In Vitro Digestibility and Models of Cumulative Gas Production of Forage-Free Diet
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
| Diet | ||
|---|---|---|
| Corn Silage | Cottonseed Cake | |
| Corn silage (g/kg DM) | 200 | - |
| Cottonseed cake (g/kg DM) | - | 230 |
| Ground corn (g/kg DM) | 619 | 740 |
| Cottonseed meal (g/kg DM) | 155 | - |
| Urea (g/kg DM) | 6 | 10 |
| Supplement mineral (g/kg DM) | 20 | 20 |
| Chemical composition (g/kg) | ||
| DM | 658 | 879 |
| OM | 954 | 959 |
| CP | 147 | 151 |
| EE | 41 | 56 |
| NDF | 277 | 267 |
| peNDF | 97 | 94 |
| TDN | 761 | 761 |
2.2. In Vitro Digestibility
2.3. In Vitro Gas Production Data
2.4. Models and Curve-Fitting
2.5. Statistical Analysis
3. Results
3.1. In vitro Digestibility
3.2. Models and Curve-Fitting
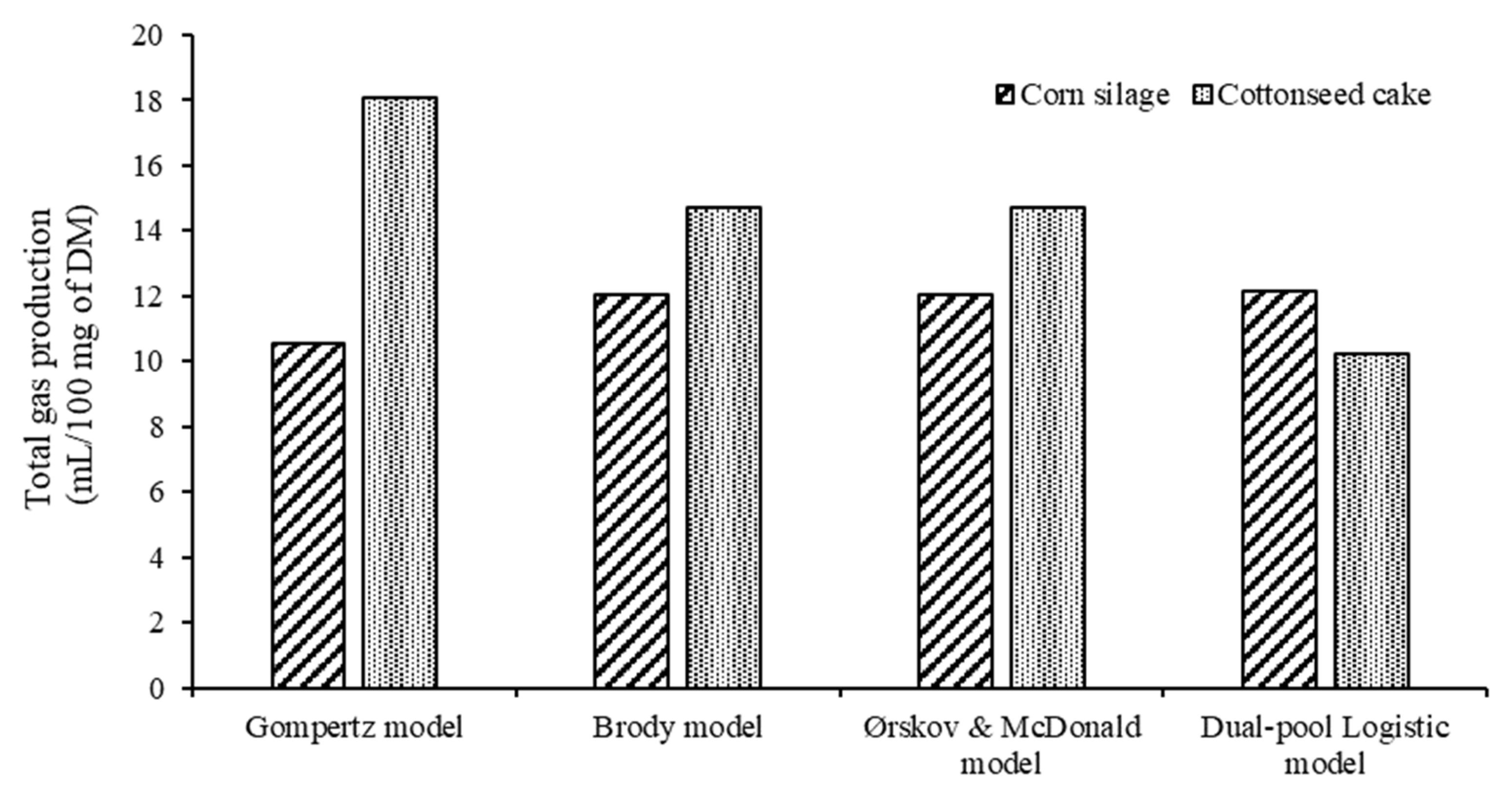
3.3. In Vitro Gas Production Data
4. Discussion
4.1. In Vitro Digestibility
4.2. Models and Curve-Fitting
4.3. In Vitro Gas Production Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Granja-Salcedo, Y.T.; Ribeiro Júnior, C.S.; Jesus, R.B.; Gomez-Insuasti, A.S.; Rivera, A.R.; Messana, J.D.; Canesin, R.C.; Berchielli, T.T. Effect of different levels of concentrate on ruminal microorganisms and rumen fermentation in Nellore steers. Arch. Anim. Nutr. 2016, 70, 17–32. [Google Scholar] [CrossRef]
- Martin, C.; Morgavi, D.P.; Doreau, M. Methane mitigation in ruminants: From microbe to the farm scale. Animal 2010, 4, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Mertens, D.R. Creating a system for meeting the fiber requirements of dairy cows. J. Dairy Sci. 1997, 80, 1463–1481. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Beef Cattle: Eighth Revised Edition; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- García-Rodríguez, J.; Ranilla, M.J.; França, J.; Alaiz-Moretón, H.; Carro, M.D.; López, S. Chemical composition, in vitro digestibility and rumen fermentation kinetics of agro-industrial by-products. Animals 2019, 9, 861. [Google Scholar] [CrossRef]
- Goulart, R.S.; Vieira, R.A.M.; Daniel, J.L.P.; Amaral, R.C.; Santos, V.P.; Toledo Filho, S.G.; Cabezas-Garcia, E.H.; Tedeschi, L.O.; Nussio, L.G. Effects of source and concentration of neutral detergent fiber from roughage in beef cattle diets: Comparison of methods to measure the effectiveness of fiber. J. Anim. Sci. 2020, 98, skaa108. [Google Scholar] [CrossRef] [PubMed]
- Goulart, R.S.; Vieira, R.A.M.; Daniel, J.L.P.; Amaral, R.C.; Santos, V.P.; Toledo Filho, S.G.; Cabezas-Garcia, E.H.; Tedeschi, L.O.; Nussio, L.G. Effects of source and concentration of neutral detergent fiber from roughage in beef cattle diets on feed intake, ingestive behavior, and ruminal kinetics. J. Anim. Sci. 2020, 98, skaa107. [Google Scholar] [CrossRef]
- Arcanjo, H.M.A.; Ítavo, L.C.V.; Ítavo, C.C.B.F.; Dias, A.M.; Difante, G.S.; Franco, G.L.; Longhini, V.Z.; Gomes, F.K.; Ali, O.; Santana, J.C.S.; et al. Effectiveness of cottonseed cake fibre included in the diet of Nellore steers finished in confinement. N. Z. J. Agric. Res. 2023, 66, 1–15. [Google Scholar] [CrossRef]
- Arcanjo, H.M.A.; Ítavo, L.C.V.; Ítavo, C.C.B.F.; Franco, G.L.; Dias, A.M.; Difante, G.S.; Lima, E.A.; Santana, J.C.S.; Gurgel, A.L.C. Cotton cake as an economically viable alternative fibre source of forage in a high-concentrate diet for finishing beef cattle in feedlots. Trop. Anim. Health Prod. 2022, 54, 112. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, K.H.; Park, P.J.; Jeon, B.T.; Oh, M.R.; Jang, S.Y.; Sung, S.H.; Moon, S.H. Effects of physically effective neutral detergent fibre content on dry-matter intake, digestibility and chewing activity in beef cattle fed total mixed ration. Anim. Prod. Sci. 2014, 55, 166–169. [Google Scholar] [CrossRef]
- Alhadas, H.M.; Valadares Filho, S.C.; Silva, F.F.; Silva, F.A.S.; Pucetti, P.; Pacheco, M.V.C.; Silva, B.C.; Tedeschi, L.O. Effects of including physically effective fiber from sugarcane in whole corn grain diets on the ingestive, digestive, and ruminal parameters of growing beef bulls. Livest. Sci. 2021, 248, 104508. [Google Scholar] [CrossRef]
- da Mata, D.G.; Ítavo, L.C.V.; Ítavo, C.C.B.F.; Ferreira, J.D.J.; Paulino, P.V.R.; Moraes, G.J.; Niwa, M.V.G.; Kozerski, N.D.; Leal, E.S.; Costa, M.C.M. Ruminal responses, digestibility, and blood parameters of beef cattle fed diets without forage with different hybrids and processing of the corn. J. Anim. Physiol. Anim. Nutr. 2023, 107, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Olivo, P.M.; Santos, G.T.; Ítavo, L.C.V.; Silva, R.C.D.; Leal, E.S.; Prado, R.M.D. Assessing the nutritional value of agroindustrial co-products and feed through chemical composition, in vitro digestibility, and gas production technique. Acta Sci.-Anim. Sci. 2017, 39, 289–295. [Google Scholar] [CrossRef]
- Souza, A.D.V.; Ítavo, L.C.V.; Favaro, S.P.; Ítavo, C.C.B.F.; Petit, H.V.; Dias, A.M.; Morais, M.G.; Reis, F.A.; Roscoe, R. Thermal decomposition, chemical composition, in vitro digestibility and gas production and in situ degradability of oilseed residues from the biofuel industry. Anim. Sci. J. 2018, 89, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Leal, E.S.; Ítavo, L.C.V.; Valle, C.B.; Ítavo, C.C.B.F.; Dias, A.M.; Difante, G.; Ferreira, M.B.; Nonato, L.M.; Melo, G.K.A.; Gurgel, A.L.C. Influence of protodioscin content on digestibility and in vitro degradation kinetics in Urochloa brizantha cultivars. Crop Pasture Sci. 2020, 72, 278–284. [Google Scholar] [CrossRef]
- Santana, J.C.S.; Morais, J.A.S.; Difante, G.S.; Ítavo, L.C.V.; Gurgel, A.L.C.; Oliveira, V.S.; Rodrigues, M.J.S.T. In vitro digestion characteristics of various combinations of elephant grass hay, gliricidia hay or silage, soybean meal and corn meal in rations for sheep. Trop. Grassl.-Forrajes Trop. 2020, 8, 147–152. [Google Scholar] [CrossRef]
- Zornitta, C.S.; Ítavo, L.C.V.; Ítavo, C.C.B.F.; Santos, G.T.; Dias, A.M.; Difante, G.S.; Gurgel, A.L.C. Kinetics of in vitro gas production and fitting mathematical models of corn silage. Fermentation 2021, 7, 298. [Google Scholar] [CrossRef]
- dos Santos, A.L.P.; Moreira, G.R.; Gomes-Silva, F.; Brito, C.C.R.; da Costa, M.L.L.; Pereira, L.G.R.; Mauricio, R.M.; Azevêdo, J.A.G.; Pereira, J.M.; Ferreira, A.L.; et al. Generation of models from existing models composition: An application to agrarian sciences. PLoS ONE 2019, 14, e0214778. [Google Scholar] [CrossRef]
- Schofield, P.; Pitt, R.E.; Pell, A.N. Kinetics of fiber digestion from in vitro gas production. J. Anim. Sci. 1994, 72, 2980–2991. [Google Scholar] [CrossRef]
- Díaz, T.G.; Branco, A.F.; Ítavo, L.C.V.; Santos, G.T.; Carvalho, S.T.; Teodoro, A.L.; Oliveira, R.L. In vitro gas production kinetics and digestibility in ruminant diets with different levels of cashew nut shell liquid. Semin. Cienc. Agrar. 2018, 39, 1669–1682. [Google Scholar] [CrossRef]
- Gurgel, A.L.C.; Morais, J.A.S.; Santana, J.C.S.; Difante, G.S.; Emerenciano Neto, J.V.; Ítavo, L.C.V.; Ítavo, C.C.B.F.; Oliveira, V.S.; Rodrigues, M.J.S.T. Mathematical models to adjust the parameters of in vitro cumulative gas production of diets containing preserved Gliricidia. Cienc. Rural 2021, 51, 993. [Google Scholar] [CrossRef]
- Velho, J.P.; Mühlbach, P.R.F.; Genro, T.C.M.; Barcellos, J.O.J.; Braccini Neto, J.; Silva, R.S.M. Mathematical models for adjustment of in vitro gas production at different incubation times and kinetics of corn silages. Semin. Cienc. Agrar. 2014, 35, 2531–2539. [Google Scholar] [CrossRef]
- Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 10, 3583–3597. [Google Scholar] [CrossRef]
- Cappelle, E.R.; Valadares Filho, S.C.; Silva, J.F.C.; Cecon, P.R. Estimativas do valor energético a partir de características químicas e bromatológicas dos alimentos. Rev. Bras. Zootec. 2001, 30, 1837–1856. [Google Scholar] [CrossRef]
- Lammers, B.P.; Buckmaster, D.R.; Heinrichs, A.J. A simple method for the analysis of particle sizes of forage and total mixed rations. J. Dairy Sci. 1996, 79, 922–928. [Google Scholar] [CrossRef]
- Valadares Filho, S.C.; Costa e Silva, L.F.; Gionbelli, M.P.; Rotta, P.P.; Marcondes, M.I.; Chizzotti, M.L.; Prados, L.F. Exigências Nutricionais de Zebuínos Puros e Cruzados BR-CORTE, 3rd ed.; UFV, DZO: Viçosa, Brazil, 2016; 327p. [Google Scholar]
- Tilley, J.M.A.; Terry, D.R. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Holden, L.A. Comparison of methods of in vitro matter digestibility for ten feeds. J. Dairy Sci. 1999, 2, 1791–1794. [Google Scholar] [CrossRef]
- Ítavo, L.C.V.; Soares, C.M.; Ítavo, C.C.B.F.; Dias, A.M.; Petit, H.V.; Leal, E.S.; Souza, A.D.V. Calorimetry, chemical composition and in vitro digestibility of oilseeds. Food Chem. 2015, 185, 219–225. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Brody, S. Bioenergetics and Growth, with Special Reference to the Efficiency Complex in Domestic Animals; Reinhold Publishing Corporation: New York, NY, USA, 1945. [Google Scholar]
- Ørskov, E.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Tedeschi, L.O. Assessment of the adequacy of mathematical models. Agric. Syst. 2006, 89, 225–247. [Google Scholar] [CrossRef]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar] [PubMed]
- Negrão, F.M.; Zanine, A.M.; Ribeiro, M.D.; Ferreira, D.J.; Souza, A.L.; Parente, M.O.M.; Parente, H.N.; Geron, L.J.V.; Lima, A.G.V.O.; Reis, R.H.P.; et al. By-product of cotton agribusiness as an alternative protein source for rams. Agriculture 2020, 10, 280. [Google Scholar] [CrossRef]
- Melo, H.S.A.; Ítavo, L.C.V.; Castro, A.P.; Ítavo, C.C.B.F.; Caldas, R.A.; Mateus, R.G.; Niwa, M.V.G.; Moraes, G.J.; Zornitta, C.S.; Gurgel, A.L.C.; et al. Bacterial species in the ruminal content of steers fed oilseeds in the diet. Trop. Anim. Health Prod. 2022, 54, 396. [Google Scholar] [CrossRef] [PubMed]
- Negrão, F.M.; Zanine, A.M.; Ribeiro, M.D.; Ferreira, D.J.; Souza, A.L.; Parente, M.O.M.; Parente, H.N.; Reis, R.H.P.; Lins, T.O.J.D.; Lima, A.G.V.O. Rumen fermentation and metabolic profile of rams fed with diets amended cottonseed cake. Trop. Anim. Health Prod. 2021, 53, 548. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xue, B.; Hu, A.; Yue, S.; Wu, M.; Hong, Q.; Wu, Y.; Wang, Z.; Wang, L.; Peng, Q.; et al. Effect of dietary peNDF levels on digestibility and rumen fermentation, and microbial community in growing goats. Front. Microbiol. 2022, 13, 598. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.X.; Goes, R.H.T.B.; Carneiro, M.Y.; Carneiro, M.Y.; Burin, P.; Oliveira, E.; Souza, K.; Ítavo, L.C.V.; Branco, A.F.; Oliveira, R.L. Total gas production and in vitro degradability of diets with sunflower crushed. Arch. Zootec. 2015, 64, 365–371. [Google Scholar] [CrossRef][Green Version]
- Cabral, Í.S.; Azevêdo, J.A.G.; Pina, D.S.; Pereira, L.G.R.; Fernandes, H.J.; Almeida, F.M.; Souza, L.L.; Lima, R.F.; Cirne, L.G.A. Evaluation of models utilized in in vitro gas production from tropical feedstuffs. Semin. Cienc. Agrar. 2019, 40, 443–456. [Google Scholar] [CrossRef]
- Wanderley, A.M.; Ítavo, L.C.V.; Santos, G.T.; Ítavo, C.C.B.F.; Cunha, C.S.; Difante, G.S.; Dias, A.M.; Mateus, R.G.; Oliveira, M.V.M. Ruminal degradation kinetics of diets with different lipid sources and its influence on intake and milk yield of early lactation crossbred Holstein × Gir cows. Trop. Anim. Health Prod. 2021, 53, 516. [Google Scholar] [CrossRef]

| Models | Equation | Parameters |
|---|---|---|
| Gompertz | V(t) = VF e(−b.e(−kt)) | 3 |
| Brody | V(t) = VF (1 − b.e(−kt)) | 3 |
| Ørskov and McDonald | V(t) = VF + b(1 − e(−kt)) | 3 |
| Dual-pool Logistic | V(t) = V1F/(1 + e(2−4.k1(t−λ))) + V2F/(1 + e(2−4.k2(t−λ))) | 5 |
| Diets | SEM | p-Value | ||
|---|---|---|---|---|
| Corn Silage | Cottonseed Cake | |||
| ivDDM | 958.0 a | 935.4 b | 9.95 | 0.0001 |
| ivDOM | 875.2 b | 883.2 a | 3.20 | 0.0001 |
| ivDNDF | 871.4 a | 846.6 b | 9.21 | 0.0001 |
| Fiber source | ||||
| Corn silage | Cottonseed cake | |||
| ivDDM | 834.7 | 570.9 | 6.68 | 0.0001 |
| ivDOM | 882.1 | 940.4 | 0.66 | 0.0001 |
| ivDNDF | 765.2 | 538.5 | 3.68 | 0.0001 |
| Mean | SD | Min | Max | R2 | p-Value | CCC | RMSEP | Decomposition of MSEP (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ME | SB | RE | |||||||||
| Corn silage diet | |||||||||||
| Observed data | 6.73 | 3.62 | 0.0 | 13.57 | |||||||
| Gompertz | 6.77 | 2.96 | 0.86 | 10.13 | 0.84 | 0.7978 | 0.82 | 1.95 | 0.038 | 0.194 | 99.767 |
| Brody | 6.72 | 3.08 | −0.43 | 10.42 | 0.85 | 0.9999 | 0.84 | 1.91 | 0.000 | 0.000 | 100.00 |
| Ørskov and McDonald | 6.73 | 3.08 | −0.43 | 10.42 | 0.85 | 0.9999 | 0.84 | 1.91 | 0.000 | 0.000 | 100.00 |
| Dual-pool Logistic | 6.94 | 2.77 | 0.85 | 10.71 | 0.85 | 0.0220 | 0.82 | 1.92 | 1.170 | 2.674 | 96.156 |
| Cottonseed cake diet | |||||||||||
| Observed data | 5.87 | 3.47 | 0.0 | 10.09 | |||||||
| Gompertz | 5.59 | 4.09 | 0.19 | 12.72 | 0.95 | 0.0001 | 0.94 | 1.33 | 4.503 | 34.754 | 60.743 |
| Brody | 5.87 | 3.44 | −1.37 | 10.54 | 0.99 | 0.9957 | 0.99 | 0.41 | 0.009 | 0.334 | 99.657 |
| Ørskov and McDonald | 5.89 | 3.44 | −1.36 | 10.54 | 0.99 | 0.9963 | 0.99 | 0.41 | 0.007 | 0.001 | 99.992 |
| Dual-pool Logistic | 5.90 | 3.41 | 0.31 | 9.94 | 0.99 | 0.0015 | 0.99 | 0.16 | 3.063 | 9.688 | 87.249 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ítavo, L.C.V.; Gurgel, A.L.C.; Ferreira Ítavo, C.C.B.; Cunha, C.S.; Longhini, V.Z.; Difante, G.d.S.; Dias, A.M.; Santana, J.C.S.; Arcanjo, A.H.M.; Niwa, M.V.G.; et al. In Vitro Digestibility and Models of Cumulative Gas Production of Forage-Free Diet. Animals 2023, 13, 3515. https://doi.org/10.3390/ani13223515
Ítavo LCV, Gurgel ALC, Ferreira Ítavo CCB, Cunha CS, Longhini VZ, Difante GdS, Dias AM, Santana JCS, Arcanjo AHM, Niwa MVG, et al. In Vitro Digestibility and Models of Cumulative Gas Production of Forage-Free Diet. Animals. 2023; 13(22):3515. https://doi.org/10.3390/ani13223515
Chicago/Turabian StyleÍtavo, Luís Carlos Vinhas, Antonio Leandro Chaves Gurgel, Camila Celeste Brandão Ferreira Ítavo, Camila Soares Cunha, Vanessa Zirondi Longhini, Gelson dos Santos Difante, Alexandre Menezes Dias, Juliana Caroline Santos Santana, Angelo Herbet Moreira Arcanjo, Marcus Vinicius Garcia Niwa, and et al. 2023. "In Vitro Digestibility and Models of Cumulative Gas Production of Forage-Free Diet" Animals 13, no. 22: 3515. https://doi.org/10.3390/ani13223515
APA StyleÍtavo, L. C. V., Gurgel, A. L. C., Ferreira Ítavo, C. C. B., Cunha, C. S., Longhini, V. Z., Difante, G. d. S., Dias, A. M., Santana, J. C. S., Arcanjo, A. H. M., Niwa, M. V. G., Nonato, L. M., Tadeu dos Santos, G., & Chay-Canul, A. J. (2023). In Vitro Digestibility and Models of Cumulative Gas Production of Forage-Free Diet. Animals, 13(22), 3515. https://doi.org/10.3390/ani13223515











