Historic Horse Family Displaying Malformations of the Cervicothoracic Junction and Their Connection to Modern German Warmblood Horses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Examination of Historical Equine Skeletons
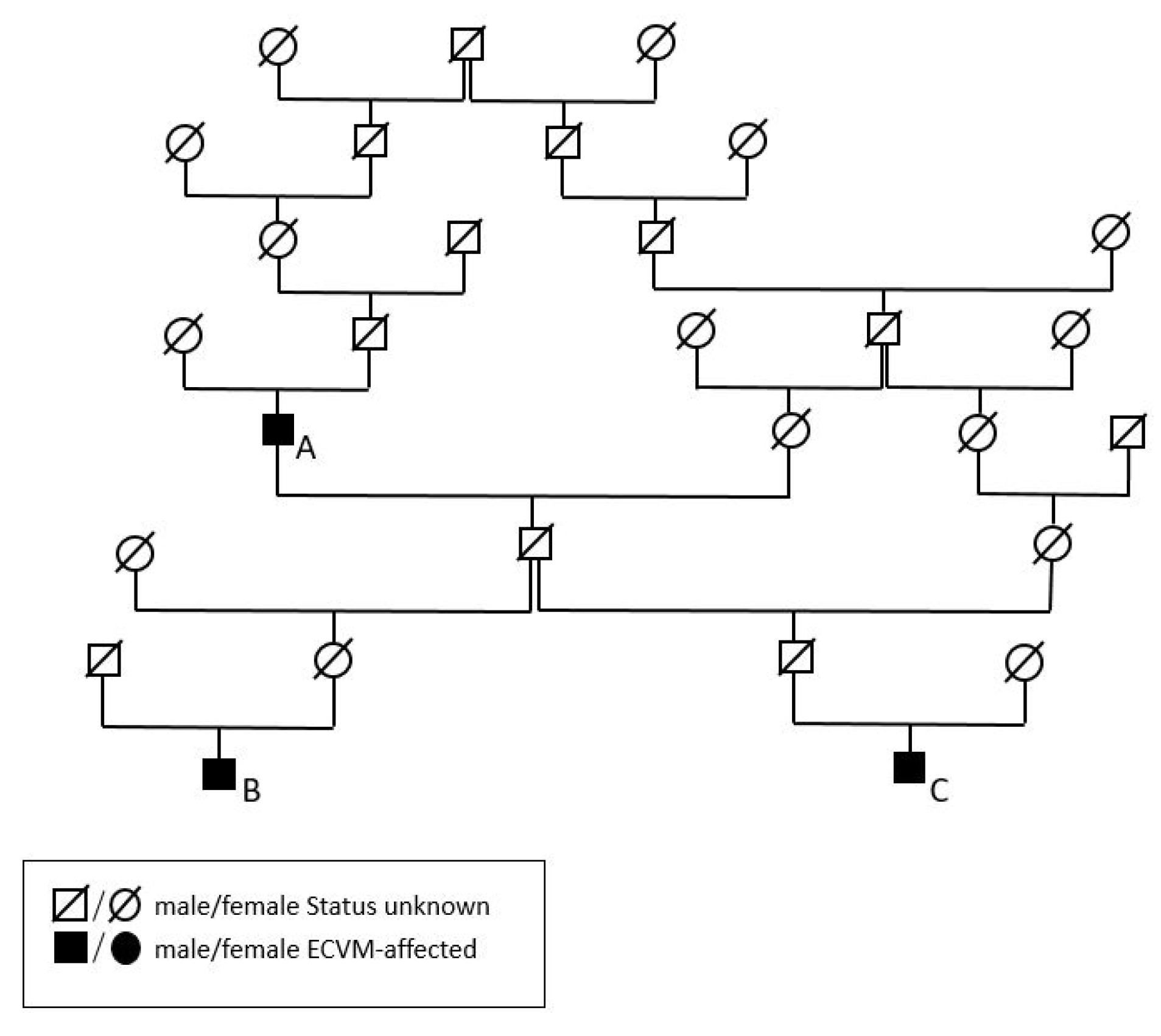
2.2. Pedigree Analysis
2.2.1. Animals
2.2.2. Data Analysis
3. Results
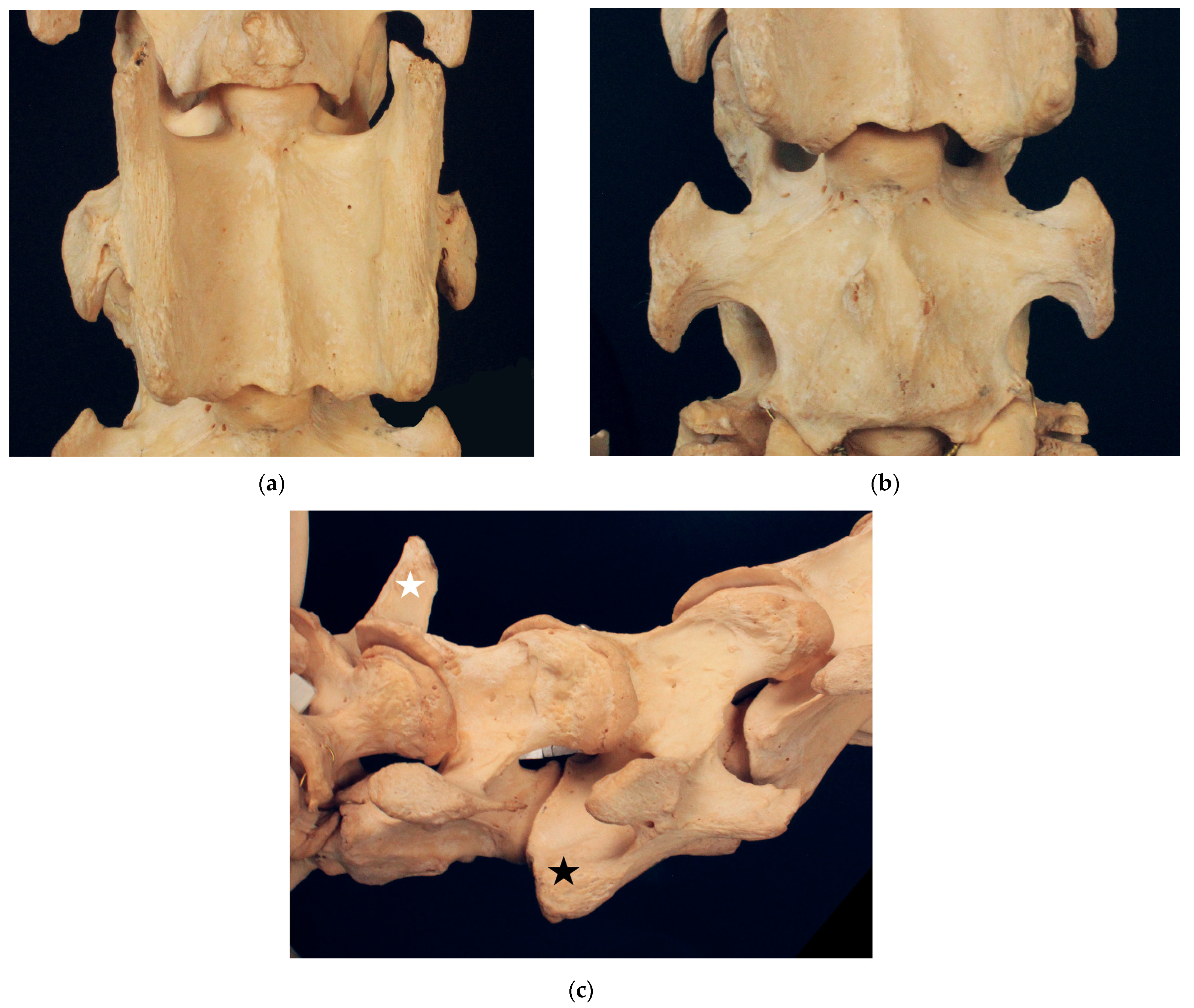
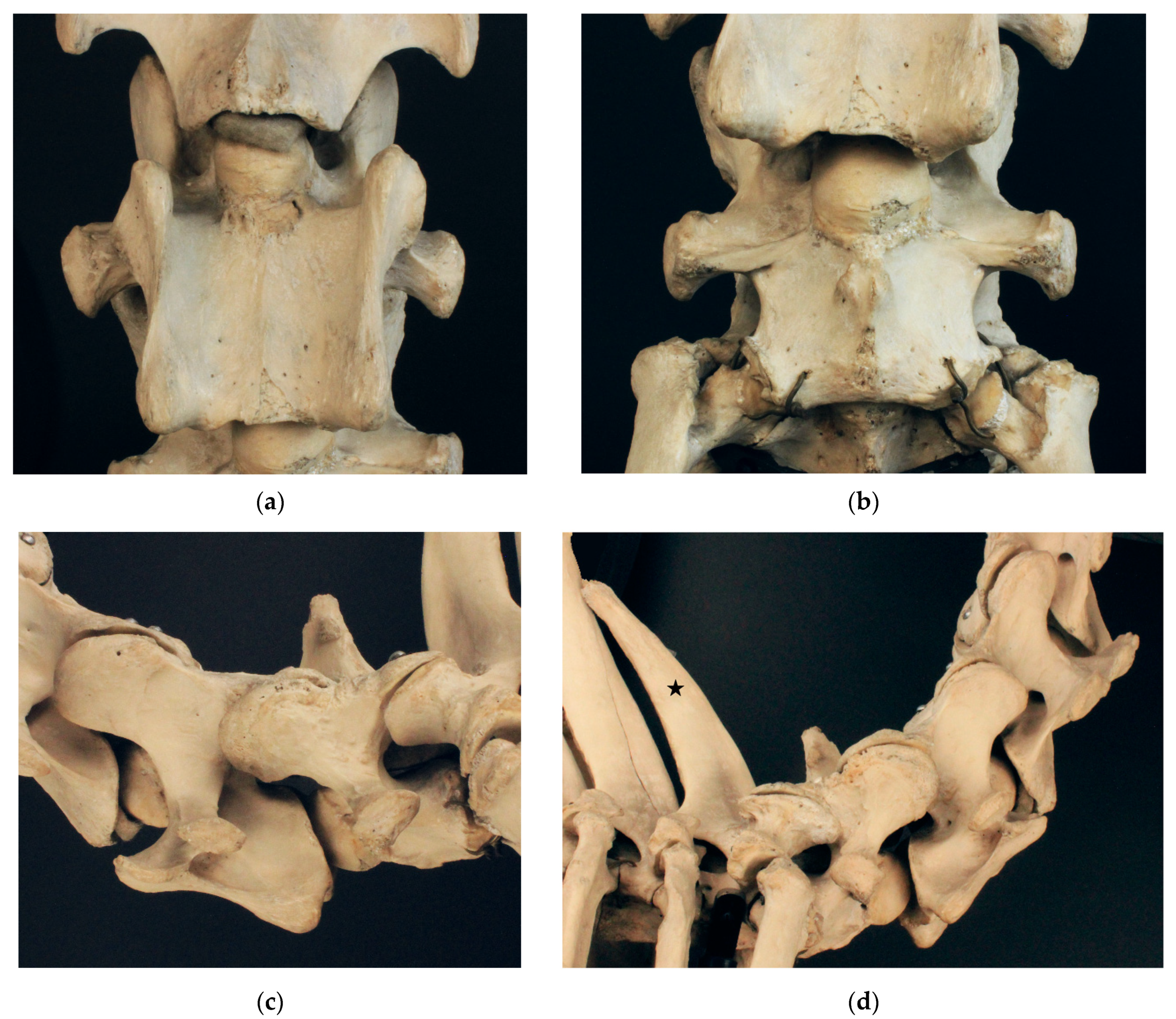
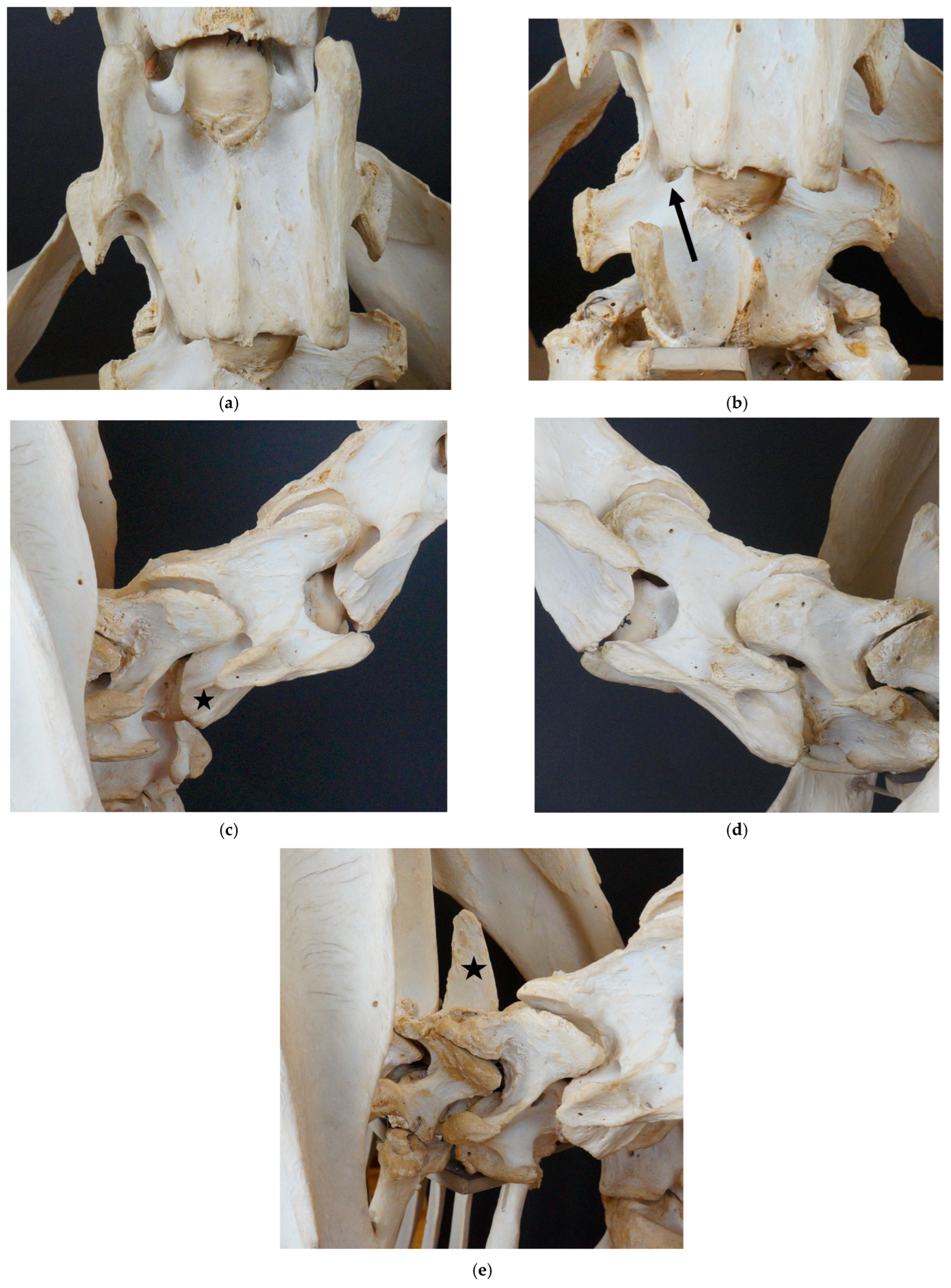
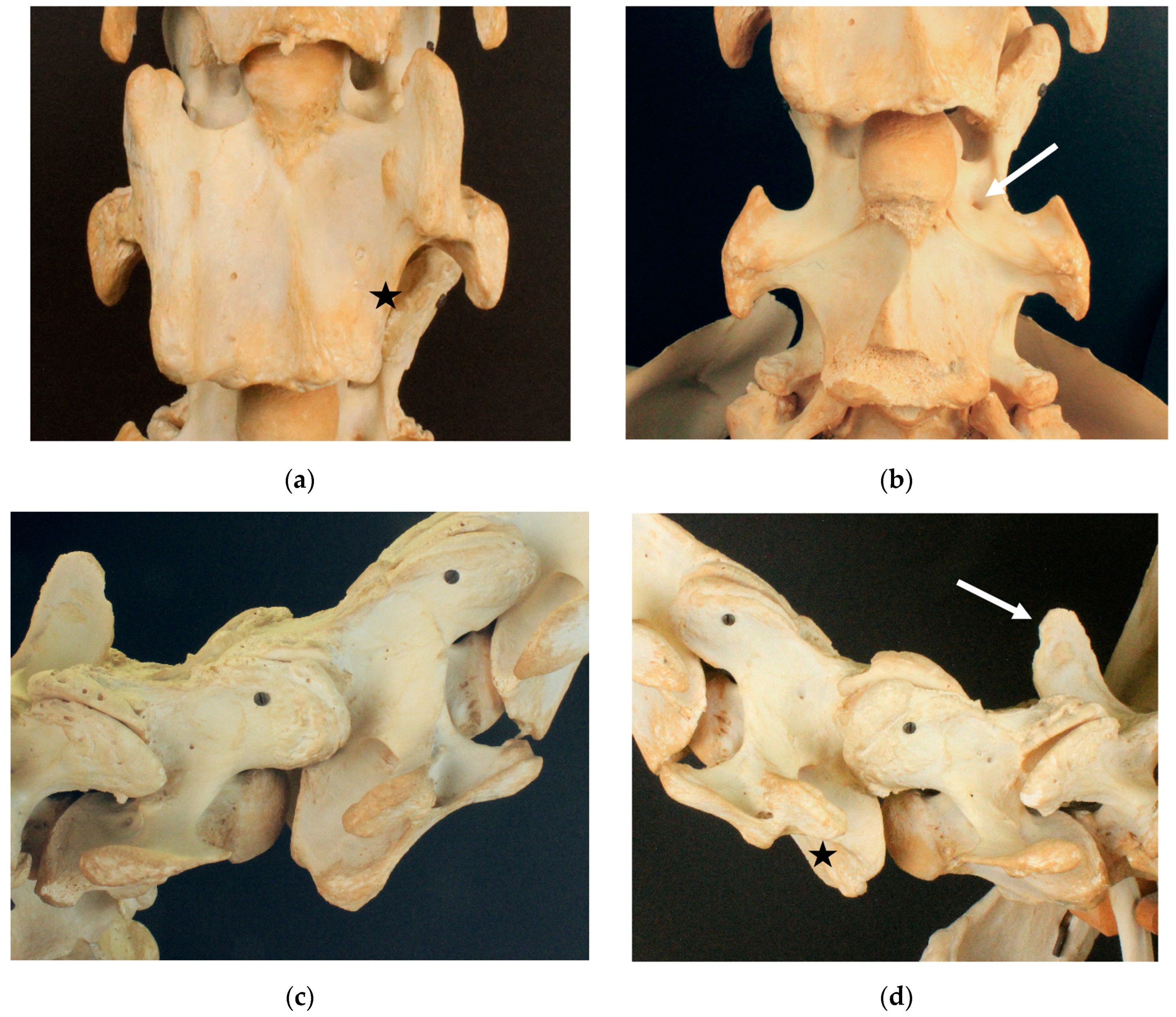
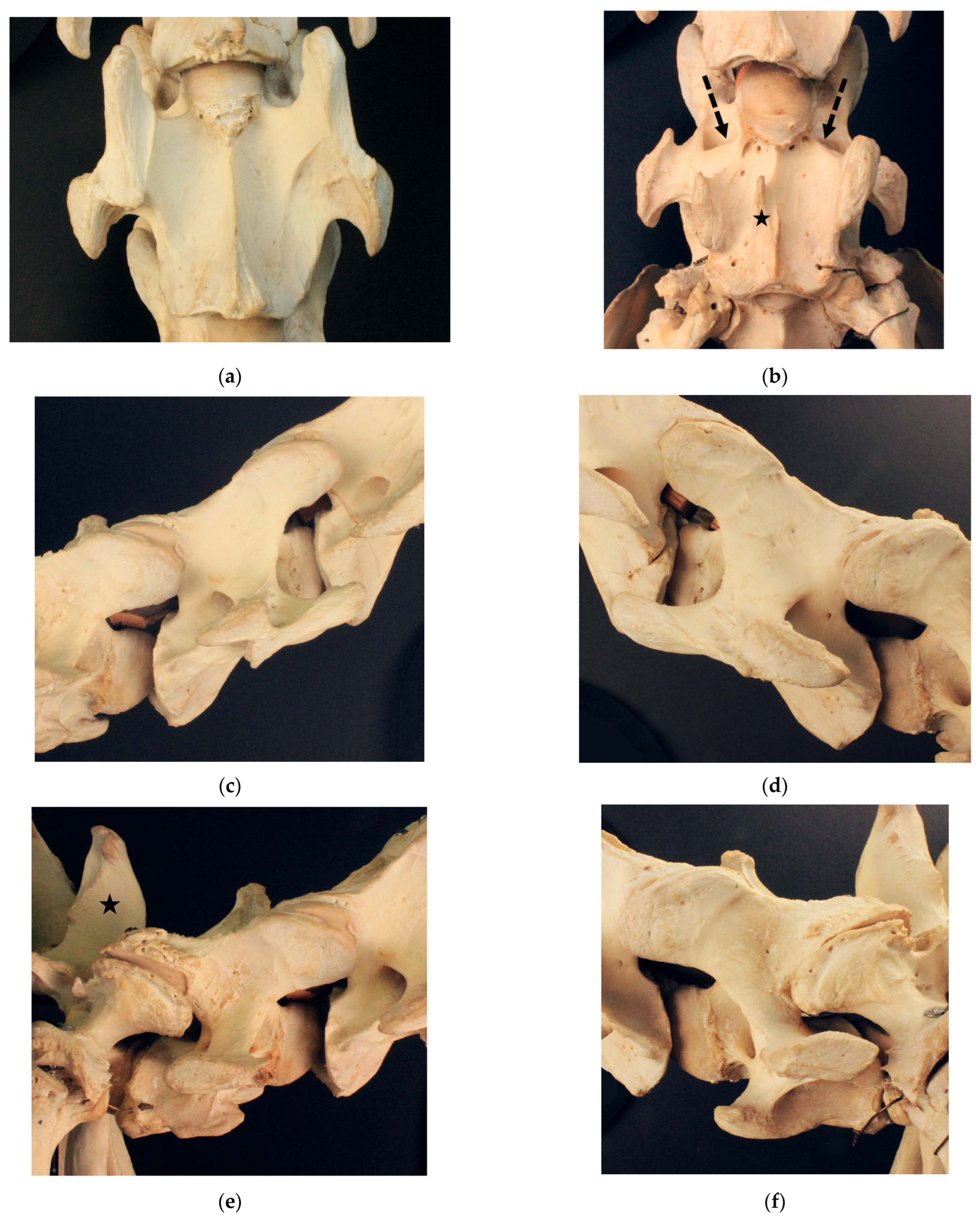
3.1. Examination of Historical Equine Skeletons
3.1.1. Dux
3.1.2. Le Destrier XX
3.1.3. Dark Ronald XX
3.1.4. Der Loewe XX
3.1.5. Birkhahn XX
3.2. Additive Genetic Relationships of the Malformation of Cervicothoracic Junction-Affected Historic Horses
3.3. Evaluation of 11 Modern Horses and Their Additive Genetic Relationships to the Historic Stallions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nickel, R.; Schummer, A.; Seiferle, E. Lehrbuch der Anatomie der Haustiere Band I. In Bewegungsapparat; Georg Thieme Verlag: New York, NY, USA, 2004. [Google Scholar] [CrossRef]
- Santinelli, I.; Beccati, F.; Arcelli, R.; Pepe, M. Anatomical variation of the spinous and transverse processes in the caudal cervical vertebrae and the first thoracic vertebra in horses. Equine Vet. J. 2016, 48, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Rombach, N.; Stubbs, N.C.; Clayton, H.M. Gross anatomy of the deep perivertebral musculature in horses. Am. J. Vet. Res. 2014, 75, 433–440. [Google Scholar] [CrossRef]
- May-Davis, S. The Occurrence of a Congenital Malformation in the Sixth and Seventh Cervical Vertebrae Predominantly Observed in Thoroughbred Horses. J. Equine Vet. Sci. 2014, 34, 1313–1317. [Google Scholar] [CrossRef]
- Veraa, S.; Bergmann, W.; van den Belt, A.J.; Wijnberg, I.; Back, W. Ex Vivo Computed Tomographic Evaluation of Morphology Variations in Equine Cervical Vertebrae. Vet. Radiol. Ultrasound 2016, 57, 482–488. [Google Scholar] [CrossRef] [PubMed]
- May-Davis, S.; Dzingle, D.; Saber, E.; Blades Eckelbarger, P. Characterization of the Caudal Ventral Tubercle in the Sixth Cervical Vertebra in Modern Equus ferus caballus. Animals 2023, 13, 2384. [Google Scholar] [CrossRef]
- Beccati, F.; Pepe, M.; Santinelli, I.; Gialletti, R.; Di Meo, A.; Romero, J.M. Radiographic findings and anatomical variations of the caudal cervical area in horses with neck pain and ataxia: Case-control study on 116 horses. Vet. Rec. 2020, 187, e79. [Google Scholar] [CrossRef]
- May-Davis, S.; Hunter, R.; White, R. Morphology of the Ventral Process of the Sixth Cervical Vertebra in Extinct and Extant Equus: Functional Implications. Animals 2023, 13, 1672. [Google Scholar] [CrossRef]
- DeRouen, A.; Spriet, M.; Aleman, M. Prevalence of Anatomical Variation of the Sixth Cervical Vertebra And Association With Vertebral Canal Stenosis And Articular Process Osteoarthritis in the Horse. Vet. Radiol. Ultrasound 2016, 57, 253–258. [Google Scholar] [CrossRef]
- Veraa, S.; de Graaf, K.; Wijnberg, I.D.; Back, W.; Vernooij, H.; Nielen, M.; Belt, A.J.M. Caudal cervical vertebral morphological variation is not associated with clinical signs in Warmblood horses. Equine Vet. J. 2020, 52, 219–224. [Google Scholar] [CrossRef]
- Gerlach, K.; Schad, P.; Offhaus, J.; Brehm, W.; Pelli, A. Vorkommen von unterschiedlichen Ausprägungen der Processus transversi im Bereich der letzten beiden Halswirbel des Pferdes. Pferdeheilkunde-Equine Med. 2021, 37, 605–610. [Google Scholar]
- Spoormakers, T.J.P.; Veraa, S.; Graat, E.A.M.; van Weeren, P.R.; Brommer, H. A comparative study of breed differences in the anatomical configuration of the equine vertebral column. J. Anat. 2021, 239, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Mur, P.; Phillips, K.L.; Galuppo, L.D.; DeRouen, A.; Benoit, P.; Anderson, E.; Shaw, K.; Puchalski, S.; Peters, D.; Kass, P.H.; et al. Radiological prevalence of osteoarthritis of the cervical region in 104 performing Warmblood jumpers. Equine Vet. J. 2021, 53, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Gee, C.; Small, A.; Shorter, K.; Brown, W.Y. A Radiographic Technique for Assessment of Morphologic Variations of the Equine Caudal Cervical Spine. Animals 2020, 10, 667. [Google Scholar] [CrossRef] [PubMed]
- Withers, J.M.; Voûte, L.C.; Hammond, G.; Lischer, C.J. Radiographic anatomy of the articular process joints of the caudal cervical vertebrae in the horse on lateral and oblique projections. Equine Vet. J. 2009, 41, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Gorton, B. Abnormal Cervical Vertebra of Horse. J. Anat. 1923, 57, 380–381. [Google Scholar]
- May-Davis, S.; Walker, C. Variations and Implications of the Gross Morphology in the Longus colli Muscle in Thoroughbred and Thoroughbred Derivative Horses Presenting With a Congenital Malformation of the Sixth and Seventh Cervical Vertebrae. J. Equine Vet. Sci. 2015, 35, 560–568. [Google Scholar] [CrossRef]
- May-Davis, S. Congenital Malformations of the First Sternal Rib. J. Equine Vet. Sci. 2017, 49, 92–100. [Google Scholar] [CrossRef]
- Martins, F.P.; Souza, E.C.; Bernardes, F.C.S.; Abidu-Figueiredo, M.; Kasper, C.B.; de Souza-Junior, P. Anatomical variations in cervical vertebrae in two species of neotropical canids: What is the meaning? Anat. Histol. Embryol. 2021, 50, 212–217. [Google Scholar] [CrossRef]
- Räikkönen, J.; Vucetich, J.A.; Peterson, R.O.; Nelson, M.P. Congenital bone deformities and the inbred wolves (Canis lupus) of Isle Royale. Biol. Conserv. 2009, 142, 1025–1031. [Google Scholar] [CrossRef]
- SAS Help Center. SAS Enterprise Guide 7.1; SAS Help Center: Cary, NC, USA, 2014. [Google Scholar]
- Wrede, J.; Schmidt, T. OPTI-MATE, Version 4.2; Institute for Animal Breeding and Genetics: Hannover, Germany, 2003.
- Wright, S. Coefficients of Inbreeding and Relationship. Am. Nat. 1922, 56, 330–338. [Google Scholar] [CrossRef]
- Veraa, S.; Scheffer, C.J.W.; Smeets, D.H.M.; de Bruin, R.B.; Hoogendoorn, A.C.; Vernooij, J.C.M.; Nielen, M.; Back, W. Cervical disc width index is a reliable parameter and consistent in young growing Dutch Warmblood horses. Vet. Radiol. Ultrasound 2020, 62, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Levine, G.J.; Hoffman, A.G.; Mez, J.; Bratton, G.R. Comparative Anatomy of the Horse, Ox, and Dog: The Vertebral Column and Peripheral Nerves. Compend. Equine 2007, 2, 279–281. [Google Scholar]
- Zibis, A.; Mitrousias, V.; Galanakis, N.; Chalampalaki, N.; Arvanitis, D.; Karantanas, A. Variations of transverse foramina in cervical vertebrae: What happens to the vertebral artery? Eur. Spine J. 2018, 27, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Zibis, A.H.; Mitrousias, V.; Baxevanidou, K.; Hantes, M.; Karachalios, T.; Arvanitis, D. Anatomical variations of the foramen transversarium in cervical vertebrae: Findings, review of the literature, and clinical significance during cervical spine surgery. Eur. Spine J. 2016, 25, 4132–4139. [Google Scholar] [CrossRef]
- Bernard, T.J.; Mull, B.R.; Handler, M.H.; Harned, R.K.; Filley, C.M.; Kumpe, D.A.; Tseng, B.S. An 18-year-old man with fenestrated vertebral arteries, recurrent stroke and successful angiographic coiling. J. Neurol. Sci. 2007, 260, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Sotirios, G.; Sofia, M.; Maria, K.; Sygliti-Henrietta, P.; Athanassios, P.K. Lateral medullary ischaemic events in young adults with hypoplastic vertebral artery. J. Neurol. 2007, 78, 987. [Google Scholar] [CrossRef]
- Ahn, S.H.; Oh, S.J.; Yook, J.W.; Choi, K.D.; Lee, T.H.; Soo Kim, J.; Park, K.H. Recurrent isolated vertigo from hypoplastic vertebral artery. Eur. J. Neurol. 2008, 15, e51–e52. [Google Scholar] [CrossRef]
- Furumoto, T.; Nagase, J.; Takahashi, K.; Itabashi, T.; Iai, H.; Ishige, N. Cervical myelopathy caused by the anomalous vertebral artery: A case report. Spine 1996, 21, 2280–2283. [Google Scholar] [CrossRef]
- Janes, J.G.; Garrett, K.S.; McQuerry, K.J.; Pease, A.P.; Williams, N.M.; Reed, S.M.; MacLeod, J.N. Comparison of magnetic resonance imaging with standing cervical radiographs for evaluation of vertebral canal stenosis in equine cervical stenotic myelopathy. Equine Vet. J. 2014, 46, 681–686. [Google Scholar] [CrossRef]
- Stenglin, C.F.V. Deutsche Pferdezucht; FN-Verlag: Warendorf, Germany, 1994; Volume 2. [Google Scholar]
- Schlie, A. Der Hannoveraner—Geschichte und Zucht des Edlen Hannoverschen Warmblutpferdes; BLV-Verlagsgesellschaft: München, Germany, 1975; Volume 2. [Google Scholar]
- Hamann, H.; Distl, O. Genetic variability in Hanoverian warmblood horses using pedigree analysis. J. Anim. Sci. 2008, 86, 1503–1513. [Google Scholar] [CrossRef]
- McGivney, B.A.; Han, H.; Corduff, L.R.; Katz, L.M.; Tozaki, T.; MacHugh, D.E.; Hill, E.W. Genomic inbreeding trends, influential sire lines and selection in the global Thoroughbred horse population. Sci. Rep. 2020, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, P.W.; Garcia-Dorado, A. Understanding Inbreeding Depression, Purging, and Genetic Rescue. Trends. Ecol. Evol. 2016, 31, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Marsden, C.D.; Ortega-Del Vecchyo, D.; O’Brien, D.P.; Taylor, J.F.; Ramirez, O.; Vilà, C.; Marques-Bonet, T.; Schnabel, R.D.; Wayne, R.K.; Lohmueller, K.E. Bottlenecks and selective sweeps during domestication have increased deleterious genetic variation in dogs. Proc. Natl. Acad. Sci. USA 2016, 113, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Todd, E.T.; Ho, S.Y.W.; Thomson, P.C.; Ang, R.A.; Velie, B.D.; Hamilton, N.A. Founder-specific inbreeding depression affects racing performance in Thoroughbred horses. Sci. Rep. 2018, 8, 6167. [Google Scholar] [CrossRef] [PubMed]
| Animal | Breed | Sex | Age | C6 Left/Right | C7 Left/Right | Inbreeding Coefficient (%) |
|---|---|---|---|---|---|---|
| Horse 1 | OLD | m | 2 | -/- | -/- | 3.177 |
| Horse 2 | OLD | f | 5 | -/- | -/- | 4.499 |
| Horse 3 | HAN | f | 8 | x/- | x/- | 3.759 |
| Horse 4 | HAN | f | 5 | x/x | x/x | 6.033 |
| Horse 5 | HAN | m | 9 | -/- | -/- | 2.781 |
| Horse 6 | HAN | m | 7 | -/- | -/- | 1.051 |
| Horse 7 | HAN | f | 16 | x/x | x/x | 3.377 |
| Horse 8 | HAN | m | 12 | -/- | -/- | 0.643 |
| Horse 9 | WEST | m | 4 | x/- | x/- | 0.356 |
| Horse 10 | WEST | f | 15 | x/x | -/- | 0.258 |
| Horse 11 | WEST | f | 4 | x/x | x/x | 2.075 |
| Horse 12 | WEST | m | 3 | x/x | x/x | 1.385 |
| Horse 13 | OLD | m | 6 | -/x | -/x | 1.496 |
| Horse 14 | OLD | m | 6 | -/x | -/x | 2.052 |
| Horse 15 | OLD | f | 6 | x/x | x/x | 1.941 |
| Horse 16 | HAN | m | 5 | -/- | -/- | 1.958 |
| Horse 17 | HAN | m | 14 | -/- | -/- | 1.190 |
| Horse 18 | HAN | f | 7 | -/- | -/- | 1.416 |
| Horse 19 | WEST | m | 8 | -/- | -/- | 1.358 |
| Horse 20 | HAN | m | 7 | -/- | -/- | 0.165 |
| Animal | Dark Ronald XX | Der Loewe XX | Birkhahn XX | ||||||
|---|---|---|---|---|---|---|---|---|---|
| i | s | d | i | s | d | i | s | d | |
| with C6/C7 malformation | |||||||||
| Horse 3 | 2.79 | 3.63 | 1.94 | 2.84 | 3.91 | 1.76 | 3.13 | 6.25 | - |
| Horse 4 | 1.99 | 2.21 | 1.76 | 2.69 | 2.74 | 2.64 | 2.16 | 3.13 | 1.18 |
| Horse 7 | 1.82 | 2.06 | 1.58 | 2.84 | 0.98 | 4.70 | 1.18 | 2.35 | - |
| Horse 9 † | 1.71 | 1.38 | 2.04 | 4.01 | 6.25 | 1.77 | 0.79 | - | 1.57 |
| Horse 10 † | 1.82 | 1.38 | 2.26 | 3.13 | 6.25 | - | 3.13 | - | 6.25 |
| Horse 11 † | 1.53 | 1.38 | 1.68 | 4.21 | 6.25 | 2.16 | 0.10 | - | 0.20 |
| Horse 12 | 1.76 | 1.83 | 1.68 | 1.58 | 0.99 | 2.16 | 0.30 | 0.40 | 0.20 |
| Horse 13 | 1.66 | 2.00 | 1.31 | 1.33 | 0.49 | 2.16 | 0.79 | 1.57 | - |
| Horse 14 | 2.75 | 3.28 | 2.21 | 2.65 | 2.84 | 2.45 | 1.57 | 3.13 | - |
| Horse 15 | 1.72 | 1.57 | 1.86 | 2.16 | 2.45 | 1.86 | 0.40 | 0.00 | 0.79 |
| Mean | 1.96 | 2.07 | 1.83 | 2.74 * | 3.32 | 2.17 | 1.36 | 1.68 | 1.02 |
| without C6/C7 malformation | |||||||||
| Horse 1 | 1.48 | 1.10 | 1.86 | 1.54 | 0.72 | 2.35 | 0.79 | - | 1.57 |
| Horse 2 | 1.87 | 1.95 | 1.78 | 1.72 | 1.67 | 1.77 | 0.60 | 0.40 | 0.79 |
| Horse 5 | 1.81 | 2.02 | 1.60 | 1.72 | 1.67 | 1.76 | 0.79 | 1.57 | - |
| Horse 6 | 2.00 | 1.48 | 2.51 | 1.28 | 0.60 | 1.96 | 1.57 | - | 3.13 |
| Horse 8 | 1.37 | 1.62 | 1.11 | 1.47 | 2.06 | 0.89 | 0.40 | - | 0.79 |
| Horse 16 | 1.76 | 1.64 | 1.88 | 1.72 | 1.38 | 2.06 | 0.79 | 0.00 | 1.57 |
| Horse 17 | 1.96 | 2.63 | 1.28 | 1.96 | 2.35 | 1.57 | 1.57 | 3.13 | - |
| Horse 18 | 1.86 | 2.63 | 1.09 | 2.35 | 2.35 | 2.35 | 1.57 | 3.13 | - |
| Horse 19 | 1.33 | 1.97 | 0.69 | 0.64 | 1.28 | - | 0.79 | 1.57 | - |
| Horse 20 | 2.41 | 3.63 | 1.18 | 1.96 | 3.91 | 0.00 | 3.13 | 6.25 | - |
| Mean | 1.79 | 2.07 | 1.50 | 1.64* | 1.80 | 1.47 | 1.20 | 1.61 | 0.79 |
| Animal | Dark Ronald XX | Der Loewe XX | Birkhahn XX | |||
|---|---|---|---|---|---|---|
| pF (%) | cp | pF (%) | cp | pF (%) | cp | |
| with C6/C7 malformation | ||||||
| Horse 3 | 0.45 | 93 | 0.33 | 1 | - | - |
| Horse 4 | 0.01 | 67 | 0.27 | 6 | 0.10 | 1 |
| Horse 7 | 0.08 | 39 | 0.11 | 2 | - | - |
| Horse 9 † | 0.95 | 44 | 17.96 | 4 | - | - |
| Horse 10 † | 2.80 | 29 | - | - | - | - |
| Horse 11 † | 0.07 | 29 | 3.29 | 3 | - | - |
| Horse 12 | 0.03 | 36 | 0.67 | 8 | - | - |
| Horse 13 | 0.08 | 27 | 0.15 | 6 | - | - |
| Horse 14 | 0.08 | 116 | 1.57 | 18 | - | - |
| Horse 15 | 0.01 | 16 | 0.61 | 34 | - | - |
| Mean | 0.46 | 50 | 2.77 | 9 | - | - |
| without C6/C7 malformation | ||||||
| Horse 1 | 0.00 | 27 | 0.12 | 10 | - | - |
| Horse 2 | 0.01 | 26 | 0.16 | 25 | - | - |
| Horse 5 | 0.04 | 59 | 0.19 | 3 | - | - |
| Horse 6 | 0.12 | 79 | 0.53 | 3 | - | - |
| Horse 8 | 0.05 | 11 | 0.88 | 15 | - | - |
| Horse 16 | 0.01 | 37 | 0.29 | 19 | - | - |
| Horse 17 | 0.42 | 73 | 1.18 | 1 | - | - |
| Horse 18 | 0.32 | 59 | 1.91 | 4 | - | - |
| Horse 19 | 0.02 | 13 | - | - | - | - |
| Horse 20 | 9.35 | 62 | - | - | - | - |
| Mean | 1.03 | 45 | 0.66 | 10 | - | - |
| Author, Date | Breeds Affected |
|---|---|
| Malformation of C6 | |
| De Rouen et al., 2016 [9] | 19/55 Warmbloods 2/12 Thoroughbreds 2/16 Quarter Horses 1/9 Arabian |
| Veraa et al., 2016 [5] | 21/65 Dutch Warmbloods 1/1 Oldenburg 1/2 Westphalian 1/1 Trotter 1/2 Friesian 1/2 crossbreed |
| Beccati et al., 2020 [7] | 15/69 Warmbloods 3/16 Thoroughbreds 4/15 Arabians 2/16 others |
| May-Davis et al., 2023 [6] * | 40 Thoroughbreds 13 Warmbloods 6 Australian Stock horses 5 crossbreed 4 Standardbreds 3 Appaloosas 2 Quarter Horses 1 Irish Sport Horse 1 Riding Pony 1 Friesian |
| Malformation of C6/C7 | |
| May-Davis, 2014 [4] | 19/50 Thoroughbreds 3/3 Thoroughbred-crossbreds |
| Santinelli et al., 2016 [2] | 21/138 Warmbloods 4/41 Thoroughbreds 3/13 Quarter Horses 3/29 Arabians 3/27 Anglo-Arabians |
| Veraa et al., 2020 [10] | 105/377 Warmblood Horses |
| Spoormakers et al., 2021 [12] | 21/49 Warmblood horses 3/48 Shetland ponies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann, E.; Ros, K.B.; Pfarrer, C.; Distl, O. Historic Horse Family Displaying Malformations of the Cervicothoracic Junction and Their Connection to Modern German Warmblood Horses. Animals 2023, 13, 3415. https://doi.org/10.3390/ani13213415
Zimmermann E, Ros KB, Pfarrer C, Distl O. Historic Horse Family Displaying Malformations of the Cervicothoracic Junction and Their Connection to Modern German Warmblood Horses. Animals. 2023; 13(21):3415. https://doi.org/10.3390/ani13213415
Chicago/Turabian StyleZimmermann, Elisa, Katharina B. Ros, Christiane Pfarrer, and Ottmar Distl. 2023. "Historic Horse Family Displaying Malformations of the Cervicothoracic Junction and Their Connection to Modern German Warmblood Horses" Animals 13, no. 21: 3415. https://doi.org/10.3390/ani13213415
APA StyleZimmermann, E., Ros, K. B., Pfarrer, C., & Distl, O. (2023). Historic Horse Family Displaying Malformations of the Cervicothoracic Junction and Their Connection to Modern German Warmblood Horses. Animals, 13(21), 3415. https://doi.org/10.3390/ani13213415





