A New Species of Nanorana (Anura: Dicroglossidae) from Northwestern Yunnan, China, with Comments on the Taxonomy of Nanorana arunachalensis and Allopaa
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
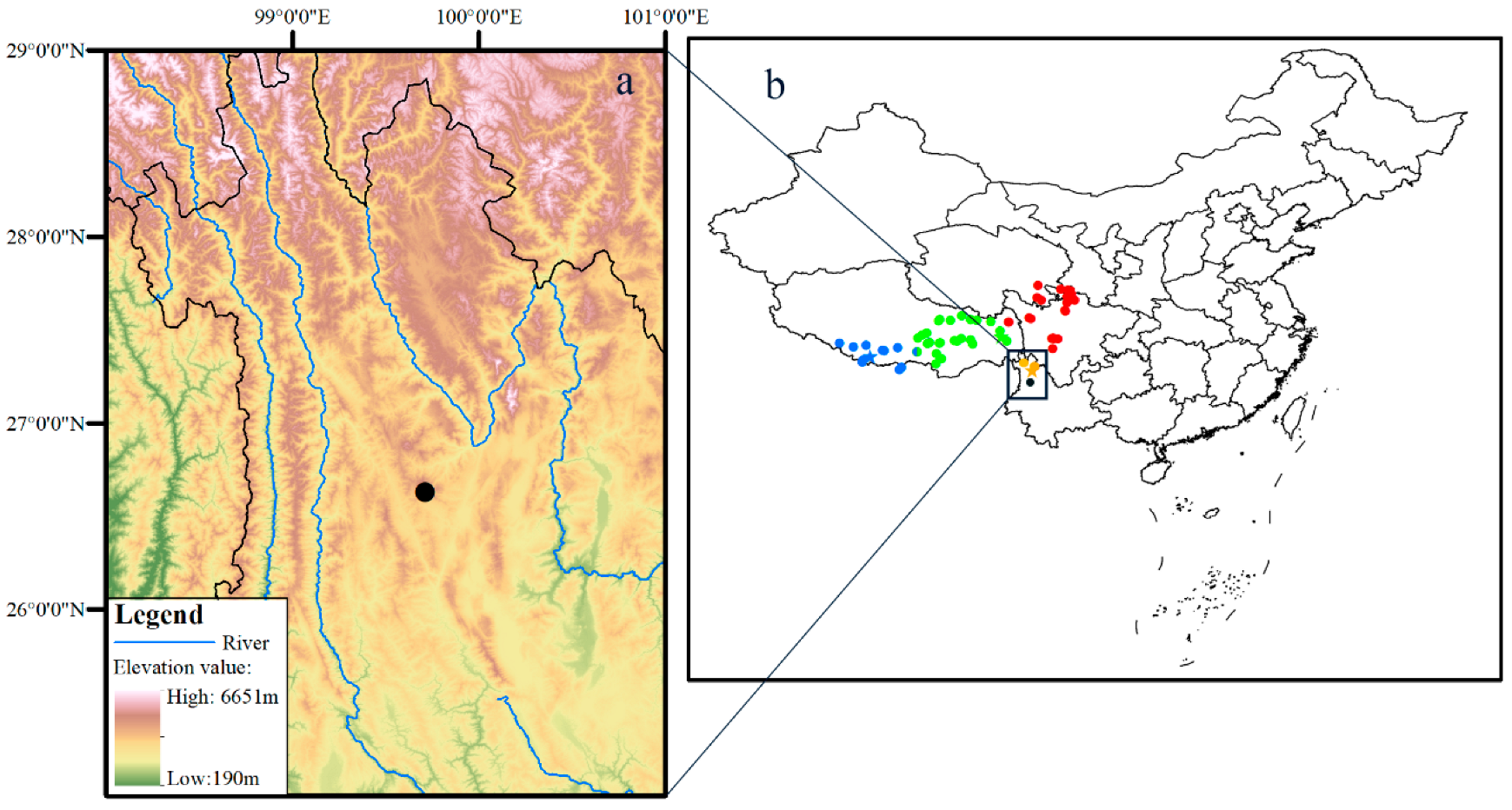
2.1. Sampling
2.2. Morphology
2.3. Molecular Phylogenetic Analyses
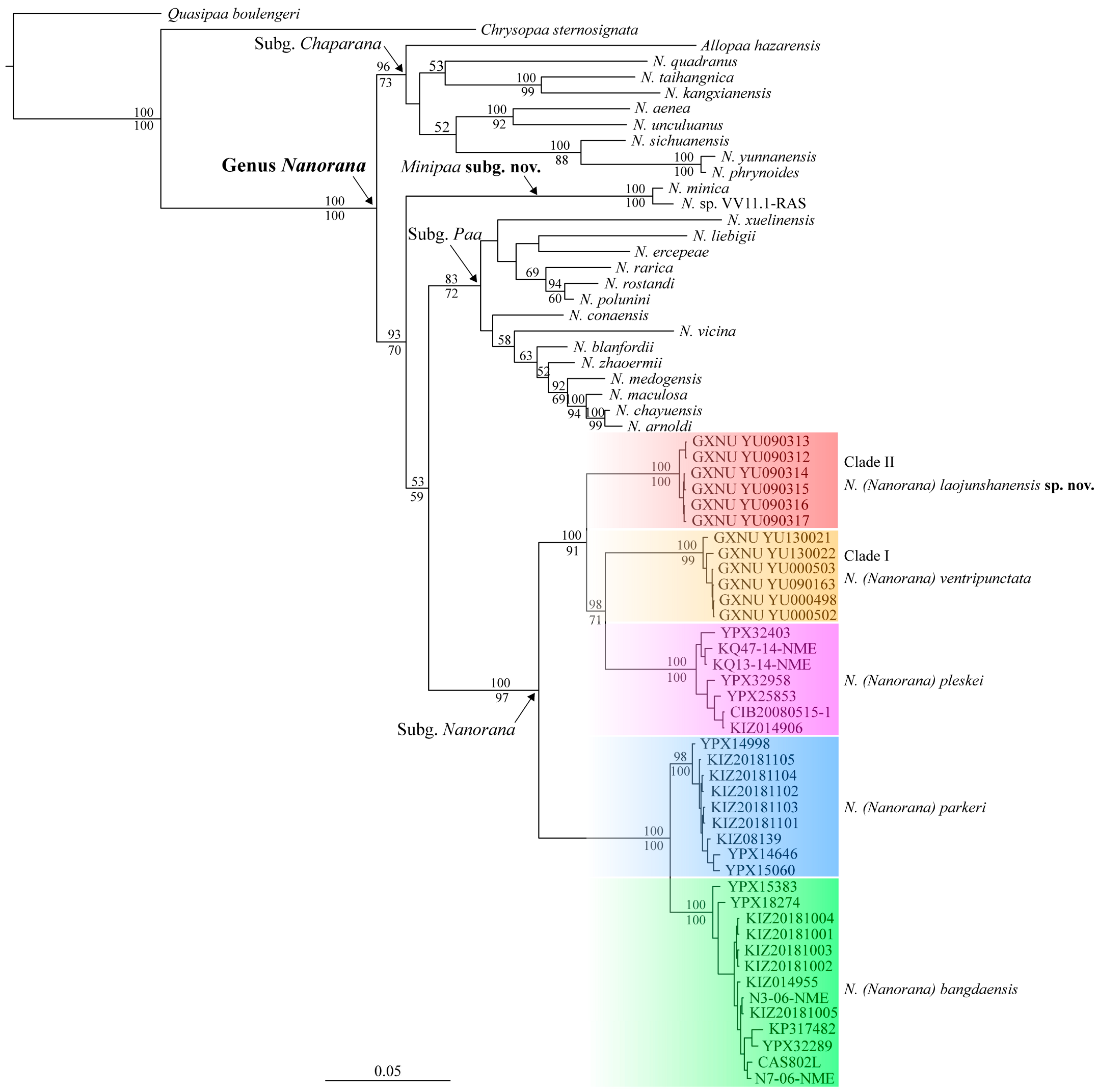
3. Results
3.1. Molecular Phylogeny
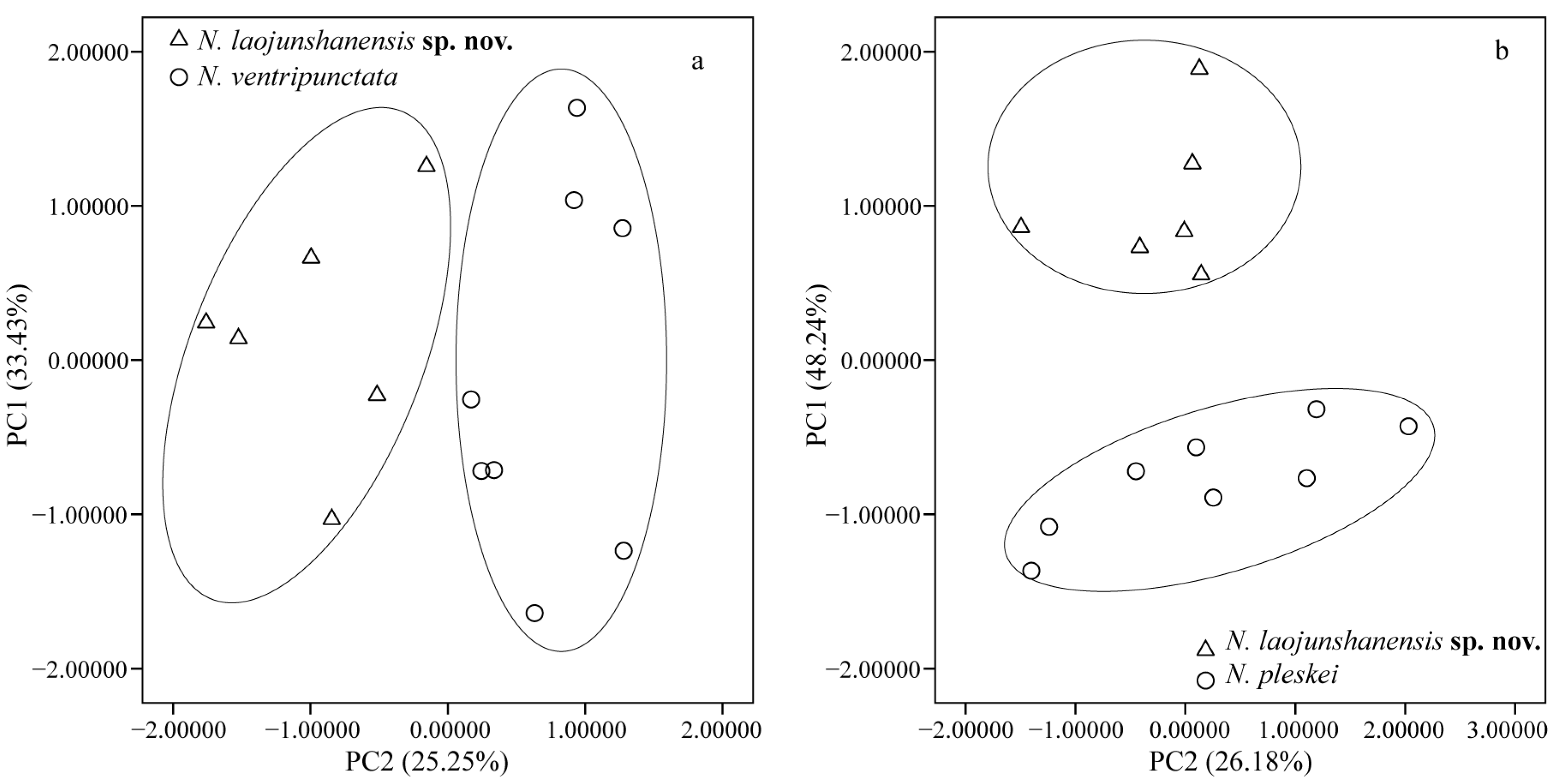
3.2. Morphometric Analyses
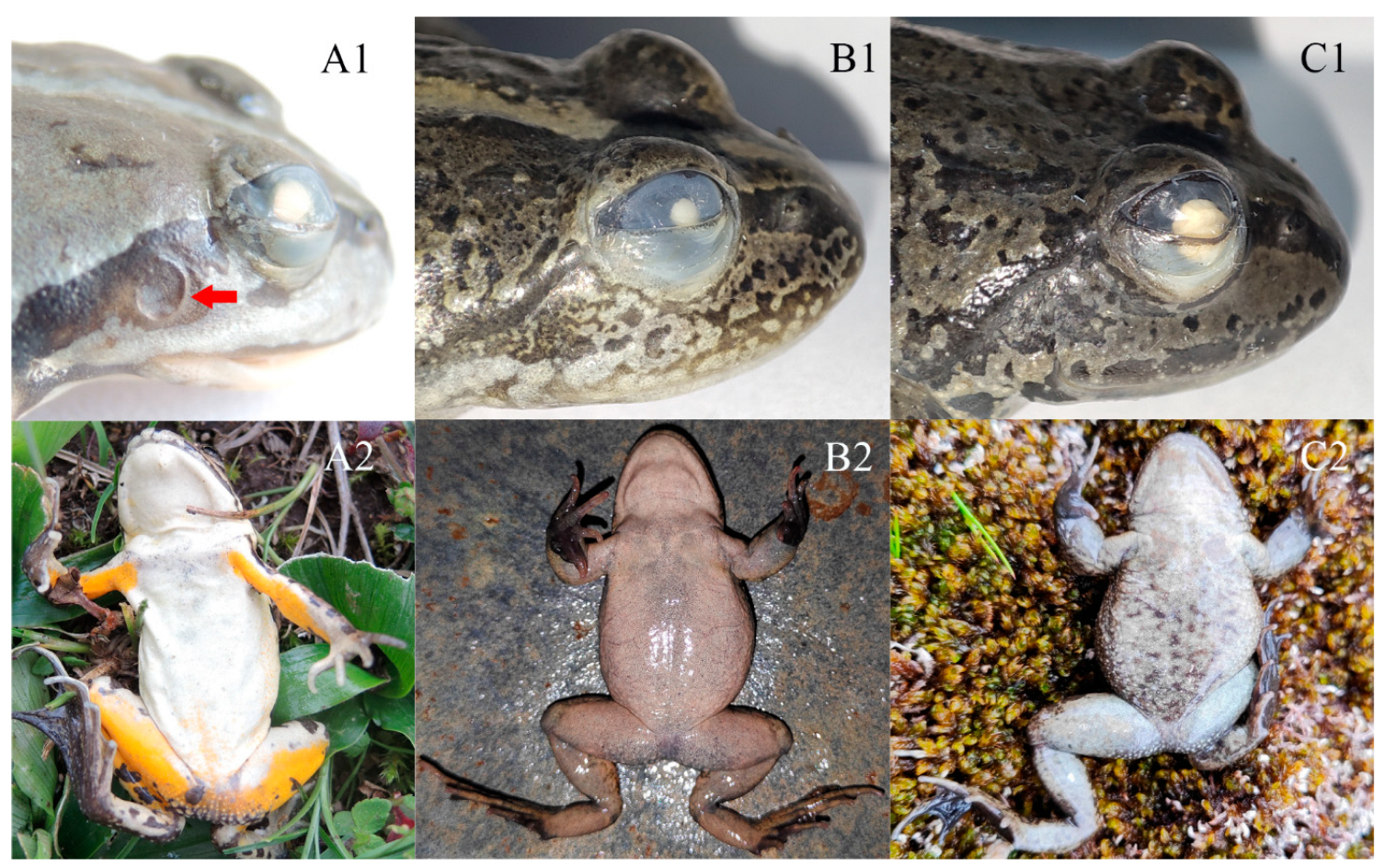
3.3. Taxonomic Account
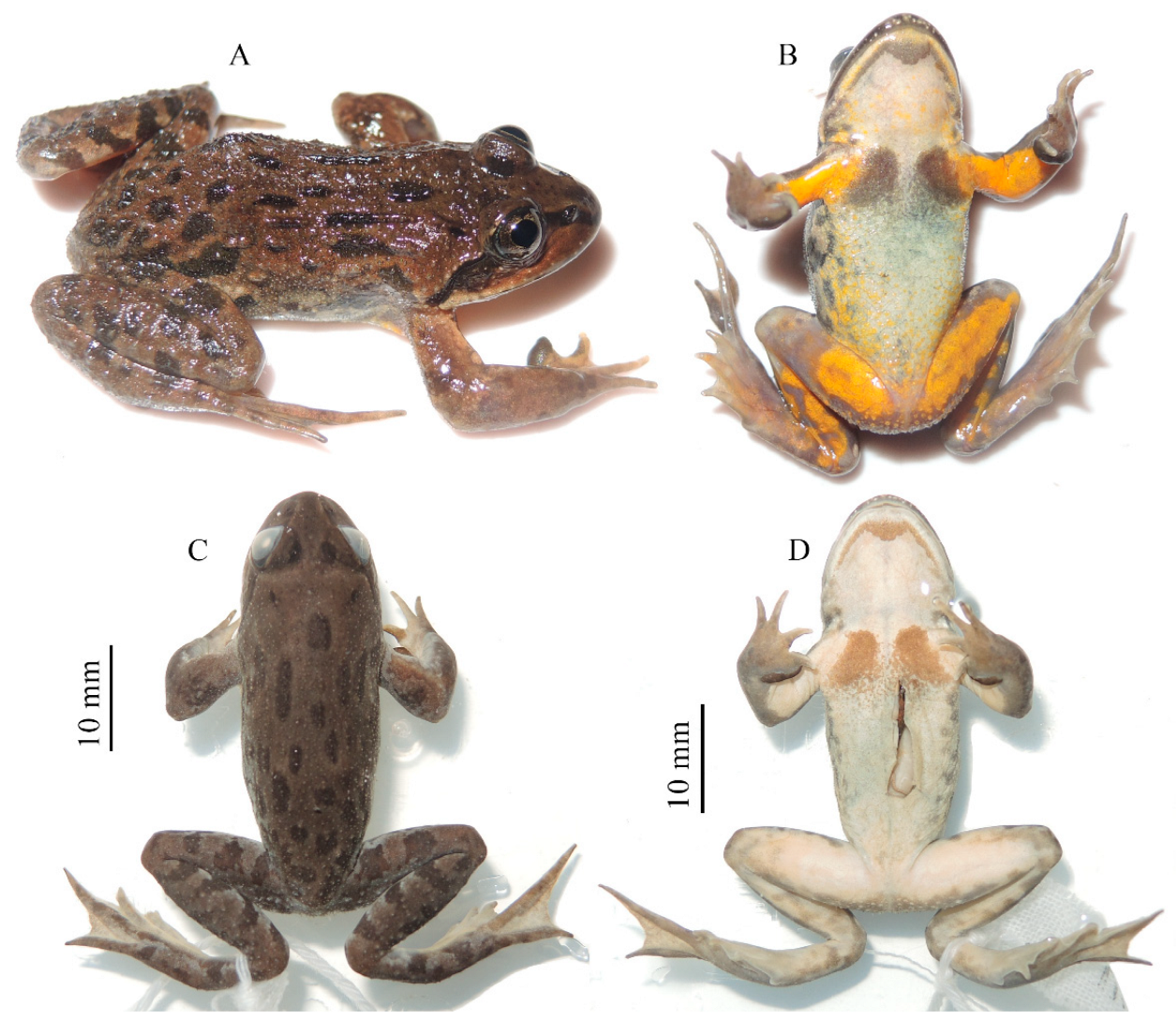
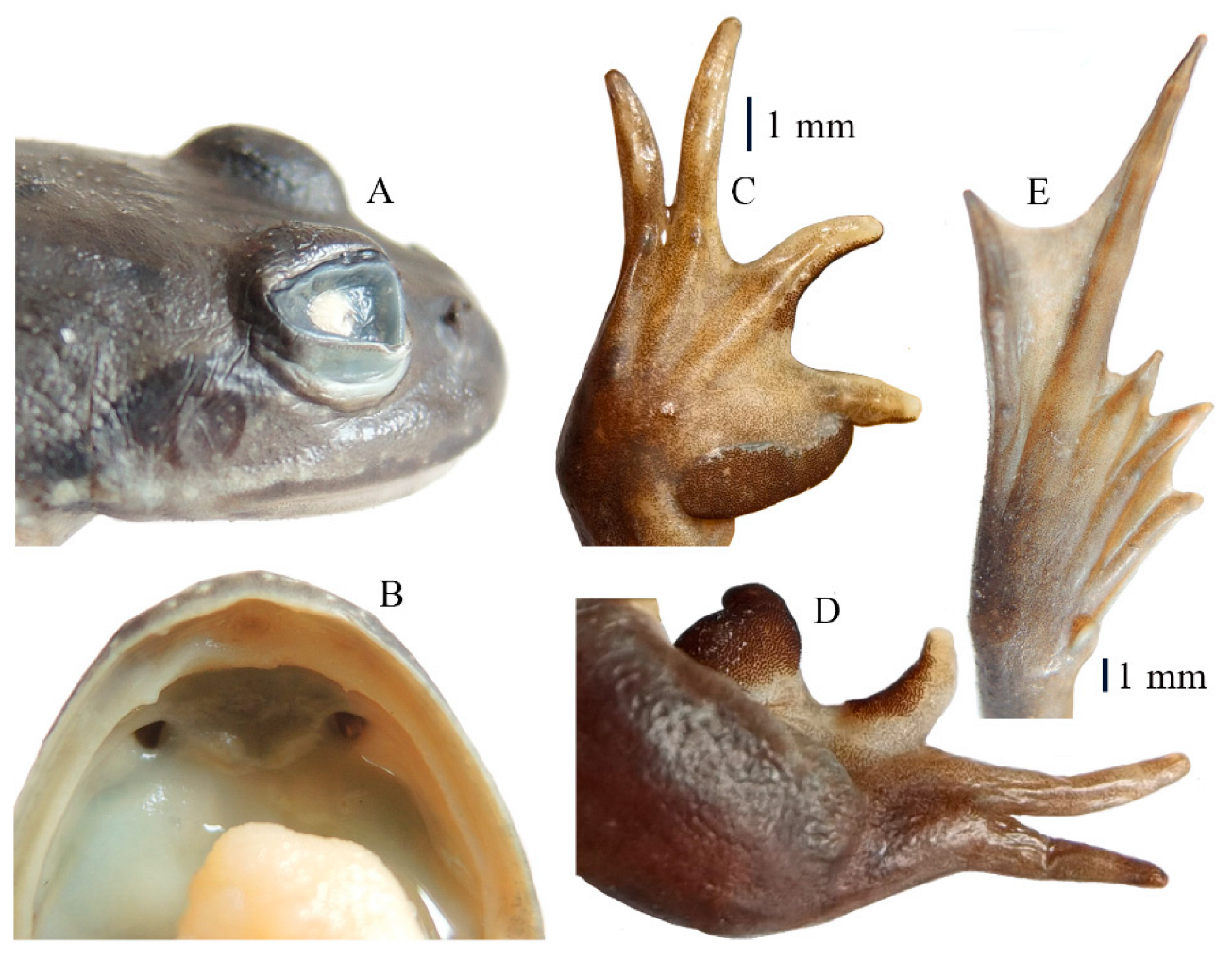

4. Discussion
5. Conclusions
- A key to members of Nanorana (Nanorana)
- 1
- Tympanum absent………………………………………………………………………….2
- –
- Tympanum present…………………………………………………………………………3
- 2
- Nuptial pad present on base of finger I……………………………………N. bangdaensis
- –
- Nuptial pad present on both fingers I and II……………………………………N. parkeri
- 3
- Finger I shorter than finger II………………………………………………………N. pleskei
- –
- Finger I equal to finger II……………………………………………………………………4
- 4
- Subarticular tubercles distinct; ventral surface grayish white scattered with dark blotches………………………………………………………………….…N. ventripunctata
- –
- Subarticular tubercles indistinct; lacking dark blotches on ventral surface and ventral surface of limbs yolk yellow………………………………….N. laojunshanensis sp. nov.
- Minipaa subgen. nov.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xing, Y.W.; Ree, R.H. Uplift-driven diversification in the Hengduan Mountains, a temperate biodiversity hotspot. Proc. Natl. Acad. Sci. USA 2017, 114, E3444–E3451. [Google Scholar] [CrossRef]
- He, K.; Gutiérrez, E.E.; Heming, N.M.; Koepfli, K.P.; Wan, T.; He, S.W.; Jin, W.; Liu, S.Y.; Jiang, X.L. Cryptic phylogeographic history sheds light on the generation of species diversity in sky-island mountains. J. Biogeogr. 2019, 46, 2232–2247. [Google Scholar] [CrossRef]
- López-Pujol, J.; Zhang, F.M.; Sun, H.Q.; Ying, T.S.; Ge, S. Mountains of Southern China as “Plant Museums” and “Plant Cradles”: Evolutionary and conservation insights. Mt. Res. Dev. 2011, 31, 261–269. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Zachos, F.E., Habel, J.C., Eds.; Springer: Berlin, Germany, 2011; pp. 3–22. [Google Scholar]
- Zhao, E.M.; Yang, D.T. Amphibians and Reptiles of the Hengduan Mountains Region; Science Press: Beijing, China, 1997. [Google Scholar]
- Liu, X.L.; He, Y.H.; Wang, Y.F.; Beukema, W.; Hou, S.B.; Li, Y.C.; Che, J.; Yuan, Z.Y. A new frog species of the genus Odorrana (Anura: Ranidae) from Yunnan, China. Zootaxa 2021, 4908, 263–275. [Google Scholar] [CrossRef]
- Zhang, D.R.; Liu, S.; Zhang, L.X.; Hui, H.; Xiao, H.; Rao, D.Q. A New Species of Glyphoglossus Günther, 1869 (Anura: Microhylidae) from Western Yunnan, China. Asian Herpetol. Res. 2021, 12, 371–380. [Google Scholar]
- Rao, D. Atlas of Wildlife in Southwest China: Amphibian. In Atlas of Wildlife in Southwest China: Amphibian; Zhu, J.G., Rao, D.Q., Eds.; Beijing Publishing Group: Beijing, China, 2022; “2020” (printed in 2020 but not distributed until 2022). [Google Scholar]
- Wu, Y.H.; Yan, F.; Stuart, B.L.; Prendini, E.; Suwannapoom, C.; Dahn, H.A.; Zhang, B.L.; Cai, H.X.; Xu, Y.B.; Jiang, K.; et al. A combined approach of mitochondrial DNA and anchored nuclear phylogenomics sheds light unrecognized diversity, phylogeny, and historical biogeography of the torrent frogs, genus Amolops (Anura: Ranidae). Mol. Phylogent. Evol. 2020, 148, 106789. [Google Scholar] [CrossRef]
- Günther, A.C.L.G. Report on the collections of reptiles, batrachians and fishes made by Messrs Potanin and Berezowski in the Chinese provinces Kansu and Sze-chuen. Annuaire du Musée Zoologique de l’Academie Impériale des Sciences de St. Pétersbourg 1896, 1, 199–219. [Google Scholar]
- Frost, D.R. Amphibian Species of the World: An Online Reference. Version 6.2. 2023. American Museum of Natural History, New York, USA. Available online: https://amphibiansoftheworld.amnh.org/index.php (accessed on 13 August 2023).
- Che, J.; Zhou, W.W.; Hu, J.S.; Papenfuss, T.J.; Wake, D.B.; Zhang, Y.P. Spiny frogs (Paini) illuminate the history of the Himalayan region and Southeast Asia. Proc. Natl. Acad. Sci. USA 2010, 107, 13765–13770. [Google Scholar] [CrossRef]
- Dubois, A. Un nouveau sous-genre (Paa) et trois nouvelles espèces du genre Rana. Remarques sur la phylogénies des Ranidés (Amphibiens, Anoures). Bull. Mus. Natl. Hist. Nat. Paris. Ser. 3 Zool. 1975, 324, 1093–1115. [Google Scholar]
- Bourret, R. Notes herpétologiques sur l’Indochine française. XVII. Reptiles et batraciens reçus au Laboratoire des Sciences Naturelles de l’Université au cors de l’année 1938. Descriptions de trois espèces nouvelles. Annexe au Bulletin Général de l’Instruction Publique (Hanoi) 1939, 1939, 13–34. [Google Scholar]
- Roelants, K.; Jiang, J.P.; Bossuyt, F. Endemic ranid (Amphibia: Anura) genera in southern mountain ranges of the Indian subcontinent represent ancient frog lineages: Evidence from the molecular data. Mol. Phylogenet. Evol. 2004, 31, 730–740. [Google Scholar] [CrossRef]
- Jiang, J.P.; Dubois, A.; Ohler, A.; Tillier, A.; Chen, X.H.; Xie, F.; Stöck, M. Phylogenetic relationships of the tribe Paini (Amphibia, Anura, Ranidae) based on partial sequences of mitochondrial 12s and 16s rRNA genes. Zool. Sci. 2005, 22, 353–362. [Google Scholar] [CrossRef]
- Chen, L.Q.; Murphy, R.W.; Lathrop, A.; Ngo, A.; Orlov, N.L.; Ho, C.T.; Somorjai, I. Taxonomic chaos in Asian ranid frogs: An initial phylogenetic resolution. Herpetol. J. Lond. 2005, 15, 231–243. [Google Scholar]
- Frost, D.R.; Grant, T.; Faivovich, J.; Bain, R.H.; Haas, A.; Haddad, C.F.B.; de Sá, R.O.; Channing, A.; Wilkinson, M.; Donnellan, S.C.; et al. The amphibian tree of life. Bull. Am. Mus. Nat. Hist. 2006, 297, 1–370. [Google Scholar] [CrossRef]
- Ohler, A.; Dubois, A. Phylogenetic relationships and generic taxonomy of the tribe Paini (Amphibia, Anura, Ranidae, Dicroglossinae) with diagnoses of two new genera. Zoosystema 2006, 28, 769–784. [Google Scholar]
- Che, J.; Hu, J.S.; Zhou, W.W.; Murphy, R.W.; Papenfuss, T.J.; Chen, M.Y.; Rao, D.Q.; Li, P.P.; Zhang, Y.P. Phylogeny of the Asian spiny frog tribe Paini (Family Dicroglossidae) sensu Dubois. Mol. Phylogenet. Evol. 2009, 50, 59–73. [Google Scholar] [CrossRef]
- Qi, S.; Zhou, Z.Y.; Lu, Y.Y.; Li, J.L.; Qin, H.H.; Hou, M.; Zhang, Y.; Ma, J.Z.; Li, P.P. A new species of Nanorana (Anura: Dicroglossidae) from southern Tibet, China. Russ. J. Herpetol. 2019, 26, 159–174. [Google Scholar] [CrossRef]
- Hofmann, S.; Baniya, C.B.; Litvinchuk, S.N.; Miehe, G.; Li, J.T.; Schmidt, J. Phylogeny of spiny frogs Nanorana (Anura: Dicroglossidae) supports a Tibetan origin of a Himalayan species group. Ecol. Evol. 2019, 9, 14498–14511. [Google Scholar] [CrossRef]
- Hofmann, S.; Masroor, R.; Jablonski, D. Morphological and molecular data on tadpoles of the westernmost Himalayan spiny frog Allopaa hazarensis (Dubois & Khan, 1979). ZooKeys 2021, 1049, 67–77. [Google Scholar]
- Hofmann, S.; Jablonski, D.; Litvinchuk, S.N.; Masroor, R.; Schmidt, J. Relict groups of spiny frogs indicate Late Paleogene-Early Neogene trans-Tibet dispersal of thermophile faunal elements. PeerJ 2021, 9, e11793. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, P.; Rao, D. A new species of Nanorana Günther, 1896 (Anura, Dicroglossidae) from Yunnan, China. ZooKeys 2021, 1048, 49–67. [Google Scholar] [CrossRef]
- Akram, A.; Rais, M.; Lopez-Hervas, K.; Tarvin, R.D.; Saeed, M.; Bolnick, D.I.; Cannatella, D.C. An insight into molecular taxonomy of bufonids, microhylids, and dicroglossid frogs: First genetic records from Pakistan. Ecol. Evol. 2021, 11, 14175–14216. [Google Scholar] [CrossRef]
- Dufresnes, C.; Litvinchuk, S.N. Diversity, distribution and molecular species delimitation in frogs and toads from the Eastern Palaearctic. Zool. J. Linn. Soc. 2022, 195, 695–760. [Google Scholar] [CrossRef]
- Shrestha, B.; Suwal, S.P.; Pandey, B.; Das, J.; Manandhar, P.; Karmacharya, D.; Ohler, A.; Dubois, A.; O’Connell, K.A. Molecular and morphological identification of frog species collected at Rara Lake in Rara National Park, Nepal. Zootaxa 2022, 5168, 222–236. [Google Scholar] [CrossRef]
- Dubois, A.; Ohler, A.; Pyron, R.A. New concepts and methods for phylogenetic taxonomy and nomenclature in zoology, exemplified by a new ranked cladonomy of recent amphibians (Lissamphibia). Megataxa 2021, 5, 1–738. [Google Scholar] [CrossRef]
- Dubois, A. Notes sur la classification des Ranidae (Amphibiens anoures). Bull. Mens. Soc. Linn. Lyon. 1992, 61, 305–352. [Google Scholar] [CrossRef]
- Stejneger, L. A new genus and species of frog from Tibet. J. Wash. Acad. Sci. 1927, 17, 317–319. [Google Scholar]
- Fei, L.; Huang, Y.Z. A new species of the genus Nanorana (Amphibia: Ranidae) from northwestern Yunnan, China. Acta Biol. Plateau Sin. 1985, 4, 71–75. [Google Scholar]
- Fei, L.; Ye, C.Y.; Jiang, J.P. Colored Atlas of Chinese Amphibians; Sichuan Publishing House of Science & Technology: Chengdu, China, 2010. [Google Scholar]
- AmphibiaChina. The Database of Chinese Amphibians. Kunming Institute of Zoology (CAS), Kunming, Yunnan, China. 2023. Available online: http://www.amphibiachina.org/ (accessed on 13 August 2023).
- Fei, L.; Ye, C.Y.; Jiang, J.P.; Xie, F. An Illustrated Key to Chinese Amphibians; Sichuan Publishing House of Science & Technology: Chengdu, China, 2005. [Google Scholar]
- Fei, L.; Hu, S.Q.; Ye, C.Y.; Huang, Y.Z. Fauna Sinica, Amphibia, Vol. 3 Anura Ranidae; Science Press: Beijing, China, 2009. [Google Scholar]
- Palumbi, S.R.; Martin, A.; Romano, S.; Owen MacMillan, W.; Stice, L.; Grabowski, G. The Simple Fool’s Guide to PCR; Department of Zoology, University of Hawaii: Honolulu, HI, USA, 1991. [Google Scholar]
- Che, J.; Chen, H.M.; Yang, J.X.; Jin, J.Q.; Jiang, K.; Yuan, Z.Y.; Murphy, R.W.; Zhang, Y.P. Universal COI primers for DNA barcoding amphibians. Mol. Ecol. Resour. 2012, 12, 247–258. [Google Scholar] [CrossRef]
- Stuart, B.L. The phylogenetic problem of Huia (Amphibia: Ranidae). Mol. Phylogenet. Evol. 2008, 46, 49–60. [Google Scholar] [CrossRef]
- Murray, J.A. A new frog (Rana sternosignata) from Sind. Ann. Mag. Nat. Hist. Ser. 5 1885, 16, 120–121. [Google Scholar] [CrossRef][Green Version]
- Günther, A.C.L.G. Third contribution to our knowledge of reptiles and fishes from the upper Yangtze-Kiang. Ann. Mag. Nat. Hist. Ser. 6 1889, 4, 218–229. [Google Scholar] [CrossRef]
- Dubois, A.; Khan, M.S. A new species of frog (genus Rana, subgenus Paa) from northern Pakistan (Amphibia, Anura). J. Herpetol. 1979, 13, 403–410. [Google Scholar] [CrossRef]
- Gravenhorst, J.L.C. Deliciae Musei Zoologici Vratislaviensis. Fasciculus primus. Chelonios et Batrachia; Leopold Voss: Leipzig, Germany, 1829. [Google Scholar]
- Lyu, Z.T.; Wang, J.; Zeng, Z.C.; Luo, L.; Zhang, Y.W.; Guo, C.P.; Ren, J.L.; Qi, S.; Mo, Y.M.; Wang, Y.Y. Taxonomic clarifications on the floating frogs (Anura: Dicroglossidae: Occidozyga sensu lato) in southeastern China. Vertebr. Zool. 2022, 72, 495–512. [Google Scholar] [CrossRef]
- Köhler, G.; Vargas, J.; Than, N.L.; Schell, T.; Janke, A.; Pauls, S.U.; Thammachoti, P. A taxonomic revision of the genus Phrynoglossus in Indochina with the description of a new species and comments on the classification within Occidozyginae (Amphibia, Anura, Dicroglossidae). Vertebr. Zool. 2021, 71, 1–26. [Google Scholar] [CrossRef]
- Annandale, N. Zoological results of the Abor Expedition, 1911–1912. I. Amphibia. Rec. Indian Mus. 1912, 8, 7–36. [Google Scholar] [CrossRef]
- Sclater, W.L. On some specimens of frogs in the Indian Museum, Calcutta with description of several new species. Proc. Zool. Soc. Lond. 1892, 1892, 341–348. [Google Scholar]
- Osbeck, P. Reise nach Ostindien und China. Nebst O. Toreens Reise nach Suratte und C. G. Ekebergs nachricht von der Landwirthschaft der Chinesen. Aus dem Schwedischen Übersetzt von J. G. Georgi; J. C. Koppe: Rostock, Germany, 1765. [Google Scholar]
- Schneider, J.G. Historia Amphibiorum Naturalis et Literarariae. Fasciculus Primus. Continens Ranas, Calamitas, Bufones, Salamandras et Hydros in Genera et Species Descriptos Notisque suis Distinctos; Friederici Frommanni: Jena, Germany, 1799. [Google Scholar]
- Yu, G.H.; Wu, Z.J.; Yang, J.X. A new species of the Amolops monticola group (Anura: Ranidae) from southwestern Yunnan, China. Zootaxa 2019, 4577, 548–560. [Google Scholar] [CrossRef]
- Boulenger, G.A. On new or little-known Indian and Malayan reptiles and batrachians. Ann. Mag. Nat. Hist. Ser. 6 1891, 8, 288–292. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution formolecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Saikia, B.; Sinha, B.; Kharkongor, I. Odorrana arunachalensis: A new species of Cascade Frog (Anura: Ranidae) from Talle Valley Wildlife Sanctuary, Arunachal Pradesh, India. J. Bioresour. 2017, 4, 30–41. [Google Scholar]
- Smith, M.A. Notes on reptiles and batrachians from Siam and Indo-China (No. 1). J. Nat. Hist. Soc. Siam 1922, 4, 203–214. [Google Scholar]
- Boulenger, G.A. A monograph of the South Asian, Papuan, Melanesian and Australian frogs of the genus Rana. Rec. Indian Mus. 1920, 20, 1–226. [Google Scholar] [CrossRef]
- Anderson, J. A list of the reptilian accession to the Indian Museum, Calcutta from 1865 to 1870, with a description of some new species. J. Asiatic Soc. Bengal 1871, 40, 12–39. [Google Scholar]
- Günther, A.C.L.G. Contribution to the knowledge of the reptiles of the Himalaya mountains. Proc. Zool. Soc. Lond. 1860, 1860, 148–175. [Google Scholar]
- Smith, M.A. On a collection of amphibians and reptiles from Nepal. Ann. Mag. Nat. Hist. Ser. 12 1951, 4, 726–728. [Google Scholar] [CrossRef]
- Dubois, A.; Matsui, M.; Ohler, A. A replacement name for Rana (Paa) rara Dubois & Matsui, 1983 (Amphibia, Anura, Ranidae, Raninae). Alytes 2001, 19, 2–4. [Google Scholar]
- Dubois, A. Diagnoses de trois espèces nouvelles d’amphibiens du Népal. Bull. Soc. Zool. Fr. 1974, 98, 495–497. [Google Scholar]
- Liu, C.C.; Hu, S.Q.; Yang, F.H. Amphibia of Yunnan collected in 1958. Acta Zool. Sin. 1960, 12, 149–174. [Google Scholar]
- Stoliczka, F. Notes on some new species of Reptilia and Amphibia, collected by Dr. W. Waagen in North-western Punjab. Proc. Asiat. Soc. Bengal 1872, 1872, 124–131. [Google Scholar]
- Anderson, J. Anatomical and Zoological Researches: Comprising an Account of the Zoological Results of the Two Expeditions to Western Yunnan in 1868 and 1875; and a Monograph of the Two Cetacean Genera Platanista and Orcella; Bernard Quaritch: London, UK, 1879. [Google Scholar]
- Boulenger, G.A. Catalogue of the Batrachia Salientia s. Ecaudata in the Collection of the British Museum, 2nd ed.; Taylor and Francis: London, UK, 1882. [Google Scholar]
- Ye, C.C. A survey of amphibians in Xizang (Tibet). Acta Zool. Sin. 1977, 23, 54–63. [Google Scholar]
- Huang, Y.Z.; Fei, L. Two new species of amphibians from Xizang. Acta Zootaxonomica Sin. 1981, 6, 211–215. [Google Scholar]
- Boulenger, G.A. An account of the batrachians obtained in Burma by M.L. Fea of the Genoa Civic Museum. Ann. Mus. Civico Storia Nat. Genova Ser. 2 1887, 5, 418–424. [Google Scholar]
- Fei, L. Atlas of Amphibians of China; Henan Press of Science and Technology: Zhengzhou, China, 1999. [Google Scholar]
- Das, I.; Chanda, S.K. A new species of Scutiger (Anura: Megophryidae) from Nagaland, north-eastern India. Herpetol. J. Lond. 2000, 10, 69–72. [Google Scholar]
- Boulenger, G.A. Descriptions of new frogs of the genus Rana. Ann. Mag. Nat. Hist. Ser. 8 1917, 20, 413–418. [Google Scholar] [CrossRef]
- Dubois, A. Miscellanea taxinomica batrachologica (I). Alytes 1987, 5, 7–95. [Google Scholar]
- Yang, X.; Wang, B.; Hu, J.H.; Jiang, J.P. A new species of the genus Feirana (Amphibia: Anura: Dicroglossidae) from the Western Qinling Mountains of China. Asian Herpetol. Res. 2011, 2, 72–86. [Google Scholar] [CrossRef]
- Liu, C.C.; Hu, S.Q.; Yang, F.H. Amphibians from Wushan, Szechwan. Acta Zool. Sin. 1960, 12, 278–292. [Google Scholar]
- Chen, X.H.; Jiang, J.P. A new species of the genus Paa from China. Herpetol. Sin. 2002, 9, 231. [Google Scholar]
- Zhou, W.W.; Zhang, B.L.; Chen, H.M.; Jin, J.Q.; Yang, J.X.; Wang, Y.Y.; Jiang, K.; Murphy, R.W.; Zhang, Y.P.; Che, J. DNA barcodes and species distribution models evaluate threats of global climate changes to genetic diversity: A case study from Nanorana parkeri (Anura: Dicroglossidae). PLoS ONE 2014, 9, e10389. [Google Scholar] [CrossRef][Green Version]
- Wang, G.D.; Zhang, B.L.; Zhou, W.W.; Li, Y.X.; Jin, J.Q.; Shao, Y.; Yang, H.C.; Liu, Y.H.; Yan, F.; Chen, H.M.; et al. Selection and environmental adaptation along a path to speciation in the Tibetan frog Nanorana parkeri. Proc. Natl. Acad. Sci. USA 2018, 115, E5056–E5065. [Google Scholar] [CrossRef]
- Vieites, D.R.; Wollenberg, K.C.; Andreone, F.; Köhler, J.; Glaw, F.; Vences, M. Vast underestimation of Madagascar’s biodiversity evidenced by an integrative amphibian inventory. Proc. Natl. Acad. Sci. USA 2009, 106, 8267–8272. [Google Scholar] [CrossRef]
- Tang, S.J.; Sun, T.; Liu, S.; Luo, S.D.; Yu, G.H.; Du, L.N. A new species of cascade frog (Anura: Ranidae: Amolops) from central Yunnan, China. Zool. Lett. 2023, 9, 15. [Google Scholar] [CrossRef]
- Hofmann, S.; Schmidt, J.; Masroor, R.; Borkin, L.J.; Litvintchuk, S.; Rödder, D.; Vershinin, V.; Jablonski, D. Endemic lineages of spiny frogs demonstrate the biogeographic importance and conservational needs of the Hindu Kush-Himalaya region. Zool. J. Linn. Soc. 2023, 198, 310–325. [Google Scholar] [CrossRef]
- Zug, G.R. Amphibians and Reptiles of Myanmar: Checklists and Keys I. Amphibians, Crocodilians, and Turtles. Smithson. Contrib. Zool. 2022, 653, 1–113. [Google Scholar] [CrossRef]
- Wangyal, J.T.; Norbu, L.; Ghalley, T.B.; Shacha, N. First record of the Arunachal cascade frog, Nanorana arunachalensis (Saikia et al., 2017), from the Himalayan Kingdom of Bhutan. Herpetol. Notes 2023, 16, 569–572. [Google Scholar]
- Pyron, R.A.; Wiens, J.J. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of advanced frogs, salamanders, and caecilians. Mol. Phylogenet. Evol. 2011, 61, 543–583. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Jiang, K.; Yang, F.; Zhang, Y.P. Amphibians and Reptiles in Tibet: Diversity and Evolution; Science Press: Beijing, China, 2020. [Google Scholar]


| Species | Voucher no. | Locality | 16S | COI | CYTB | RAG-1 |
|---|---|---|---|---|---|---|
| Odorrana hosii | USNM:Herp:586991 | Yeybu Village, Tanintharyi, Myanmar | MG935960 | MG935666 | - | - |
| Amolops mengdingensis | KIZ20160265 | Mengding, Yunnan, China | MK501808 | MK501811 | - | - |
| Occidozyga lima | USNM:Herp:520376 | Chatthin, Sagaing, Myanmar | MG935924 | MG935630 | - | - |
| Occidozyga lingnanica | SYS a005585 | Shenzhen, Guangdong, China | ON615075 | ON615615 | - | - |
| Occidozyga myanhessei | USNM:Herp:587105 | Dawei, Bago, Myanmar | MG935916 | MG935622 | - | - |
| Ingerana tenasserimensis | CAS 205064 | Myanmar | AY322302 | - | - | |
| Ingerana borealis | MZMU1644 | Tibet, China | MT799709 | - | - | KU243100 |
| Fejervarya cancrivora | HW6 | Guangxi, China | EU652694 | EU652694 | EU652694 | HM163581 |
| Hoplobatrachus chinensis | THW1 | Jinhua, Zhejiang, China | JX181763 | JX181763 | JX181763 | - |
| Sphaerotheca breviceps | USNM:Herp:537466 | Sagaing, Myanmar | MG935993 | MG935699 | - | - |
| Limnonectes bannaensis | ZNAC 21020 | Yunnan, China | AY899242 | AY899242 | AY899242 | |
| Quasipaa boulengeri | XM3632 | Sichuan, China | KX645665 | KX645665 | KX645665 | - |
| Chrysopaa sternosignata | USNM:Herp:589844 | Parvan, Afghanistan | MG700155 | MG699938 | - | - |
| Allopaa hazarensis | 9386 | Pakistan | MW598397 | MW603006 | - | MW598465 |
| Nanorana arunachalensis | ZSIS-M40 | Cona, Tibet, China | MN636773 | - | - | - |
| Nanorana (Chaparana) aenea | 2001.0277 | Yunnan, China | KR827954 | KR087830 | - | HM163609 |
| Nanorana (Chaparana) quadranus | CIB 20060644 | Sichuan, China | GQ225907 | OL762449 | - | HM163591 |
| Nanorana (Chaparana) taihangnica | LGW-LC-001 | Henan, China | KF199146 | KF199146 | KF199146 | - |
| Nanorana (Chaparana) yunnanensis | STJW-LP-001 | Yunnan, China | KF199150 | KF199150 | KF199150 | HM163592 |
| Nanorana (Paa) chayuensis | MNU20190419 | Chayu, China | MN411630 | MN411630 | MN411630 | HM163587 |
| Nanorana (Paa) liebigii | SH080524-NME | Nepal | MN012113 | MN012241 | - | MN032536 |
| Nanorana (Paa) medogensis | SYNU-XZ35 | Motuo, China | MH315960 | - | - | HM163590 |
| Nanorana rarica | A1961-13-NME | Nepal | MN012202 | MN012322 | ||
| Nanorana (Paa) rostandi | SYNU-1507058 | Tibet, China | MH315964 | - | - | MW111374 |
| Nanorana ercepeae | A2017-13-NME | Nepal | MN012076 | MN012212 | MN032500 | |
| Nanorana zhaoermii | SYNU-1706063 | Tibet, China | MH315958 | - | - | - |
| Nanorana xuelinensis | KIZL2019013 | Lancang, Yunnan, Chna | MZ416027 | - | - | - |
| Nanorana vicina | WLM:NV289171 | Murree, Pakistan | MW898174 | - | - | - |
| Nanorana unculuanus | KizYP010 | Yunnan, China | DQ118491 | - | - | HM163595 |
| Nanorana sichuanensis | CIBYY20080693 | Sichuan, China | KU140064 | - | - | - |
| Nanorana polunini | 2003.3085 | Pangum, Nepal | KR827957 | MW111375 | ||
| Nanorana phrynoides | CIBYN090223 | Yunnan, China | KU140002 | - | - | - |
| Nanorana maculosa | YNU-HU2002308 | Yunnan, China | EU979835 | - | - | HM163588 |
| Nanorana kangxianensis | CIB1247625 | Gansu, China | MZ895123 | MZ895123 | MZ895123 | - |
| Nanorana conaensis | KizYP152 | Tibet, China | DQ118513 | - | - | HM163589 |
| Nanorana blanfordii | SYNU-1507011 | Tibet, China | MH315963 | - | - | MW111370 |
| Nanorana arnoldi | YNU-HU200109006 | Yunnan, China | EU979837 | - | - | - |
| Nanorana minica | WT008 | Himachal Pradesh, India | OQ079488 | - | - | - |
| Nanorana sp. | VV11.1-RAS | - | OP173783 | OP174426 | - | OP204887 |
| Nanorana (Nanorana) bangdaensis | N7_06_NME | Nimu, Lasa, Tibet, China | MN012126 | MN012249 | - | MN032549 |
| Nanorana (Nanorana) bangdaensis | CAS802L | Dangxiong, Lasa, Tibet, China | MN012138 | MN012261 | - | MN032555 |
| Nanorana (Nanorana) bangdaensis | KIZ014955 | Bangda, Baxoi, Tibet, China | - | KJ811447 | - | - |
| Nanorana (Nanorana) bangdaensis | YPX32289 | Daritang, Uzi, Tibet | - | KJ811502 | - | - |
| Nanorana (Nanorana) bangdaensis | YPX18274 | Lulang, Nyingchi, Tibet | - | KJ811344 | - | - |
| Nanorana (Nanorana) bangdaensis | YPX15383 | Lhunze, Tibet | - | KJ811253 | - | - |
| Nanorana (Nanorana) bangdaensis | N3_06_NME | Seni, Naqu, Tibet, China | MN012150 | MN012272 | - | MN032561 |
| Nanorana (Nanorana) bangdaensis | KP317482 | Dangxiong, Lasa, Tibet, China | KP317482 | KP317482 | KP317482 | - |
| Nanorana (Nanorana) bangdaensis | KIZ20181001 | Bangda, Baxoi, Tibet | OR678588 | OR671665 | - | OR678566 |
| Nanorana (Nanorana) bangdaensis | KIZ20181002 | Bangda, Baxoi, Tibet | OR678589 | OR671666 | - | OR678567 |
| Nanorana (Nanorana) bangdaensis | KIZ20181003 | Bangda, Baxoi, Tibet | OR678590 | OR671667 | - | OR678568 |
| Nanorana (Nanorana) bangdaensis | KIZ20181004 | Bangda, Baxoi, Tibet | OR678591 | OR671668 | - | OR678569 |
| Nanorana (Nanorana) bangdaensis | KIZ20181005 | Bangda, Baxoi, Tibet | OR678592 | OR671669 | - | OR678570 |
| Nanorana (Nanorana) parkeri | KIZ20181101 | Jilong, Rikaze, Tibet, China | OR678593 | OR671670 | - | OR678571 |
| Nanorana (Nanorana) parkeri | KIZ20181102 | Jilong, Rikaze, Tibet, China | OR678594 | OR671671 | - | OR678572 |
| Nanorana (Nanorana) parkeri | KIZ20181103 | Jilong, Rikaze, Tibet, China | OR678595 | OR671672 | - | OR678573 |
| Nanorana (Nanorana) parkeri | KIZ20181104 | Jilong, Rikaze, Tibet, China | OR678596 | OR671673 | - | OR678574 |
| Nanorana (Nanorana) parkeri | KIZ20181105 | Jilong, Rikaze, Tibet, China | OR678597 | OR671674 | - | OR678575 |
| Nanorana (Nanorana) parkeri | KIZ08139 | Gangga, Tingri, Tibet, China | - | KJ811162 | - | - |
| Nanorana (Nanorana) parkeri | YPX14998 | Nierixiong, Rikaze, Tibet | - | KJ811364 | - | - |
| Nanorana (Nanorana) parkeri | YPX15060 | Saga, Tibet | - | KJ811396 | - | - |
| Nanorana (Nanorana) parkeri | YPX14646 | Nierixiong, Rikaze, Tibet, China | - | KY172326 | KY172509 | - |
| Nanorana (Nanorana) pleskei | YPX32958 | Maqu, Ganan, Gansu, China | - | KY172146 | KY172329 | KY172539 |
| Nanorana (Nanorana) pleskei | YPX25853 | Banma, Guoluo, Qinghai, China | - | KY172155 | KY172338 | KY172541 |
| Nanorana (Nanorana) pleskei | KIZ014906 | Jiangda, Changdu, Tibet | - | KY172183 | KY172366 | KY172552 |
| Nanorana (Nanorana) pleskei | YPX32403 | Kangding, Sichuan, China | - | KY172218 | KY172401 | KY172605 |
| Nanorana (Nanorana) pleskei | KQ13_14_NME | Ganzi, Sichuan, China | MN012160 | MN012282 | - | MN032566 |
| Nanorana (Nanorana) pleskei | KQ47_14_NME | Kangding, Ganzi, Sichuan, China | MN012156 | MN012278 | - | MN032562 |
| Nanorana (Nanorana) pleskei | CIB20080515-1 | Shiqu, Sichuan, China | HQ324232 | HQ324232 | HQ324232 | - |
| Nanorana (Nanorana) ventripunctata | GXNU YU130021 | Xiaozhongdian, Zhongdian, Yunnan, China | OR678598 | OR671675 | OR678554 | OR678576 |
| Nanorana (Nanorana) ventripunctata | GXNU YU130022 | Xiaozhongdian, Zhongdian, Yunnan, China | OR678599 | OR671676 | OR678555 | OR678577 |
| Nanorana (Nanorana) ventripunctata | GXNU YU090163 | Bitahai, Zhongdian, Yunnan, China | OR678600 | OR671677 | OR678556 | OR678578 |
| Nanorana (Nanorana) ventripunctata | GXNU YU000498 | Bitahai, Zhongdian, Yunnan, China | OR678601 | OR671678 | OR678557 | OR678579 |
| Nanorana (Nanorana) ventripunctata | GXNU YU000502 | Bitahai, Zhongdian, Yunnan, China | OR678602 | OR671679 | OR678558 | OR678580 |
| Nanorana (Nanorana) ventripunctata | GXNU YU000503 | Bitahai, Zhongdian, Yunnan, China | OR678603 | OR671680 | OR678559 | OR678581 |
| Nanorana (Nanorana) laojunshanensis sp. nov. | GXNU YU090312 | Mt. Laojun, Lijiang, Yunnan, China | OR678604 | OR671681 | OR678560 | OR678582 |
| Nanorana (Nanorana) laojunshanensis sp. nov. | GXNU YU090313 | Mt. Laojun, Lijiang, Yunnan, China | OR678605 | OR671682 | OR678561 | OR678583 |
| Nanorana (Nanorana) laojunshanensis sp. nov. | GXNU YU090314 | Mt. Laojun, Lijiang, Yunnan, China | OR678606 | OR671683 | OR678562 | OR678584 |
| Nanorana (Nanorana) laojunshanensis sp. nov. | GXNU YU090315 | Mt. Laojun, Lijiang, Yunnan, China | OR678607 | OR671684 | OR678563 | OR678585 |
| Nanorana (Nanorana) laojunshanensis sp. nov. | GXNU YU090316 | Mt. Laojun, Lijiang, Yunnan, China | OR678608 | OR671685 | OR678564 | OR678586 |
| Nanorana (Nanorana) laojunshanensis sp. nov. | GXNU YU090317 | Mt. Laojun, Lijiang, Yunnan, China | OR678609 | OR671686 | OR678565 | OR678587 |
| Locus | Primer Name | Primer Sequence | Source |
|---|---|---|---|
| 16S | 16Sar | 5′-CGCCTGTTTATCAAAAACAT-3′ | [38] |
| 16Sbr | 5′-CCGGTCTGAACTCAGATCACGT-3′ | [38] | |
| cytb | CytbF | 5′-ACACCCGCCAATTTGTCTTC-3′ | This study |
| CytbR | 5′-TGAGAGGGGAAGGAGAAGGA-3′ | This study | |
| COI | Chmf4 | 5′-TYTCWACWAAYCAYAAAGAYATCGG-3′ | [39] |
| Chmr4 | 5′-ACYTCRGGRTGRCCRAARAATCA-3′ | [39] | |
| RAG1 | L-RAG1Ran | 5′-CTGGTCGTCAGATCTTTCAGC-3′ | [40] |
| H-RAG1Ran | 5′-GCAAAACGTTGAGAGTGATAAC-3′ | [40] |
| Subset | Partitions | Model |
|---|---|---|
| 1 | 16S rRNA | GTR + I + G |
| 2 | COI_pos1 | SYM + I |
| 3 | COI_pos2 | HKY + I |
| 4 | COI_pos3 | GTR + G |
| 5 | cytb_pos1 | SYM + G |
| 6 | cytb_pos2 | HKY + I |
| 7 | cytb_pos3 | GTR + G |
| 8 | Rag1_pos1 | GTR + G |
| 9 | Rag1_pos2 | GTR + I |
| 10 | Rag1_pos3 | GTR + G |
| Species | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| 1 | N. laojunshanensis sp. nov. | 7.4 | 8.7 | 10.6 | 10.0 | |
| 2 | N. ventripunctata | 1.6 | 8.8 | 11.2 | 10.9 | |
| 3 | N. pleskei | 1.6 | 1.9 | 12.9 | 12.4 | |
| 4 | N. bangdaensis | 2.0 | 2.1 | 2.8 | 3.4 | |
| 5 | N. parkeri | 1.6 | 1.8 | 2.7 | 1.0 |
| Character | GXNU YU090314 | GXNU YU090313 | GXNU YU090315 | GXNU YU090316 | GXNU YU090317 | GXNU YU090312 |
|---|---|---|---|---|---|---|
| Sex | M | M | M | M | M | F |
| SVL (snout–vent length) | 36.1 | 38.5 | 35.9 | 34.9 | 33.3 | 42.9 |
| HL (head length) | 10.7 | 11.0 | 10.6 | 10.2 | 10.1 | 11.4 |
| HW (head width) | 11.3 | 12.9 | 11.9 | 11.5 | 10.7 | 13.6 |
| SL (snout length) | 4.8 | 4.9 | 5.0 | 4.5 | 4.5 | 5.0 |
| IND (internarial distance) | 2.3 | 2.4 | 2.3 | 2.1 | 2.1 | 2.7 |
| IOD (interorbital distance) | 1.9 | 2.2 | 2.1 | 1.9 | 1.8 | 2.3 |
| UEW (upper eyelid width) | 2.4 | 2.4 | 2.0 | 2.0 | 1.9 | 2.6 |
| ED (eye diameter) | 3.9 | 4.5 | 3.9 | 3.8 | 3.9 | 4.6 |
| TD (tympanum diameter) | 1.3 | 1.8 | 1.2 | 1.1 | 1.2 | 1.5 |
| DNE (nostril–eye distance) | 1.7 | 2.1 | 1.7 | 1.6 | 1.6 | 2.1 |
| FHL (forearm and hand length) | 15.1 | 15.1 | 15.4 | 16.2 | 13.8 | 17.4 |
| TL (tibia length) | 15.3 | 15.8 | 15.7 | 15.5 | 14.2 | 16.9 |
| TFL (length of foot and tarsus) | 26.5 | 27.6 | 26.2 | 27.3 | 24.1 | 30.7 |
| FL (foot length) | 18.9 | 20.8 | 18.9 | 20.1 | 17.1 | 22.4 |
| N. laojunshanensis sp. nov. and N. ventripunctata | N. laojunshanensis sp. nov. and N. pleskei | |||
|---|---|---|---|---|
| Character | PC1 | PC2 | PC1 | PC2 |
| Eigenvalue | 4.346 | 3.283 | 6.272 | 3.403 |
| % variation | 33.43% | 25.25% | 48.24% | 26.18% |
| HL | 0.742 | −0.123 | −0.552 | 0.711 |
| HW | 0.878 | −0.015 | 0.383 | 0.810 |
| SN | 0.384 | −0.574 | −0.411 | 0.783 |
| IND | 0.126 | 0.563 | −0.840 | 0.289 |
| IOD | 0.677 | −0.297 | 0.753 | −0.109 |
| UEW | −0.249 | 0.729 | −0.800 | 0.320 |
| ED | 0.251 | −0.755 | −0.89 | 0.698 |
| TD | −0.09 | 0.162 | −0.638 | 0.346 |
| NED | 0.390 | −0.733 | −0.680 | 0.224 |
| FHL | 0.600 | 0.650 | 0.871 | 0.321 |
| TL | 0.629 | 0.596 | 0.770 | 0.605 |
| TFL | 0.838 | 0.191 | 0.854 | 0.418 |
| FL | 0.818 | 0.230 | 0.884 | 0.359 |
| Species | Suggested Subgenus | ||||
|---|---|---|---|---|---|
| Subg. Nanorana | Subg. Paa | Subg. Chaparana | Subg. Minipaa | Unknown | |
| N. aenea | yes | ||||
| N. annandalii | yes | ||||
| N. arnoldi | yes | ||||
| N. bangdaensis | yes | ||||
| N. blanfordii | yes | ||||
| N. chayuensis | yes | ||||
| N. conaensis | yes | ||||
| N. ercepeae | yes | ||||
| N. feae | yes | ||||
| N. gammii | yes | ||||
| N. hazarensis | yes | ||||
| N. kangxianensis | yes | ||||
| N. liebigii | yes | ||||
| N. maculosa | yes | ||||
| N. medogensis | yes | ||||
| N. minica | yes | ||||
| N. mokokchungensis | yes | ||||
| N. parkeri | yes | ||||
| N. phrynoides | yes | ||||
| N. pleskei | yes | ||||
| N. polunini | yes | ||||
| N. quadranus | yes | ||||
| N. rarica | yes | ||||
| N. rostandi | yes | ||||
| N. sichuanensis | yes | ||||
| N. taihangnica | yes | ||||
| N. unculuanus | yes | ||||
| N. ventripunctata | yes | ||||
| N. vicina | yes | ||||
| N. xuelinensis | yes | ||||
| N. yunnanensis | yes | ||||
| N. zhaoermii | yes | ||||
| N. laojunshanensis sp. nov. | yes | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Liu, S.; Yu, G. A New Species of Nanorana (Anura: Dicroglossidae) from Northwestern Yunnan, China, with Comments on the Taxonomy of Nanorana arunachalensis and Allopaa. Animals 2023, 13, 3427. https://doi.org/10.3390/ani13213427
Tang S, Liu S, Yu G. A New Species of Nanorana (Anura: Dicroglossidae) from Northwestern Yunnan, China, with Comments on the Taxonomy of Nanorana arunachalensis and Allopaa. Animals. 2023; 13(21):3427. https://doi.org/10.3390/ani13213427
Chicago/Turabian StyleTang, Shangjing, Shuo Liu, and Guohua Yu. 2023. "A New Species of Nanorana (Anura: Dicroglossidae) from Northwestern Yunnan, China, with Comments on the Taxonomy of Nanorana arunachalensis and Allopaa" Animals 13, no. 21: 3427. https://doi.org/10.3390/ani13213427
APA StyleTang, S., Liu, S., & Yu, G. (2023). A New Species of Nanorana (Anura: Dicroglossidae) from Northwestern Yunnan, China, with Comments on the Taxonomy of Nanorana arunachalensis and Allopaa. Animals, 13(21), 3427. https://doi.org/10.3390/ani13213427






