777. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.M.; Price, T.D. Chromosomal inversion differences correlate with range overlap in passerine birds. Nat. Ecol. Evol. 2017, 1, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Dapper, A.L.; Payseur, B.A. Connecting theory and data to understand recombination rate evolution. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160469. [Google Scholar] [CrossRef]
- Neupane, S.; Xu, S. Adaptive divergence of meiotic recombination rate in ecological speciation. Genome Biol. Evol. 2020, 12, 1869–1881. [Google Scholar] [CrossRef]
- Peters, A.H.F.M.; Plug, A.W.; van Vugt, M.J.; de Boer, P.; de Boer, P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosom. Res. 1997, 5, 66–68. [Google Scholar] [CrossRef]
- Anderson, L.K.; Reeves, A.; Webb, L.M.; Ashley, T. Distribution of crossing over on mouse synaptonemal complexes using immunofluorescent localization of MLH1 protein. Genetics 1999, 151, 1569–1579. [Google Scholar] [CrossRef]
- Reeves, A. MicroMeasure: A new computer program for the collection and analysis of cytogenetic data. Genome 2001, 44, 439–443. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28–30 June 2010; pp. 92–96. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Torgasheva, A.A.; Malinovskaya, L.P.; Zadesenets, K.S.; Karamysheva, T.V.; Kizilova, E.A.; Akberdina, E.A.; Pristyazhnyuk, I.E.; Shnaider, E.P.; Volodkina, V.A.; Saifitdinova, A.F.; et al. Germline-restricted chromosome (GRC) is widespread among songbirds. Proc. Natl. Acad. Sci. USA 2019, 116, 11845–11850. [Google Scholar] [CrossRef]
- Gržan, T.; Despot-Slade, E.; Meštrović, N.; Plohl, M.; Mravinac, B. CenH3 distribution reveals extended centromeres in the model beetle Tribolium castaneum. PLoS Genet. 2020, 16, e1009115. [Google Scholar] [CrossRef]
- Drpic, D.; Almeida, A.C.; Aguiar, P.; Larkin, D.M.; Khodjakov, A.; Maiato, H. Chromosome segregation is biased by kinetochore size. Curr. Biol. 2018, 28, 1344–1356.e5. [Google Scholar] [CrossRef]
- Christidis, L. Animal Cytogenetics 4: Chordata 3; B, Aves. Animal Cytogenetics; Gebr üder Borntraeger: Stuttgart, Germany, 1990; ISBN 9783443260149. [Google Scholar]
- dos Santos, M.d.S.; Kretschmer, R.; Frankl-Vilches, C.; Bakker, A.; Gahr, M.; O’Brien, P.C.M.; Ferguson-Smith, M.A.; de Oliveira, E.H.C. Comparative cytogenetics between two important songbird, models: The zebra finch and the canary. PLoS ONE 2017, 12, e0170997. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.P.; Payseur, B.A. Crossover interference: Shedding light on the evolution of recombination. Annu. Rev. Genet. 2019, 53, 19–44. [Google Scholar] [CrossRef] [PubMed]
- Borodin, P.M.; Karamysheva, T.V.; Belonogova, N.M.; Torgasheva, A.A.; Rubtsov, N.B.; Searle, J.B. Recombination map of the common shrew, Sorex araneus (Eulipotyphla, Mammalia). Genetics 2008, 178, 621–632. [Google Scholar] [CrossRef]
- Calderón, P.L.; Pigozzi, M.I. MLH1-focus mapping in birds shows equal recombination between sexes and diversity of crossover patterns. Chromosom. Res. 2006, 14, 605–612. [Google Scholar] [CrossRef]
- Backström, N.; Forstmeier, W.; Schielzeth, H.; Mellenius, H.; Nam, K.; Bolund, E.; Webster, M.T.; Öst, T.; Schneider, M.; Kempenaers, B.; et al. The recombination landscape of the zebra finch Taeniopygia guttata genome. Genome Res. 2010, 20, 485–495. [Google Scholar] [CrossRef]
- Kawakami, T.; Mugal, C.F.; Suh, A.; Nater, A.; Burri, R.; Smeds, L.; Ellegren, H. Whole-genome patterns of linkage disequilibrium across flycatcher populations clarify the causes and consequences of fine-scale recombination rate variation in birds. Mol. Ecol. 2017, 26, 4158–4172. [Google Scholar] [CrossRef]
- Malinovskaya, L.; Shnaider, E.; Borodin, P.; Torgasheva, A. Karyotypes and recombination patterns of the Common Swift (Apus apus Linnaeus, 1758) and Eurasian Hobby (Falco subbuteo Linnaeus, 1758). Avian Res. 2018, 9, 4. [Google Scholar] [CrossRef]
- Pigozzi, M.I. Distribution of MLH1 foci on the synaptonemal complexes of chicken oocytes. Cytogenet. Cell Genet. 2001, 95, 129–133. [Google Scholar] [CrossRef]
- The World Bird Database. Available online: https://avibase.bsc-eoc.org (accessed on 27 October 2023).
- Lisachov, A.P.; Zadesenets, K.S.; Rubtsov, N.B.; Borodin, P.M. Sex chromosome synapsis and recombination in male guppies. Zebrafish 2015, 12, 174–180. [Google Scholar] [CrossRef]
- Lisachov, A.P.; Trifonov, V.A.; Giovannotti, M.; Ferguson-Smith, M.A.; Borodin, P.M.P.M. Immunocytological analysis of meiotic recombination in two anole lizards (Squamata, Dactyloidae). Comp. Cytogenet. 2017, 11, 129–141. [Google Scholar] [CrossRef]
- Zickler, D.; Kleckner, N. A few of our favorite things: Pairing, the bouquet, crossover interference and evolution of meiosis. Semin. Cell Dev. Biol. 2016, 54, 135–148. [Google Scholar] [CrossRef]
- Lisachov, A.P.; Trifonov, V.A.; Giovannotti, M.; Ferguson-Smith, M.A.; Borodin, P.M. Heteromorphism of “homomorphic” sex chromosomes in two anole species (Squamata, Dactyloidae) revealed by synaptonemal complex analysis. Cytogenet. Genome Res. 2017, 151, 89–95. [Google Scholar] [CrossRef]
- Vozdova, M.; Sebestova, H.; Kubickova, S.; Cernohorska, H.; Vahala, J.; Rubes, J. A comparative study of meiotic recombination in cattle (Bos taurus) and three wildebeest species (Connochaetes gnou, C. taurinus taurinus and C. t. albojubatus). Cytogenet. Genome Res. 2013, 140, 36–45. [Google Scholar] [CrossRef]
- Gorlov, I.P.; Ladygina, T.Y.; Serov, O.L.; Borodin, P.M. Positional control of chiasma distribution in the house mouse. Chiasma distribution in mice homozygous and heterozygous for an inversion in chromosome 1. Heredity (Edinb.) 1991, 66, 453–458. [Google Scholar] [CrossRef][Green Version]
- Beadle, G.W. A Possible Influence of the Spindle Fibre on Crossing-Over in Drosophila. Proc. Natl. Acad. Sci. USA 1932, 18, 160–165. [Google Scholar] [CrossRef]
- Mather, K. Crossing over and heterochromatin in the X chromosome of Drosophila melanogaster. Genetics 1939, 24, 413. [Google Scholar] [CrossRef]
- Roberts, P.A. Difference in the behaviour of eu- and hetero-chromatin: Crossing-over. Nature 1965, 205, 725–726. [Google Scholar] [CrossRef]
- Rick, C.M. Controlled introgression of chromosomes of Solanum pennellii into Lycopersicon esculentum: Segregation and recombination. Genetics 1969, 62, 753–768. [Google Scholar] [CrossRef]
- Khush, G.S.; Rick, C.M. Studies on the linkage map of chromosome 4 of the tomato and on the transmission of induced deficiencies. Genetica 1967, 38, 74–94. [Google Scholar] [CrossRef]
- Fowler, K.R.; Sasaki, M.; Milman, N.; Keeney, S.; Smith, G.R. Evolutionarily diverse determinants of meiotic DNA break and recombination landscapes across the genome. Genome Res. 2014, 24, 1650–1664. [Google Scholar] [CrossRef][Green Version]
- Ludin, K.; Mata, J.; Watt, S.; Lehmann, E.; Bähler, J.; Kohli, J. Sites of strong Rec12/Spo11 binding in the fission yeast genome are associated with meiotic recombination and with centromeres. Chromosoma 2008, 117, 431–444. [Google Scholar] [CrossRef][Green Version]
- Yelina, N.E.; Lambing, C.; Hardcastle, T.J.; Zhao, X.; Santos, B.; Henderson, I.R. DNA methylation epigenetically silences crossover hot spots and controls chromosomal domains of meiotic recombination in Arabidopsis. Genes Dev. 2015, 29, 2183–2202. [Google Scholar] [CrossRef]
- Papazyan, R.; Voronina, E.; Chapman, J.R.; Luperchio, T.R.; Gilbert, T.M.; Meier, E.; Mackintosh, S.G.; Shabanowitz, J.; Tackett, A.J.; Reddy, K.L.; et al. Methylation of histone H3K23 blocks DNA damage in pericentric heterochromatin during meiosis. eLife 2014, 3, 2996. [Google Scholar] [CrossRef]


| Species | N Birds | N Cells | MLH1 Focus Number per Cell | Total SC Length (µm) |
|---|---|---|---|---|
| Linnet | 3 | 114 | 52.9 ± 2.8 | 313.3 ± 60.1 |
| Bullfinch | 4 | 177 | 49.5 ± 3.5 | 219.8 ± 41.3 |
| Siskin | 3 | 163 | 61.5 ± 6.3 | 245.1 ± 50.9 |
| Goldfinch | 3 | 119 | 52.2 ± 2.7 | 209.5 ± 28.8 |
| Species | N Cells | Relative Length of the SC1 * | Centromeric Index | SC Arm | Mean MLH1 Focus Number | SC Length, μm | Mean Relative Distance ** between Neighboring MLH1 Foci |
|---|---|---|---|---|---|---|---|
| Linnet | 114 | 0.11 ± 0.01 | 0.33 ± 0.04 | p | 1.2 ± 0.4 | 10.7 ± 2.8 | 0.57 ± 0.02 |
| q | 2.2 ± 0.7 | 22.9 ± 6.0 | 0.43 ± 0.03 | ||||
| Bullfinch | 164 | 0.11 ± 0.01 | 0.37 ± 0.03 | p | 1.2 ± 0.4 | 8.1 ± 1.9 | 0.51 ± 0.02 |
| q | 1.7 ± 0.6 | 14.3 ± 2.8 | 0.50 ± 0.03 | ||||
| Siskin | 157 | 0.11 ± 0.01 | 0.34 ± 0.03 | p | 1.9 ± 0.6 | 9.1 ± 2.3 | 0.45 ± 0.02 |
| q | 2.9 ± 0.9 | 18.1 ± 4.8 | 0.31 ± 0.02 | ||||
| Goldfinch | 102 | 0.11 ± 0.01 | 0.32 ± 0.03 | p | 1.1 ± 0.3 | 7.4 ± 1.5 | 0.58 ± 0.02 |
| q | 1.4 ± 0.6 | 15.8 ± 3.6 | 0.39 ± 0.03 |
| Species | R within the Arm | R across the Centromere |
|---|---|---|
| Linnet | 0.60 * | 0.03 |
| Bullfinch | 0.30 * | 0.09 |
| Siskin | 0.70 * | −0.01 |
| Goldfinch | 0.25 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grishko, E.; Malinovskaya, L.; Slobodchikova, A.; Kotelnikov, A.; Torgasheva, A.; Borodin, P. Cytological Analysis of Crossover Frequency and Distribution in Male Meiosis of Cardueline Finches (Fringillidae, Aves). Animals 2023, 13, 3624. https://doi.org/10.3390/ani13233624
Grishko E, Malinovskaya L, Slobodchikova A, Kotelnikov A, Torgasheva A, Borodin P. Cytological Analysis of Crossover Frequency and Distribution in Male Meiosis of Cardueline Finches (Fringillidae, Aves). Animals. 2023; 13(23):3624. https://doi.org/10.3390/ani13233624
Chicago/Turabian StyleGrishko, Ekaterina, Lyubov Malinovskaya, Anastasia Slobodchikova, Artemiy Kotelnikov, Anna Torgasheva, and Pavel Borodin. 2023. "Cytological Analysis of Crossover Frequency and Distribution in Male Meiosis of Cardueline Finches (Fringillidae, Aves)" Animals 13, no. 23: 3624. https://doi.org/10.3390/ani13233624
APA StyleGrishko, E., Malinovskaya, L., Slobodchikova, A., Kotelnikov, A., Torgasheva, A., & Borodin, P. (2023). Cytological Analysis of Crossover Frequency and Distribution in Male Meiosis of Cardueline Finches (Fringillidae, Aves). Animals, 13(23), 3624. https://doi.org/10.3390/ani13233624