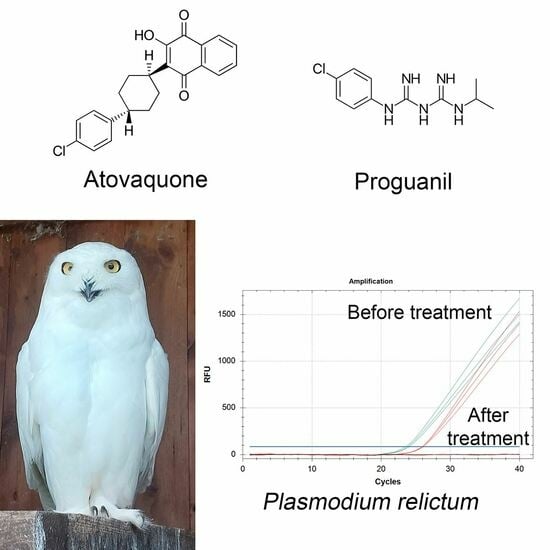
A Safe and Effective Atovaquone-Proguanil Therapeutic Protocol for the Treatment of Avian Malaria by Plasmodium relictum in Snowy Owl (Bubo scandiacus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case History
2.2. Molecular Investigations
2.3. Molecular Quantification of Plasmodium relictum
2.4. Hematological Investigations
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valkiūnas, G.; Iezhova, T.A. Keys to the avian malaria parasites. Malar. J. 2018, 17, 212. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Valkiūnas, G.; Iezhova, T.A. Exo-erythrocytic development of avian malaria and related haemosporidian parasites. Malar. J. 2017, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Fecchio, A.; Wells, K.; Bell, J.A.; Tkach, V.V.; Lutz, H.L.; Weckstein, J.D.; Clegg, S.M.; Clark, N.J. Climate variation influences host specificity in avian malaria parasites. Ecol. Lett. 2019, 22, 547–557. [Google Scholar] [CrossRef]
- Fecchio, A.; Chagas, C.R.F.; Bell, J.A.; Kirchgatter, K. Evolutionary ecology, taxonomy, and systematics of avian malaria and related parasites. Acta Trop. 2020, 204, 105364. [Google Scholar] [CrossRef] [PubMed]
- Martínez-de la Puente, J.; Santiago-Alarcon, D.; Palinauskas, V.; Bensch, S. Plasmodium relictum. Trends Parasitol. 2021, 37, 355–356. [Google Scholar] [CrossRef]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef]
- Dimitrov, D.; Palinauskas, V.; Iezhova, T.A.; Bernotienė, R.; Ilgūnas, M.; Bukauskaitė, D.; Zehtindjiev, P.; Ilieva, M.; Shapoval, A.P.; Bolshakov, C.V.; et al. Plasmodium spp.: An experimental study on vertebrate host susceptibility to avian malaria. Exp. Parasitol. 2015, 148, 1–16. [Google Scholar] [CrossRef]
- Schoener, E.R.; Banda, M.; Howe, L.; Castro, I.C.; Alley, M.R. Avian malaria in New Zealand. N. Z. Vet. J. 2014, 62, 189–198. [Google Scholar] [CrossRef]
- Ritchie, B.W.; Harrison, G.J.; Harrison, L.R. Avian Medicine: Principles and Application; Wingers Publishing: Lake Worth, FL, USA, 2000. [Google Scholar]
- Grilo, M.L.; Vanstreels, R.E.; Wallace, R.; García-Párraga, D.; Braga, É.M.; Chitty, J.; Catão-Dias, J.L.; Madeira de Carvalho, L.M. Malaria in penguins-current perceptions. Avian Pathol. 2016, 45, 393–407. [Google Scholar] [CrossRef]
- Palinauskas, V.; Valkiūnas, G.; Bolshakov, C.V.; Bensch, S. Plasmodium relictum (lineage P-SGS1): Effects on experimentally infected passerine birds. Exp. Parasitol. 2008, 120, 372–380. [Google Scholar] [CrossRef]
- Ings, K.; Denk, D. Avian malaria in penguins: Diagnostics and future direction in the context of climate change. Animals 2022, 12, 600. [Google Scholar] [CrossRef] [PubMed]
- Schoenle, L.A.; Kernbach, M.; Haussmann, M.F.; Bonier, F.; Moore, I.T. An experimental test of the physiological consequences of avian malaria infection. J. Anim. Ecol. 2017, 86, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Sohsuebngarm, D.; Sasipreeyajan, J.; Nithiuthai, S.; Chansiripornchai, N. The efficacy of artesunate, chloroquine, doxycycline, primaquine and a combination of artesunate and primaquine against avian malaria in broilers. J. Vet. Med. Sci. 2014, 76, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Palinauskas, V.; Valkiūnas, G.; Krizanauskiene, A.; Bensch, S.; Bolshakov, C.V. Plasmodium relictum (lineage P-SGS1): Further observation of effects on experimentally infected passeriform birds, with remarks on treatment with MalaroneTM. Exp. Parasitol. 2009, 123, 134–139. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwak, D.; Kim, K.T. The first clinical cases of Haemoproteus infection in a snowy owl (Bubo scandiacus) and a goshawk (Accipiter gentilis) at a zoo in the Republic of Korea. J. Vet. Med. Sci. 2018, 80, 1255–1258. [Google Scholar] [CrossRef]
- Beerahee, M. Clinical pharmacology of atovaquone and proguanil hydrochloride. J. Travel. Med. 1999, 6, S13–S17. [Google Scholar] [CrossRef]
- Hellgren, O.; Waldenström, J.; Bensch, S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef]
- Pugliese, N.; Circella, E.; Pazzani, C.; Pupillo, A.; Camarda, A. Validation of a seminested PCR approach for rapid detection of Salmonella enterica subsp. enterica serovar Gallinarum. J. Microbiol. Methods 2011, 85, 22–27. [Google Scholar] [CrossRef]
- Huang, X.; Madan, A. CAP3: A DNA sequence assembly program. Genome Res. 1999, 9, 868–877. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef]
- OligoArchitectTM Primer and Probe Design. Available online: http://www.oligoarchitect.com/ShowToolServlet?TYPE=DPROBE (accessed on 15 September 2023).
- Pugliese, N.; Circella, E.; Marino, M.; De Virgilio, C.; Cocciolo, G.; Lozito, P.; Cafiero, M.A.; Camarda, A. Circulation dynamics of Salmonella enterica subsp. enterica ser. Gallinarum biovar Gallinarum in a poultry farm infested by Dermanyssus gallinae. Med. Vet. Entomol. 2019, 33, 162–170. [Google Scholar] [CrossRef]
- Koepfli, C.; Nguitragool, W.; Hofmann, N.E.; Robinson, L.J.; Ome-Kaius, M.; Sattabongkot, J.; Felger, I.; Mueller, I. Sensitive and accurate quantification of human malaria parasites using droplet digital PCR (ddPCR). Sci. Rep. 2016, 6, 39183. [Google Scholar] [CrossRef] [PubMed]
- Preiser, P.R.; Wilson, R.J.; Moore, P.W.; McCready, S.; Hajibagheri, M.A.; Blight, K.J.; Strath, M.; Williamson, D.H. Recombination associated with replication of malarial mitochondrial DNA. EMBO J. 1996, 15, 684–693. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org (accessed on 15 September 2023).
- Valkiūnas, G.; Ilgūnas, M.; Bukauskaitė, D.; Žiegytė, R.; Bernotienė, R.; Jusys, V.; Eigirdas, V.; Fragner, K.; Weissenböck, H.; Iezhova, T.A. Plasmodium delichoni n. sp.: Description, molecular characterisation and remarks on the exoerythrocytic merogony, persistence, vectors and transmission. Parasitol. Res. 2016, 115, 2625–2636. [Google Scholar] [CrossRef]
- Pacheco, M.A.; Matta, N.E.; Valkiunas, G.; Parker, P.G.; Mello, B.; Stanley, C.E., Jr.; Lentino, M.; Garcia-Amado, M.A.; Cranfield, M.; Kosakovsky Pond, S.L.; et al. Mode and rate of evolution of haemosporidian mitochondrial genomes: Timing the radiation of avian parasites. Mol. Biol. Evol. 2018, 35, 383–403. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.; Fachet, K.; Dinkel, A.; Mackenstedt, U.; Woog, F. Carrion crows (Corvus corone) of southwest Germany: Important hosts for haemosporidian parasites. Malar. J. 2017, 16, 369. [Google Scholar] [CrossRef]
- Sekar, V.; Rivero, A.; Pigeault, R.; Gandon, S.; Drews, A.; Ahren, D.; Hellgren, O. Gene regulation of the avian malaria parasite Plasmodium relictum, during the different stages within the mosquito vector. Genomics 2021, 113, 2327–2337. [Google Scholar] [CrossRef]
- Cocumelli, C.; Iurescia, M.; Diaconu, E.L.; Galietta, V.; Raso, C.; Buccella, C.; Stravino, F.; Grande, F.; Fiorucci, L.; De Liberat, C.; et al. Plasmodium matutinum causing avian malaria in lovebirds (Agapornis roseicollis) hosted in an Italian zoo. Microorganisms 2021, 9, 1356. [Google Scholar] [CrossRef]
- González-Olvera, M.; Hernandez-Colina, A.; Himmel, T.; Eckley, L.; Lopez, J.; Chantrey, J.; Baylis, M.; Jackson, A.P. Molecular and epidemiological surveillance of Plasmodium spp. during a mortality event affecting Humboldt penguins (Spheniscus humboldti) at a zoo in the UK. Int. J. Parasitol. Parasites Wildl. 2022, 19, 26–37. [Google Scholar] [CrossRef]
- Theodosopoulos, A.N.; Grabenstein, K.C.; Bensch, S.; Taylor, S.A. A highly invasive malaria parasite has expanded its range to non-migratory birds in North America. Biol. Lett. 2021, 17, 20210271. [Google Scholar] [CrossRef]
- Meister, S.L.; Wyss, F.; Wenker, C.; Hoby, S.; Basso, W.U. Avian haemosporidian parasites in captive and free-ranging, wild birds from zoological institutions in Switzerland: Molecular characterization and clinical importance. Int. J. Parasitol. Parasites Wildl. 2022, 20, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Chitty, J. Use of doxycycline in treatment of avian malaria. In Proceedings of the Association of Avian Veterinarians 32nd Annual Conference and Expo with the Association of Exotic Mammal Veterinarians, Seattle, WA, USA, 6–12 August 2011. [Google Scholar]
- Thorel, M.; Chavatte, J.M.; Landau, I.; Lemberger, K.; Leclerc, A. First case of Plasmodium relictum lineage pGRW11 infection in a captive-bred common eider (Somateria Mollissima) in Europe. Vet. Parasitol. Reg. Stud. Reports. 2021, 23, 100529. [Google Scholar] [CrossRef] [PubMed]
- Kurotschka, P.K.; Serafini, A.; Massari, M.; Da Cas, R.; Figueiras, A.; Forte, V.; Moro, M.F.; Massidda, M.; Contu, F.; Minerba, L.; et al. Broad Spectrum project: Factors determining the quality of antibiotic use in primary care: An observational study protocol from Italy. BMJ Open 2020, 10, e038843. [Google Scholar] [CrossRef]
- Remple, J.D. Intracellular hematozoa of raptors: A review and update. J. Avian Med. Surg. 2004, 18, 75–88. [Google Scholar] [CrossRef]
- Ovung, A.; Bhattacharyya, J. Sulfonamide drugs: Structure, antibacterial property, toxicity, and biophysical interactions. Biophys. Rev. 2021, 13, 259–272. [Google Scholar] [CrossRef]
- Srivastava, I.K.; Rottenberg, H.; Vaidya, A.B. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. J. Biol. Chem. 1997, 272, 3961–3966. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, A.; Bergqvist, Y.; Takechi, M.; Kalkoa, M.; Kaneko, O.; Kobayakawa, T.; Ishizaki, T.; Björkman, A. Intrinsic efficacy of proguanil against falciparum and vivax malaria independent of the metabolite cycloguanil. J. Infect. Dis. 1999, 179, 974–979. [Google Scholar] [CrossRef][Green Version]
- Cohn, L.A.; Birkenheuer, A.J.; Brunker, J.D.; Ratcliff, E.R.; Craig, A.W. Efficacy of atovaquone and azithromycin or imidocarb dipropionate in cats with acute cytauxzoonosis. J. Vet. Intern. Med. 2011, 25, 55–60. [Google Scholar] [CrossRef]
- Kirk, S.K.; Levy, J.K.; Crawford, P.C. Efficacy of azithromycin and compounded atovaquone for treatment of Babesia gibsoni in dogs. J. Vet. Intern. Med. 2017, 31, 1108–1112. [Google Scholar] [CrossRef]
- Savitz, D.A.; Styka, A.N. Assessment of Long-Term Health Effects of Antimalarial Drugs When Used for Prophylaxis; The National Academies Press: Washington, DC, USA, 2020. [Google Scholar]
- Looareesuwan, S.; Chulay, J.D.; Canfield, C.J.; Hutchinson, D.B. Malarone (atovaquone and proguanil hydrochloride): A review of its clinical development for treatment of malaria. Malarone Clinical Trials Study Group. Am. J. Trop. Med. Hyg. 1999, 60, 533–541. [Google Scholar] [CrossRef]
- Deye, G.A.; Miller, R.S.; Miller, L.; Salas, C.J.; Tosh, D.; Macareo, L.; Smith, B.L.; Fracisco, S.; Clemens, E.G.; Murphy, J.; et al. Prolonged protection provided by a single dose of atovaquone-proguanil for the chemoprophylaxis of Plasmodium falciparum malaria in a human challenge model. Clin. Infect. Dis. 2012, 54, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Malarone® Product Monograph. GlaxoSmithKline, Mississauga. Available online: https://pdf.hres.ca/dpd_pm/00031541.PDF (accessed on 1 August 2023).
- Sabchareon, A.; Attanath, P.; Phanuaksook, P.; Chanthavanich, P.; Poonpanich, Y.; Mookmanee, D.; Chongsuphajaisiddhi, T.; Sadler, B.M.; Hussein, Z.; Canfield, C.J.; et al. Efficacy and pharmacokinetics of atovaquone and proguanil in children with multidrug-resistant Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, J.B. Gamma glutamyl transferase. Crit. Rev. Clin. Lab. Sci. 2001, 38, 263–355. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E. The safety of atovaquone/proguanil in long-term malaria prophylaxis of nonimmune adults. J. Travel. Med. 2003, 10, S13–S15; discussion S21. [Google Scholar] [CrossRef] [PubMed]
- Lell, B.; Luckner, D.; Ndjavé, M.; Scott, T.; Kremsner, P.G. Randomised placebo-controlled study of atovaquone plus proguanil for malaria prophylaxis in children. Lancet 1998, 351, 709–713. [Google Scholar] [CrossRef]
- Mayer, R.C.; Tan, K.R.; Gutman, J.R. Safety of atovaquone-proguanil during pregnancy. J. Travel Med. 2019, 26, tay138. [Google Scholar] [CrossRef]
- Abugroun, A.; Colina Garcia, I.; Ahmed, F.; Potts, S.; Flicker, M. The first report of atovaquone/proguanil-induced vanishing bile duct syndrome: Case report and mini-review. Travel Med. Infect. Dis. 2019, 32, 101439. [Google Scholar] [CrossRef]
- Wiegmann, A.; Rinaud, T.; Ottensmann, M.; Krüger, O.; Springer, A.; Legler, M.; Fehr, M.; Strube, C.; Chakarov, N. Tolerability of atovaquone-proguanil application in common buzzard nestlings. Vet. Sci. 2022, 9, 397. [Google Scholar] [CrossRef]
- Knowles, S.C.; Palinauskas, V.; Sheldon, B.C. Chronic malaria infections increase family inequalities and reduce parental fitness: Experimental evidence from a wild bird population. J. Evol. Biol. 2010, 23, 557–569. [Google Scholar] [CrossRef]
| Time | SO1 | SO2 | SO3 | |||
|---|---|---|---|---|---|---|
| Nested PCR | Estimated Parasitic Load (Cells/μL Blood) | Nested PCR | Estimated Parasitic Load (Cells/μL Blood) | Nested PCR | Estimated Parasitic Load (Cells/μL Blood) | |
| D-7 | Positive | 855 ± 138 | Positive | 2753 ± 825 | Positive | 3802 ± 1847 |
| D1 1 | Positive | 254 ± 77 | Positive | 3700 ± 774 | Positive | 633 ± 137 |
| D20 | Negative | ND | Negative | ND | Negative | ND |
| D43 | Negative | ND | Negative | ND | Negative | ND |
| D73 | Negative | ND | Negative | ND | Negative | ND |
| Parameter | Reference Interval | D-7 | D20 | D43 | D73 | D-7 | D20 | D43 | D73 | D-7 | D20 | D43 | D73 | ANOVA p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SO1 | SO1 | SO1 | SO1 | SO2 | SO2 | SO2 | SO2 | SO3 | SO3 | SO3 | SO3 | |||
| RBC (1012/L) | 2.4–4.7 | 2.24 a | 2.61 | 2.33 a | 2.40 | 2.76 | 2.41 | 2.92 | 2.54 | 2.78 | 2.26 a | 2.78 | 2.37 a | 0.683 |
| HGB (g/dL) | NA | 12.6 | 14.4 | 14.2 | 14.8 | 12.6 | 15.6 | 16.4 | 16.3 | 14.2 | 15.5 | 15.3 | 15.0 | 0.024 ** |
| HCT (%) | NA | 40 | 43 | 44 | 47 | 40 | 48 | 53 | 52 | 45 | 49 | 48 | 50 | 0.081 * |
| WBC (109/L) | 5.1–26.4 | 8.2 | 6.0 | 9.8 | 5.6 | 8.0 | 6.2 | 7.0 | 7.4 | 8.4 | 5.0 a | 6.2 | 6.4 | 0.350 |
| Lymphocytes (109/L) | 0.3–10.7 | 5.4 | 2.8 | 2.5 | 2.5 | 3.7 | 3.3 | 2.1 | 3.6 | 4.7 | 2.3 | 3.0 | 4.0 | 0.022 ** |
| Monocytes (109/L) | 0.2–1.8 | 0.3 | 0.1 a | 1.0 | 0.0 a | 0.3 | 0.2 | 0.1 a | 0.1 a | 0.7 | 0.3 | 0.1 | 0.4 | 0.421 |
| Eosinophils (109/L) | 0.1–4.2 | 0.2 | 1.1 | 1.8 | 0.9 | 0.6 | 0.6 | 1.3 | 1.0 | 0.5 | 1.0 | 0.7 | 0.4 | 0.276 |
| Basophils (109/L) | 0.0–0.3 | ND | 0.2 | 0.9 b | 0.4 | ND | 0.2 | 0.0 | 0.5 b | ND | 0.2 | 0.1 | 0.4 b | 0.322 |
| CPK (IU/L) | 20–3338 | 632 | 380 | 316 | 377 | 764 | 317 | 244 | 419 | 692 | 857 | 554 | 422 | 0.170 |
| AST (IU/L) | 171–301 | 224 | 230 | 175 | 285 | 269 | 230 | 276 | 323 b | 251 | 303 b | 288 | 309 b | 0.294 |
| ALT(IU/L) | NA | 27 | 22 | 17 | 23 | 39 | 31 | 36 | 47 | 27 | 33 | 36 | 38 | 0.781 |
| ALP (IU/L) | NA | 30 | 72 | 46 | 60 | 46 | 45 | 50 | 74 | 76 | 60 | 84 | 66 | 0.740 |
| GGT (IU/L) | 0–5 | ND | 2.1 | 0.1 | 1.2 | ND | 2.1 | 1.3 | 0.1 | ND | 2.6 | 0.1 | 0.9 | 0.015 * |
| Bilirubin (mmol/L) | 0–3 | 1.20 | 2.57 | 2.57 | 3.08 b | 2.22 | 2.57 | 2.22 | 3.42 b | 2.05 | 2.39 | 1.20 | 2.39 | 0.099 |
| Total protein (g/L) | 26–47 | 34 | 38 | 46 | 35 | 37 | 35 | 33 | 34 | 37 | 40 | 36 | 40 | 0.879 |
| Albumin (g/L) | 8–19 | 12 | 14 | 16 | 12 | 14 | 14 | 14 | 14 | 13 | 15 | 13 | 13 | 0.281 |
| Globulins (g/L) | 13–34 | 22 | 24 | 30 | 23 | 23 | 21 | 19 | 20 | 24 | 25 | 23 | 27 | 0.987 |
| Cholesterol (mmol/L) | 3.94–21.70 | 5.90 | 7.22 | 7.06 | 6.21 | 7.19 | 4.68 | 5.64 | 5.20 | 5.66 | 6.47 | 7.76 | 5.61 | 0.576 |
| Triglycerides (g/L) | NA | 0.81 | 1.07 | 0.85 | 0.91 | 0.91 | 2.05 | 0.97 | 2.26 | 0.84 | 1.14 | 1.04 | 0.90 | 0.422 |
| Amylase (IU/L) | 151–285 | ND | 316 | 318 | 282 | ND | 273 | 210 | 313 | ND | 409 | 233 | 302 | 0.260 |
| Creatinine (mg/L) | NA | 3.6 | 4.0 | 4.5 | 3.1 | 4.9 | 4.0 | 4.1 | 3.0 | 4.7 | 5.0 | 4.1 | 4.1 | 0.180 |
| Glucose (mmol/L) | 12.1–26.0 | 17.4 | 19.4 | 19.7 | 20.9 | 17.4 | 19.8 | 21.0 | 19.9 | 22.1 | 20.5 | 18.2 | 23.0 | 0.462 |
| Calcium (mmol/L) | 1.83–2.78 | 2.4 | 2.5 | 2.7 | 2.4 | 2.5 | 2.6 | 2.5 | 2.4 | 2.5 | 2.6 | 2.5 | 2.4 | 0.052 |
| Phosphorus (mmol/L) | 0.53–2.51 | 7.3 b | 3.2 b | 3.1 b | 3.1 b | 5.3 b | 5.8 b | 4 b | 5.7 b | 4.8 b | 4.9 b | 7 b | 4.8 b | 0.723 |
| Sodium (mmol/L) | 156–170 | 149 a | 160 | 161 | 162 | 159 | 165 | 163 | 163 | 156 | 166 | 152 a | 163 | 0.105 |
| Potassium (mmol/L) | 1.8–5.5 | 1.9 | 2.2 | 3.4 | 1.9 | 1.8 | 1.7 a | 2.3 | 1.7 a | 2.1 | 2.3 | 2.4 | 2.0 | 0.076 |
| Chloride (mmol/L) | 109–121 | 107 a | 114 | 115 | 114 | 114 | 115 | 110 | 115 | 117 | 109 | 109 | 115 | 0.706 |
| Uric acid (mmol/L) | 328–1329 | 410 | 275 | 169 | 269 | 668 | 522 | 442 | 1041 | 318 | 778 | 1936 b | 561 | 0.827 |
| LDH (IU/L) | 132–861 | 814 | 481 | 223 | 403 | 601 | 190 | 1128 b | 553 | 789 | 362 | 362 | 376 | 0.366 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugliese, N.; Samarelli, R.; Lombardi, R.; Schiavone, A.; Crescenzo, G.; Circella, E.; Zizzadoro, C.; Lai, O.; Saleh, M.S.; Prioletti, M.; et al. A Safe and Effective Atovaquone-Proguanil Therapeutic Protocol for the Treatment of Avian Malaria by Plasmodium relictum in Snowy Owl (Bubo scandiacus). Animals 2023, 13, 3457. https://doi.org/10.3390/ani13223457
Pugliese N, Samarelli R, Lombardi R, Schiavone A, Crescenzo G, Circella E, Zizzadoro C, Lai O, Saleh MS, Prioletti M, et al. A Safe and Effective Atovaquone-Proguanil Therapeutic Protocol for the Treatment of Avian Malaria by Plasmodium relictum in Snowy Owl (Bubo scandiacus). Animals. 2023; 13(22):3457. https://doi.org/10.3390/ani13223457
Chicago/Turabian StylePugliese, Nicola, Rossella Samarelli, Roberto Lombardi, Antonella Schiavone, Giuseppe Crescenzo, Elena Circella, Claudia Zizzadoro, Olimpia Lai, Medhat S. Saleh, Michela Prioletti, and et al. 2023. "A Safe and Effective Atovaquone-Proguanil Therapeutic Protocol for the Treatment of Avian Malaria by Plasmodium relictum in Snowy Owl (Bubo scandiacus)" Animals 13, no. 22: 3457. https://doi.org/10.3390/ani13223457
APA StylePugliese, N., Samarelli, R., Lombardi, R., Schiavone, A., Crescenzo, G., Circella, E., Zizzadoro, C., Lai, O., Saleh, M. S., Prioletti, M., & Camarda, A. (2023). A Safe and Effective Atovaquone-Proguanil Therapeutic Protocol for the Treatment of Avian Malaria by Plasmodium relictum in Snowy Owl (Bubo scandiacus). Animals, 13(22), 3457. https://doi.org/10.3390/ani13223457