Communities of Uropodina (Acari: Mesostigmata) in Nest Boxes Inhabited by Dormice (Glis glis and Muscardinus avellanarius) and Differences in Percentages of Nidicoles in Nests of Various Hosts
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Uropodina Communities in Nests of Species from Gliridae Family
3.2. Frequency and Abundance of Nidicoles Leiodinychus Orbicularis and Apionoseius Infirmus in Material from Nests of Different Hosts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polechová, J.; Storch, D. Ecological Niche. Encycl. Ecol. 2019, 3, 72–80. [Google Scholar]
- Aitken, K.E.H.; Martin, K. The importance of excavators in hole-nesting communities: Availability and use of natural tree holes in old mixed forests of western Canada. J. Ornithol. 2007, 148 (Suppl. S2), 425–434. [Google Scholar] [CrossRef]
- Cockle, K.L.; Martin, K.; Robledo, G. Linking fungi, trees, and hole-using birds in a Neotropical tree-cavity network: Pathways of cavity production and implications for conservation. For. Ecol. Manag. 2012, 264, 210–219. [Google Scholar] [CrossRef]
- Walankiewicz, W.; Czeszczewik, D.; Stański, T.; Sahel, M.; Ruczyński, I. Tree cavity resources in spruce-pine managed and protected stands of the Białowieża Forest, Poland. Nat. Areas J. 2014, 34, 423–428. [Google Scholar] [CrossRef]
- Zawadzka, D. Dziuple w ekosystemach leśnych: Formowanie, rozmieszczenie, znaczenie ekologiczne i wskazania ochronne. Sylwan 2018, 162, 509–520. [Google Scholar] [CrossRef]
- Morris, P.A.; Bright, P.W.; Woods, D. Use of nestboxes by the dormouse Muscardinus avellanarius. Biol. Conserv. 1990, 51, 1–13. [Google Scholar] [CrossRef]
- Koppmann-Rumpf, B.; Heberer, C.; Schmidt, K.H. Long term study of the reaction of the edible dormouse Glis glis (Rodentia: Gliridae) to climatic changes and its interactions with hole-breeding passerines. Acta Zool. Acad. Sci. Hung. 2003, 49 (Suppl. S1), 69–76. [Google Scholar]
- Rueegger, N. Bat boxes—A review of their use and application, past, present and future. Acta Chiropt. 2016, 18, 279–299. [Google Scholar] [CrossRef]
- Nordberg, S. Biologisch-ökologische Untersuchungen über die Vogelnidicolen. Acta Zool. Fenn. 1936, 21, 1–168. [Google Scholar]
- Woodroffe, G.E. An ecological study of the insects and mites in the nests of certain birds in Britain. Bull. Entomol. Res. 1953, 44, 739–772. [Google Scholar] [CrossRef]
- McComb, W.C.; Noble, R.E. Invertebrate use of natural tree cavities and vertebrate nest boxes. Am. Midl. Nat. 1982, 107, 163–172. [Google Scholar] [CrossRef]
- Tajovský, K.; Mock, A.; Krumpál, M. Millipedes (Diplopoda) in birds’ nests. Eur. J. Soil Biol. 2001, 37, 321–323. [Google Scholar] [CrossRef]
- Turienzo, P.; Di Iorio, O.; Mahnert, V. Global checklist of pseudoscorpions (Arachnida) found in birds’ nests. Rev. Suisse Zool. 2010, 117, 557–598. [Google Scholar]
- Broughton, R.K.; Hebda, G.; Maziarz, M.; Smith, K.W.; Smith, L.; Hinsley, S.A. Nest-site competition between bumblebees (Bombidae), social wasps (Vespidae) and cavity-nesting birds in Britain and the Western Palearctic. Bird Study 2015, 62, 427–437. [Google Scholar] [CrossRef]
- Boyes, D.H.; Lewis, O.T. Ecology of Lepidoptera associated with bird nests in mid-Wales, UK. Ecol. Entomol. 2018, 44, 1–10. [Google Scholar] [CrossRef]
- Jaworski, T.; Gryz, J.; Krauze-Gryz, D.; Plewa, R.; Bystrowski, C.; Dobosz, R.; Horák, J. My home is your home: Nest boxes for birds and mammals provide habitats for diverse insect communities. Insect Conserv. Divers. 2022, 15, 461–469. [Google Scholar] [CrossRef]
- Heeb, P.; Kolliker, M.; Richner, H. Bird-Ectoparasite Interactions, Nest Humidity, and Ectoparasite Community Structure. Ecology 2000, 81, 958–968. [Google Scholar] [CrossRef][Green Version]
- Eeva, T.; Andersson, T.; Berglund, Å.M.; Brommer, J.E.; Hyvönen, R.; Klemola, T.; Laaksonen, T.; Loukola, O.; Morosinotto, C.; Rainio, K.; et al. Species and abundance of ectoparasitic flies (Diptera) in pied flycatcher nests in Fennoscandia. Parasites Vectors 2015, 8, 648. [Google Scholar] [CrossRef] [PubMed]
- Sosnina, E.F. Parasites of Glis glis caspicus Satun. in the Caucasus National Park. Proc. Leningrad. Univ. (Biol.) 1949, 101, 128–144. [Google Scholar]
- Skuratowicz, W.A.; Bartkowska, K. Pchły (Siphonaptera) zebrane w Jugosławii. Fragm. Faunist. 1977, 23, 51–65. [Google Scholar] [CrossRef]
- Merino, S.; Potti, J. Weather dependent effects of nest ectoparasites on their bird hosts. Ecography 1996, 19, 107–113. [Google Scholar] [CrossRef]
- Mašán, P.; Fenda, P. A Review of the Laelapid Mites Associated with Terrestrial Mammals in Slovakia, with a Key to the European Species; Institute of Zoology, Slovak Academy of Sciences: Bratislava, Slovakia, 2010. [Google Scholar]
- Medvedev, S.G.; Tretyakov, K.A. Fleas of small mammals in St. Petersburg. Parazitologiia 2014, 48, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Kirillov, A.A.; Kirillova, N.Y.; Ruchin, A.B. Parasites, Bacteria and Viruses of the Edible Dormouse Glis glis (Rodentia: Gliridae) in the Western Palaearctic. Diversity 2022, 14, 562. [Google Scholar] [CrossRef]
- Błoszyk, J.; Gwiazdowicz, D.J.; Kupczyk, M.; Książkiewicz-Parulska, Z. Parasitic mesostigmatid mites (Acari)—Common inhabitants of the nest boxes of starlings (Sturnus vulgaris) in a Polish urban habitat. Biologia 2016, 71, 1034–1037. [Google Scholar] [CrossRef]
- Cosandey, V.; Séchaud, R.; Béziers, P.; Chittaro, Y.; Sanchez, A.; Roulin, A. Nidicolous beetle species richness is driven by Barn Owl’s nests occupancy and landscape structure. J. Ornithol. 2021, 162, 857–864. [Google Scholar] [CrossRef]
- Hansell, M. Bird Nests and Construction Behaviour; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Roy, L.; Bouvier, J.-C.; Lavigne, C.; Galès, M.; Buronfosse, T. Impact of pest control strategies on the arthropodofauna living in bird nests built in nestboxes in pear and apple orchards. Bull. Entomol. Res. 2013, 103, 458–465. [Google Scholar] [CrossRef]
- Boyes, D.H. Natural history of Lepidoptera associated with bird nests in mid-Wales. Entomol. Rec. J. Var. 2018, 130, 249–259. [Google Scholar]
- Krištofík, J.; Mašán, P.; Šustek, Z.; Nuhličková, S. Arthropods (Acarina, Coleoptera, Siphonaptera) in nests of hoopoe (Upupa epops) in Central Europe. Biologia 2013, 68, 155–161. [Google Scholar] [CrossRef]
- Rendell, W.B.; Verbeek, N.A.M. Are avian ectoparasites more numerous in nest boxes with old nest material? Can. J. Zool. 1996, 74, 1819–1825. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Schatz, B.; Bourgault, P. Interactions between ants and breeding Paridae in two distinct Corsican oak habitats. Folia Zool. 2008, 57, 264–268. [Google Scholar]
- Mašán, P.; Krištofik, J. Mesostigmatid mites (Acarina, Mesostigmata) in the nests of penduline tit (Remiz pendulinus). Biologia 1995, 50, 481–485. [Google Scholar]
- Błoszyk, J.; Olszanowski, J. Materiały do znajomości fauny roztoczy gniazd i budek lęgowych ptaków. II. Różnice w liczebności i składzie gatunkowym populacji Uropodina (Acari, Anactinotrichida) budek lęgowych na Mierzei Wiślanej na podstawie dwuletnich obserwacji. Przegl. Zool. 1986, 30, 63–66. [Google Scholar]
- Błoszyk, J.; Bajaczyk, R. The first record of Phaulodinychus borealis (Sellnick, 1949) phoresy (Acari, Uropodina) associated with fleas (Siphonaptera) in mole nests. In Soil Zoology in Central Europe; AS CR Institute of Soil Biology: České Budějovice, Czech Republic, 1999; pp. 13–17. [Google Scholar]
- Bajerlein, D.; Błoszyk, J.; Gwiazdowicz, D.J.; Ptaszyk, J.; Halliday, B. Community structure and dispersal of mites (Acari, Mesostigmata) in nests of the white stork (Ciconia ciconia). Biologia 2006, 61, 525–530. [Google Scholar] [CrossRef]
- Mašán, P.; Krištofík, J. Mites and ticks (Acarina: Mesostigmata et Ixodida) from the nests of Riparia riparia L. in South Slovakia. Biologia 1993, 48, 155–162. [Google Scholar]
- Gwiazdowicz, D.J.; Błoszyk, J.; Bajerlein, D.; Halliday, R.B.; Mizera, T. Mites (Acari: Mesostigmata) inhabiting nests of the white-tailed sea eagle Haliaeetus albicilla (L.) in Poland. Entomol. Fennica 2006, 17, 366–372. [Google Scholar] [CrossRef]
- Błoszyk, J.; Bajerlein, D.; Gwiazdowicz, D.J.; Halliday, R.B.; Dylewska, M. Uropodine mite communities (Acari: Mesostigmata) in birds’ nests in Poland. Belg. J. Zool. 2006, 136, 145–153. [Google Scholar]
- Błoszyk, J.; Gwiazdowicz, D.J.; Halliday, B.; Dolata, P.T.; Gołdyn, B. Nests of the black stork Ciconia nigra as a habitat for mesostigmatid mites (Acari: Mesostigmata). Biologia 2009, 64, 962–968. [Google Scholar] [CrossRef]
- Błoszyk, J.; Dražina, T.; Gwiazdowicz, D.; Halliday, R.B.; Gołdyn, B.; Napierała, A.; Rybska, E. Mesostigmatic mites (Acari: Mesostigmata) in nests of the Eurasian griffon vulture (Gyps fulvus) in Croatia. Biologia 2011, 66, 335–339. [Google Scholar] [CrossRef]
- Błoszyk, J.; Hebda, G.; Adamski, Z.; Zacharyasiewicz, M. Redescription of Chiropturopoda nidiphila Wiśniewski & Hirschmann (Acari: Uropodina) from a woodpecker’s tree holes, including all development stages and first notes on its ecology. Syst. Appl. Acarol. 2021, 26, 1867–1899. [Google Scholar] [CrossRef]
- Napierała, A.; Maziarz, M.; Hebda, G.; Broughton, R.K.; Rutkowski, T.; Zacharyasiewicz, M.; Błoszyk, J. Lack of specialist nidicoles as a characteristic of mite assemblages inhabiting nests of the ground-nesting wood warbler, Phylloscopus sibilatrix (Aves: Passeriformes). Exp. Appl. Acarol. 2021, 84, 149–170. [Google Scholar] [CrossRef]
- Mašán, P.; Kalúz, S.; Babjaková, A. Mites (Acarina) from the winter nests of the common mole (Talpa europaea L.) in South Slovakia. Biologia 1994, 49, 667–673. [Google Scholar]
- Kurek, P.; Nowakowski, K.; Rutkowski, T.; Ważna, A.; Cichocki, J.; Zacharyasiewicz, M.; Błoszyk, J. Underground diversity: Uropodina mites (Acari: Mesostigmata) from European badger (Meles meles) nests. Exp. Appl. Acarol. 2020, 82, 503–513. [Google Scholar] [CrossRef]
- Napierała, A.; Mądra, A.; Leszczyńska-Deja, K.; Gwiazdowicz, D.J.; Gołdyn, B.; Błoszyk, J. Community structure variability of Uropodina mites (Acari: Mesostigmata) in nests of the common mole, Talpa europaea, in Central Europe. Exp. Appl. Acarol. 2016, 68, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Błoszyk, J.; Rutkowski, T.; Wojtaszyn, G.; Książkiewicz-Parulska, Z.; Zacharyasiewicz, M.; Napierała, A. Leiodinychus orbicularis (CL Koch, 1839) in bat boxes in Poland. Eur. J. Biol. Res. 2020, 10, 150–155. [Google Scholar] [CrossRef]
- Gwiazdowicz, D.J.; Mizera, T.; Skorupski, M. Mites in greater spotted eagle nests. J. Raptor Res. 1999, 33, 257–260. [Google Scholar]
- Gwiazdowicz, D.J.; Mizera, T.; Skorupski, M. Mites (Acari, Gamasida) from the nests of birds of prey in Poland. Buteo 2000, 11, 97–100. [Google Scholar]
- Gwiazdowicz, D.J. Mites (Acari: Mesostigmata) occurring in the nests of birds of prey (Falconiformes) and owls (Strigi formes). Acarina 2003, 11, 235–239. [Google Scholar]
- Gwiazdowicz, D.J. Mites (Acari, Mesostigmata) appearing in Poland, in the birds’s nests of Passeriformes, Falconiformes and Strigiformes orders. In Kształtowanie i Ochrona Środowiska Leśnego; Miler, A.T., Ed.; Wydawnictwo Akademii Rolniczej: Poznań, Poland, 2003; pp. 562–572. [Google Scholar]
- Gwiazdowicz, D.J.; Błoszyk, J.; Mizera, T.; Tryjanowski, P. Mesostigmatic mites eagle nests (Haliaeetus albicilla). J. Raptor Res. 2005, 39, 60–65. [Google Scholar]
- Bloszyk, J.; Gwiazdowicz, D.J.; Bajerlein, D.; Halliday, R.B. Nests of the white stork Ciconia ciconia (L.) as a habitat for mesostigmatic mites (Acari, Mesostigmata). Acta Parasitol. 2005, 50, 171–175. [Google Scholar]
- Kurek, P.; Piechnik, Ł.; Wiatrowska, B.; Ważna, A.; Nowakowski, K.; Pardavila, X.; Cichocki, J.; Seget, B. Badger Meles meles as Ecosystem Engineer and Its Legal Status in Europe. Animals 2022, 12, 898. [Google Scholar] [CrossRef]
- Napierała, A.; Błoszyk, J. Unstable microhabitats (merocenoses) as specific habitats of Uropodina mites (Acari: Mesostigmata). Exp. Appl. Acarol. 2013, 60, 163–180. [Google Scholar] [CrossRef]
- Fenďa, P.; Lengyel, J. Roztoče (Acarina, Mesostigmata) v hniezdach orliaka morského (Haliaeetus albicilla) na Slovensku [Mites (Acarina, Mesostigmata) in nests of the white-tailed sea eagle Haliaeetus albicilla (L.) in Slovakia]. Entomofauna Carpath. 2007, 19, 48–50. [Google Scholar]
- Gwiazdowicz, D.J.; Mizera, M. Preliminary research on mites (Acari, Gamasida) occurring in the pellets of birds of prey and owls. Sci. Pap. Agric. Univ. Pozn. Anim. Sci. 2002, 4, 117–125. [Google Scholar]
- Fedyń, I.; Figarski, T.; Kajtoch, Ł. Overview of the impact of forest habitats quality and landscape disturbances on the ecology and conservation of dormice species. Eur. J. For. Res. 2021, 140, 511–526. [Google Scholar] [CrossRef]
- Schlund, W.; Scharfe, F.; Ganzhorn, J.U. Long-term comparison of food availability and reproduction in the edible dormouse (Glis glis). Mamm. Biol. 2002, 67, 219–232. [Google Scholar] [CrossRef]
- Bakó, B.; Hecker, K. Factors determining the distribution of coexisting dormouse species (Gliridae, Rodentia). Pol. J. Ecol. 2006, 54, 379–386. [Google Scholar]
- Trout, R.C.; Brooks, S.; Morris, P. Nest box usage by old edible dormice (Glis glis) in breeding and non-breeding years. Folia Zool. 2015, 64, 320–324. [Google Scholar] [CrossRef]
- Juškaitis, R. Interactions between dormice (Gliridae) and hole-nesting birds in nestboxes. Folia Zool. 2006, 55, 225–236. [Google Scholar]
- Adamík, P.; Král, M. Nest losses of cavity nesting birds caused by dormice (Gliridae, Rodentia). Acta Theriol. 2008, 53, 185–192. [Google Scholar] [CrossRef]
- Karg, W. Acari (Acarina) Milben, Unterordnung Parasitiformes (Anactinochaeta). Uropodina Kramer, Schildkrötenmilben. In Die Tierwelt Deutschlands und der Angrenzenden Meeresteile; Senglaub, K., Hannemann, H.-J., Schumann, H., Eds.; Gustav Fischer Verlag: Jena, Germany, 1989; Volume 67, p. 203. [Google Scholar]
- Błoszyk, J. Geograficzne i Ekologiczne Zróżnicowanie Zgrupowań Roztoczy z Kohoryty Uropodina (Acari: Mesostigmata) w Polsce: Uropodina Lasów Grądowych (Carpinion betuli), 1st ed.; Kontekst: Poznań, Poland, 1999; p. 245. [Google Scholar]
- Mašan, P. Mites of the Cohort Uropodina (Acarina, Mesostigmata) in Slovakia, 1st ed.; Annotationes Zoologicae et Botanicae: Bratislava, Slovakia, 2001; p. 320. [Google Scholar]
- StatSoft Inc. STATISTICA (Data Analysis Software System), version 12; StatSoft Inc.: Tulsa, OK, USA, 2014. [Google Scholar]
- Radinovsky, S. The biology and ecology of Granary Mites the Pacific Northwest. III. Life history and development of Leiodinychus krameri (Acarina: Uropodidae). Ann. Entomol. Soc. Am. 1965, 58, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Maziarz, M.; Broughton, R.K.; Wesołowski, T. Microclimate in tree cavities and nest-boxes: Implications for hole-nesting birds. For. Ecol. Manag. 2017, 389, 306–313. [Google Scholar] [CrossRef]
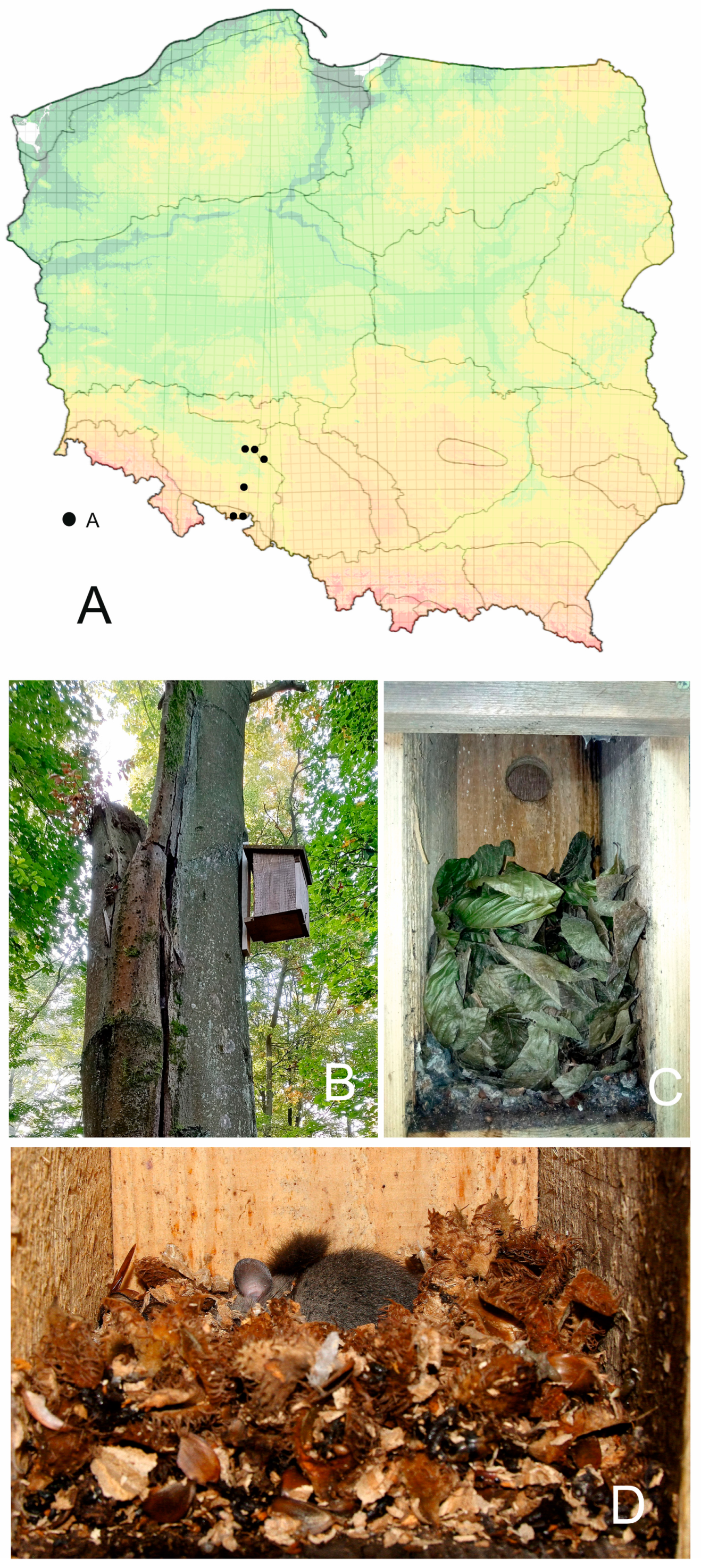
| Study Site; GPS of Central Point of the Study Site | Habitat, Dominated Tree Species; Type of Nest Boxes | Dormice Species: Nest Box Content during Sampling |
|---|---|---|
| Opawskie Mts: Pokrzywna | Deciduous forest, beech; bird nest boxes (19) | Edible dormice: leaves of trees, gnawed beech and oak seeds, droppings |
| GPS: 50.2780, 17.4514 | ||
| Opawskie Mts: Dębowiec | Deciduous forest, beech; bird nest boxes (4) | Edible dormice: leaves of trees, gnawed oak seeds, droppings |
| GPS: 50.2787, 17.5398 | ||
| Stobrawski Landscape Park: Kup | Mixed forest, pine, and beech; dormice nest boxes (6) | Edible dormice: leaves of trees, gnawed beech seeds, droppings |
| GPS: 50.8613, 17.9292 | ||
| Stobrawski Landscape Park: Lubsza | Deciduous forest, beech, oak; dormice nest boxes (4) | Hazel dormice: nests |
| GPS: 50.9328, 17.5658 | ||
| Stobrawski Landscape Park: Kozuby | Deciduous forest, oak; dormice nest boxes (3) | Edible and hazel dormice: nests, gnawed oak and beech seeds, leaves of trees, droppings |
| GPS: 50.9381, 17.8124 | ||
| “Niemodlin Forest”: Goszczowice | Deciduous forest, beech, dormice nest boxes (2) | Edible dormice: leaves of trees, gnawed beech seeds, droppings |
| GPS: 50.5784, 17.6084 |
| Species | N | D% | F% | Average ± SD | Max. | F | M | D | P | L |
|---|---|---|---|---|---|---|---|---|---|---|
| Leiodinychus orbicularis (C.L. Koch) | 559 | 99.1 | 46.0 | 32.9 ± 69.1 | 285 | 184 | 153 | 165 | 57 | - |
| Trachytes aegrota (C.L. Koch) | 2 | 0.5 | 2.7 | 2.0 | 2 | 2 | - | - | - | - |
| T. irenae Pecina | 1 | 0.2 | 2.7 | 1.0 | 1 | 1 | - | - | - | - |
| Neodiscopoma splendida (Kramer) | 1 | 0.2 | 2.7 | 1.0 | 1 | 1 | - | - | - | - |
| Nenteria sp. | 1 | 0.2 | 2.7 | 1.0 | 1 | - | 1 | - | - | - |
| Total | 564 | 100.0 | 50.0 | 29.7 ± 65.8 | 285 |
| A | B | C | D | E | F | G | H | I | J | K | L | M | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of boxes or nests | 58 | 38 | 170 | 103 | 38 | 47 | 39 | 52 | 23 | 132 | 34 | 66 | 32 |
| Number of Uropodina species | 2 | 5 | 3 | 2 | 11 | 15 | 11 | 11 | 24 | 15 | 11 | 14 | 16 |
| Number of specimens | 119 | 564 | 453 | 1525 | 2827 | 275 | 373 | 942 | 782 | 4718 | 925 | 595 | 413 |
| L. orbicularis | |||||||||||||
| Number of specimens | 118 | 559 | 443 | 1281 | 904 | 42 | 49 | 7 | 2 | 5 | 0 | 0 | 0 |
| Dominance (%) | 99 | 99 | 98 | 84 | 32 | 15 | 13 | >1 | >1 | >1 | |||
| Frequency (%) | 19 | 46 | 11 | 21 | 74 | 6 | 5 | 4 | 4 | >1 | |||
| Average number of specimens in a nest ± SD | 10 ± 14 | 32.9 ± 69.1 | 23 ± 52 | 58 ± 151 | 32 ± 91 | 14 ± 14 | 24 ± 16 | 0.1 ± 0.7 | 2 | 5 | |||
| A. infirmus | |||||||||||||
| Number of specimens | 0 | 0 | 0 | 244 | 26 | 0 | 270 | 225 | 1 | 0 | 289 | 0 | 4 |
| Dominance (%) | 16 | >1 | 21 | 24 | >1 | 10 | 1 | ||||||
| Frequency % | 10 | 26 | 31 | 18 | 4 | 32 | 9 | ||||||
| Average number of specimens in a nest ± SD | 24 ± 28 | 2 ± 2 | 22 ± 141 | 22 ± 140 | 1 | 26 ± 53 | 1 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błoszyk, J.; Hebda, G.; Kulczak, M.; Zacharyasiewicz, M.; Rutkowski, T.; Napierała, A. Communities of Uropodina (Acari: Mesostigmata) in Nest Boxes Inhabited by Dormice (Glis glis and Muscardinus avellanarius) and Differences in Percentages of Nidicoles in Nests of Various Hosts. Animals 2023, 13, 3567. https://doi.org/10.3390/ani13223567
Błoszyk J, Hebda G, Kulczak M, Zacharyasiewicz M, Rutkowski T, Napierała A. Communities of Uropodina (Acari: Mesostigmata) in Nest Boxes Inhabited by Dormice (Glis glis and Muscardinus avellanarius) and Differences in Percentages of Nidicoles in Nests of Various Hosts. Animals. 2023; 13(22):3567. https://doi.org/10.3390/ani13223567
Chicago/Turabian StyleBłoszyk, Jerzy, Grzegorz Hebda, Marta Kulczak, Michał Zacharyasiewicz, Tomasz Rutkowski, and Agnieszka Napierała. 2023. "Communities of Uropodina (Acari: Mesostigmata) in Nest Boxes Inhabited by Dormice (Glis glis and Muscardinus avellanarius) and Differences in Percentages of Nidicoles in Nests of Various Hosts" Animals 13, no. 22: 3567. https://doi.org/10.3390/ani13223567
APA StyleBłoszyk, J., Hebda, G., Kulczak, M., Zacharyasiewicz, M., Rutkowski, T., & Napierała, A. (2023). Communities of Uropodina (Acari: Mesostigmata) in Nest Boxes Inhabited by Dormice (Glis glis and Muscardinus avellanarius) and Differences in Percentages of Nidicoles in Nests of Various Hosts. Animals, 13(22), 3567. https://doi.org/10.3390/ani13223567







