Metabolomic Profiling Reveals Differences in Hypoxia Response between Far Eastern and Siberian Frogs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Maintenance
2.2. Metabolites Extraction
2.3. NMR Measurements
2.4. Data Analysis
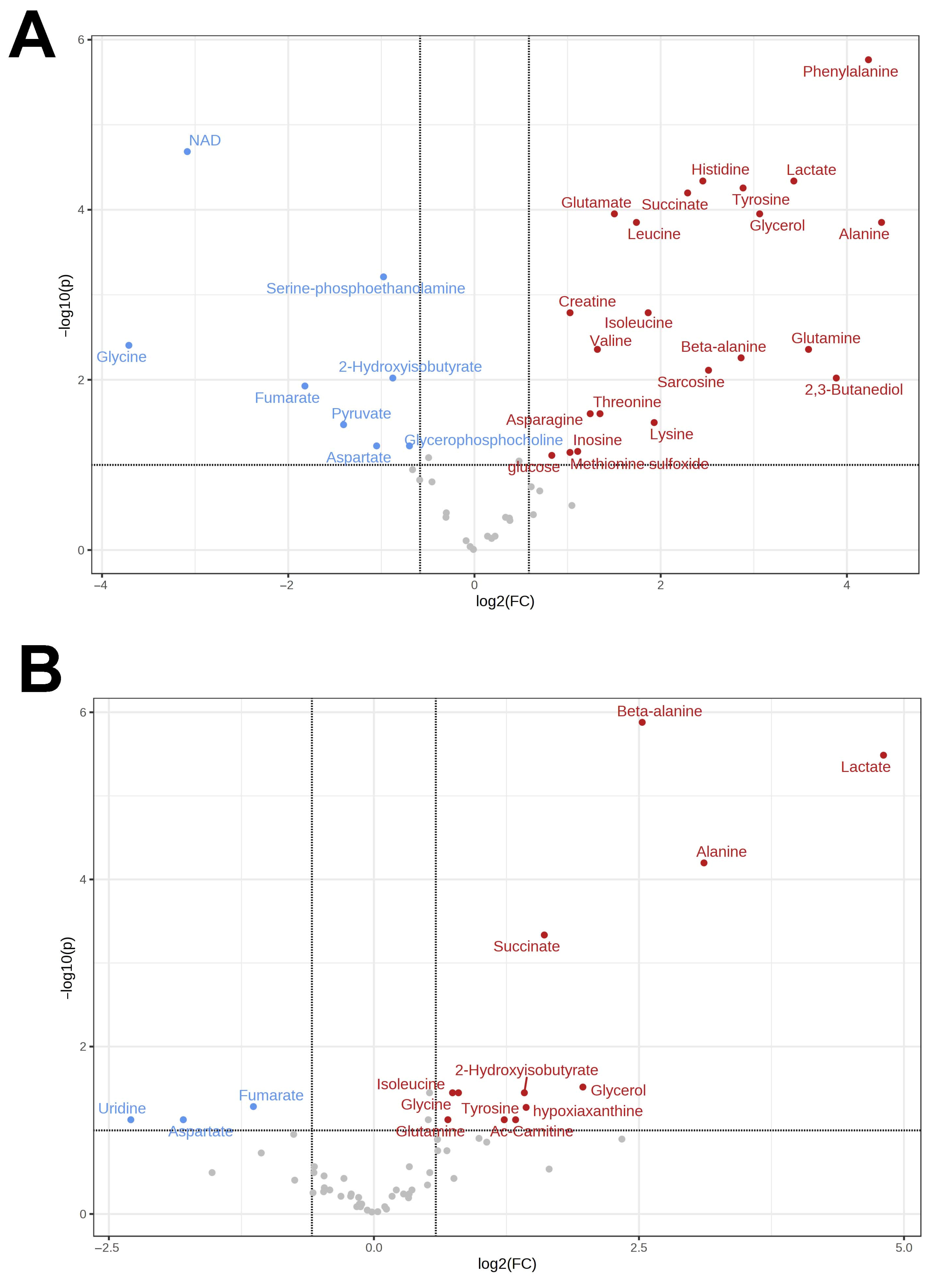
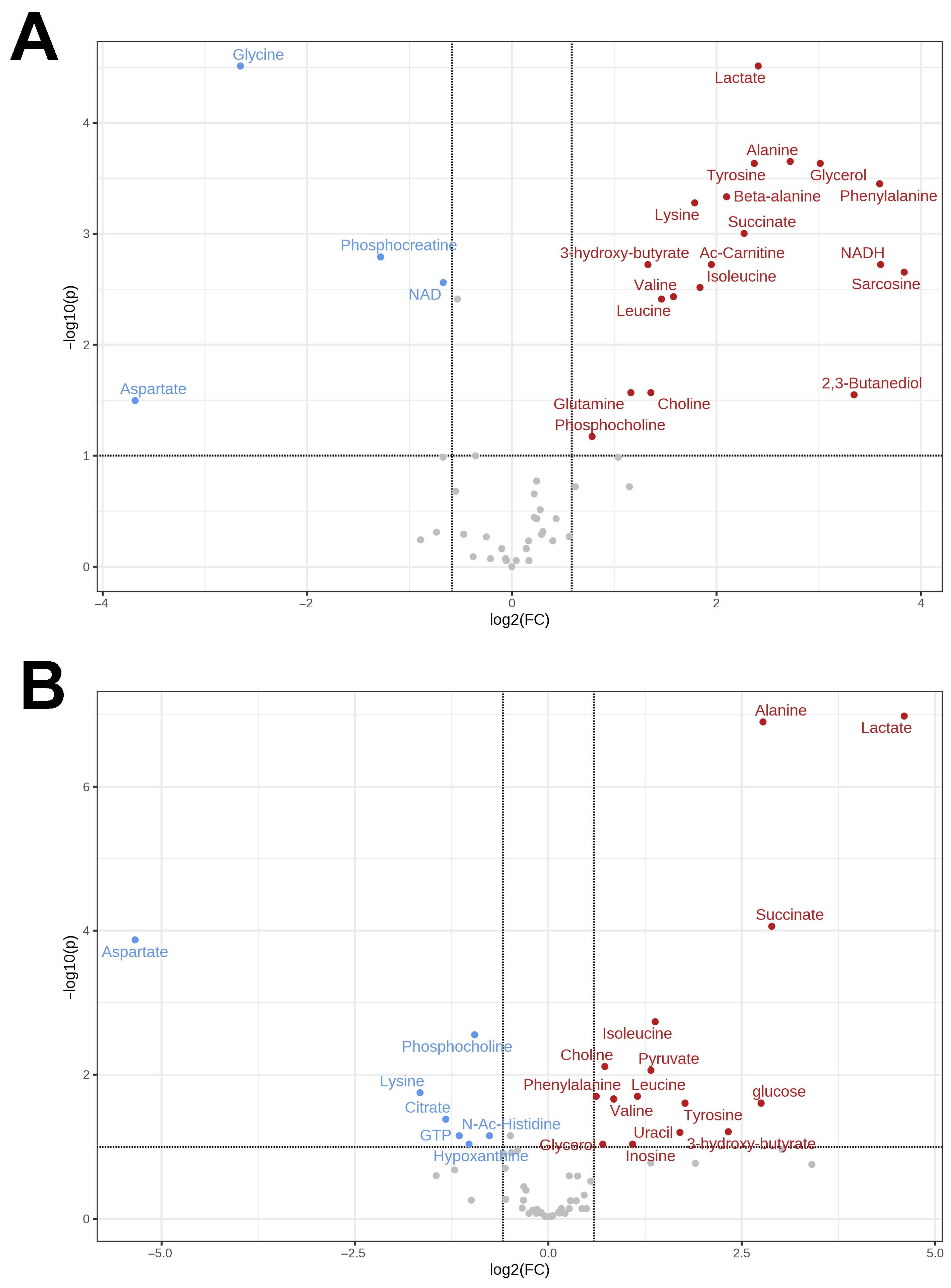
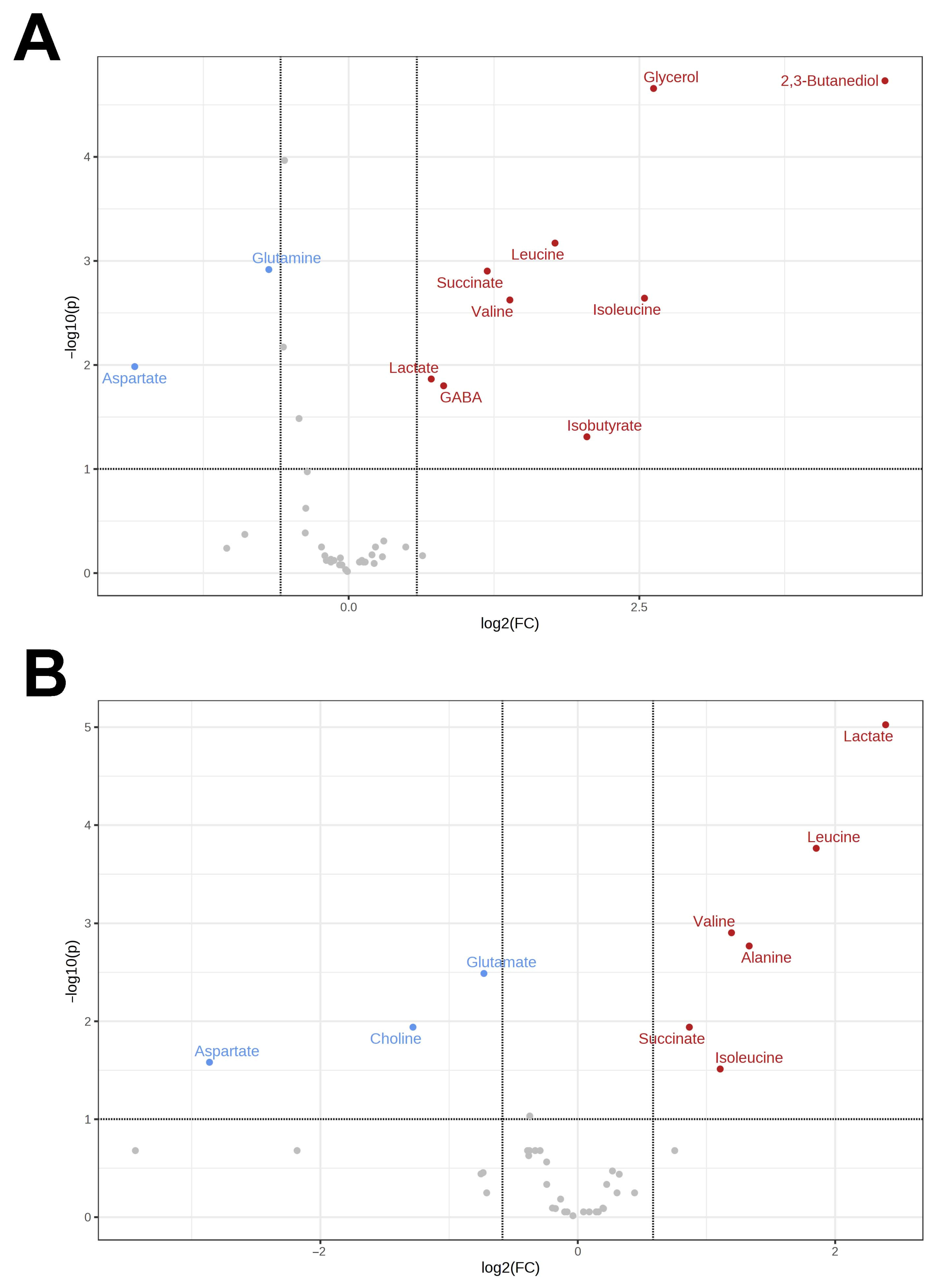
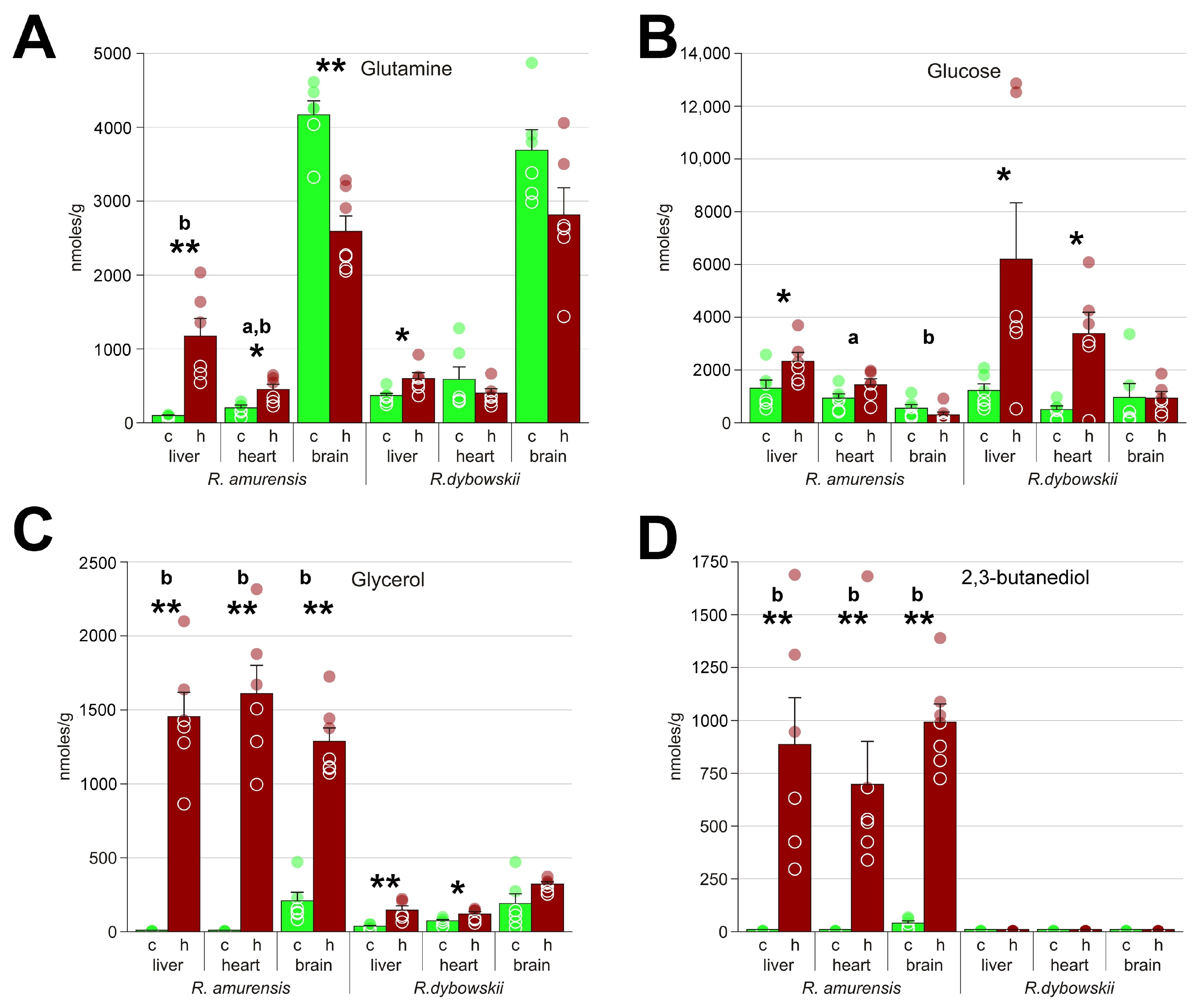
3. Results
3.1. Metabolomes of the Heart and Liver of the Far Eastern Frog
3.2. Brain Metabolomes of the Siberian and the Far Eastern Frogs
4. Discussion
4.1. Metabolomic Changes in the Far Eastern and Siberian Frogs in Response to Hypoxia
4.2. Response of Energy Pathways to Hypoxia in the Siberian Frog
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larson, J.; Park, T.J. Extreme Hypoxia Tolerance of Naked Mole-Rat Brain. Neuroreport 2009, 20, 1634–1637. [Google Scholar] [CrossRef]
- Ivy, C.M.; Sprenger, R.J.; Bennett, N.C.; Jaarsveld, B.; Hart, D.W.; Kirby, A.M.; Yaghoubi, D.; Storey, K.B.; Milsom, W.K.; Pamenter, M.E. The Hypoxia Tolerance of Eight Related African Mole-rat Species Rivals That of Naked Mole-rats, despite Divergent Ventilatory and Metabolic Strategies in Severe Hypoxia. Acta Physiol. 2020, 228, e13436. [Google Scholar] [CrossRef]
- Bickler, P.E.; Buck, L.T. Hypoxia Tolerance in Reptiles, Amphibians, and Fishes: Life with Variable Oxygen Availability. Annu. Rev. Physiol. 2007, 69, 145–170. [Google Scholar] [CrossRef]
- Ultsch, G.R. The Viability of Nearctic Freshwater Turtles Submerged in Anoxia and Normoxia at 3 and 10 °C. Comp. Biochem. Physiol. A 1985, 81, 607–611. [Google Scholar] [CrossRef]
- Ultsch, G.R. Ecology and Physiology of Hibernation and Overwintering among Freshwater Fishes, Turtles, And Snakes. Biol. Rev. 1989, 64, 435–515. [Google Scholar] [CrossRef]
- Reese, S.A.; Jackson, D.C.; Ultsch, G.R. The Physiology of Overwintering in a Turtle That Occupies Multiple Habitats, the Common Snapping Turtle (Chelydra serpentina). Physiol. Biochem. Zool. 2002, 75, 432–438. [Google Scholar] [CrossRef]
- Berman, D.I.; Bulakhova, N.A.; Meshcheryakova, E.N. The Siberian Wood Frog Survives for Months Underwater without Oxygen. Sci. Rep. 2019, 9, 13594. [Google Scholar] [CrossRef]
- Bulakhova, N.A.; Meshcheryakova, E.N.; Berman, D.I. Pallas’ Spadefoot Pelobates vespertinus (Pelobatidae, Amphibia) Tolerates Extreme Hypoxia. Eur. Zool. J. 2023, 90, 431–442. [Google Scholar] [CrossRef]
- Wishart, D.S. NMR Metabolomics: A Look Ahead. J. Magn. Reson. 2019, 306, 155–161. [Google Scholar] [CrossRef]
- Zelentsova, E.A.; Yanshole, L.V.; Tsentalovich, Y.P.; Sharshov, K.A.; Yanshole, V.V. The Application of Quantitative Metabolomics for the Taxonomic Differentiation of Birds. Biology 2022, 11, 1089. [Google Scholar] [CrossRef]
- Niu, Y.; Cao, W.; Wang, J.; He, J.; Storey, K.B.; Ding, L.; Tang, X.; Chen, Q. Freeze Tolerance and the Underlying Metabolite Responses in the Xizang Plateau Frog, Nanorana parkeri. J. Comp. Physiol. B 2021, 191, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhang, X.; Men, S.; Storey, K.B.; Chen, Q. Integrated Analysis of Transcriptome and Metabolome Data Reveals Insights for Molecular Mechanisms in Overwintering Tibetan Frogs, Nanorana parkeri. Front. Physiol. 2023, 13, 1104476. [Google Scholar] [CrossRef]
- Shekhovtsov, S.V.; Bulakhova, N.A.; Tsentalovich, Y.P.; Zelentsova, E.A.; Meshcheryakova, E.N.; Poluboyarova, T.V.; Berman, D.I. Metabolomic Analysis Reveals That the Moor Frog Rana arvalis Uses Both Glucose and Glycerol as Cryoprotectants. Animals 2022, 12, 1286. [Google Scholar] [CrossRef] [PubMed]
- Shekhovtsov, S.V.; Bulakhova, N.A.; Tsentalovich, Y.P.; Zelentsova, E.A.; Meshcheryakova, E.N.; Poluboyarova, T.V.; Berman, D.I. Biochemical Response to Freezing in the Siberian Salamander Salamandrella keyserlingii. Biology 2021, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- Shekhovtsov, S.V.; Bulakhova, N.A.; Tsentalovich, Y.P.; Zelentsova, E.A.; Yanshole, L.V.; Meshcheryakova, E.N.; Berman, D.I. Metabolic Response of the Siberian Wood Frog Rana amurensis to Extreme Hypoxia. Sci. Rep. 2020, 10, 14604. [Google Scholar] [CrossRef]
- Bulakhova, N.A.; Mazanaeva, L.F.; Mescheryakova, E.N.; Berman, D.I. Resistance of Iranian Long-Legged Wood Frog Rana macrocnemis (Amphibia, Anura) to Negative Temperatures on Land and to Hypoxia in Water during Overwintering. Herpetol. Notes 2020, 13, 1079–1086. [Google Scholar]
- Tsentalovich, Y.P.; Yanshole, V.V.; Yanshole, L.V.; Zelentsova, E.A.; Melnikov, A.D.; Sagdeev, R.Z. Seasonal Variations and Interspecific Differences in Metabolomes of Freshwater Fish Tissues: Quantitative Metabolomic Profiles of Lenses and Gills. Metabolites 2019, 9, 264. [Google Scholar] [CrossRef]
- Jackson, D.C. Living without Oxygen: Lessons from the Freshwater Turtle. Comp. Biochem. Physiol. A 2000, 125, 299–315. [Google Scholar] [CrossRef]
- Gallazzini, M.; Burg, M.B. What’s New About Osmotic Regulation of Glycerophosphocholine. Physiology 2009, 24, 245–249. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Somero, G.N. Biochemical Adaptation: Mechanism and Process in Physiological Evolution; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Clark, V.M.; Miller, A.T. Studies on Anaerobic Metabolism in the Fresh-Water Turtle (Pseudemys scripta elegans). Comp. Biochem. Physiol. A 1973, 44, 55–62. [Google Scholar] [CrossRef]
- Pamenter, M.E.; Richards, M.D.; Buck, L.T. Anoxia-Induced Changes in Reactive Oxygen Species and Cyclic Nucleotides in the Painted Turtle. J. Comp. Physiol. B 2007, 177, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Dahl, H.-A.; Johansen, A.; Nilsson, G.E.; Lefevre, S. The Metabolomic Response of Crucian Carp (Carassius carassius) to Anoxia and Reoxygenation Differs between Tissues and Hints at Uncharacterized Survival Strategies. Metabolites 2021, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Buck, L. Succinate and Alanine as Anaerobic End-Products in the Diving Turtle (Chrysemys picta bellii). Comp. Biochem. Physiol. B 2000, 126, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, A.; James, A.M.; Gruszczyk, A.V.; Martin, J.; Murphy, M.P.; Fago, A. Metabolic Adaptations during Extreme Anoxia in the Turtle Heart and Their Implications for Ischemia-Reperfusion Injury. Sci. Rep. 2019, 9, 2850. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.B. Organ-Specific Metabolism during Freezing and Thawing in a Freeze-Tolerant Frog. Am. J. Physiol. Integr. Comp. Physiol. 1987, 253, R292–R297. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Metabolic Rate Depression in Animals: Transcriptional and Translational Controls. Biol. Rev. 2004, 79, 207–233. [Google Scholar] [CrossRef]
- Vornanen, M.; Stecyk, J.A.W.; Nilsson, G.E. Chapter 9 The Anoxia-Tolerant Crucian Carp (Carassius carassius L.). In Functional Metabolism: Regulation and Adaptation; John Wiley & Sons: New York, NY, USA, 2009; pp. 397–441. [Google Scholar]
- Johnston, I.A.; Bernard, L.M. Utilization of the Ethanol Pathway in Carp Following Exposure to Anoxia. J. Exp. Biol. 1983, 104, 73–78. [Google Scholar] [CrossRef]
- Jackson, D. Lactate Accumulation in the Shell of the Turtle Chrysemys picta bellii during Anoxia at 3 °C and 10 °C. J. Exp. Biol. 1997, 200, 2295–2300. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Lactic Acidosis. N. Engl. J. Med. 2014, 371, 2309–2319. [Google Scholar] [CrossRef]
- Davis, E.C.; Jackson, D.C. Lactate Uptake by Skeletal Bone in Anoxic Turtles, Trachemys scripta. Comp. Biochem. Physiol. A 2007, 146, 299–304. [Google Scholar] [CrossRef]
- Nilsson, G.E. A Comparative Study of Aldehyde Dehydrogenase and Alcohol Dehydrogenase Activities in Crucian Carp and Three Other Vertebrates: Apparent Adaptations to Ethanol Production. J. Comp. Physiol. B 1988, 158, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.S.; Mandic, M.; Yao, L.; Cao, Z.-D.; Fu, S.-J.; Brauner, C.J.; Wang, Y.S.; Richards, J.G. Ethanol Metabolism Varies with Hypoxia Tolerance in Ten Cyprinid Species. J. Comp. Physiol. B 2018, 188, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Paulusma, C.C.; Lamers, W.H.; Broer, S.; van de Graaf, S.F.J. Amino Acid Metabolism, Transport and Signalling in the Liver Revisited. Biochem. Pharmacol. 2022, 201, 115074. [Google Scholar] [CrossRef]
- Nordström, K. Yeast Growth and Glycerol Formation. Acta Chem. Scand. 1966, 20, 1016–1025. [Google Scholar] [CrossRef]
- Ansell, R.; Granath, K.; Hohmann, S.; Thevelein, J.M.; Adler, L. The two isoenzymes for yeast NAD+-dependent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 Have Distinct Roles in Osmoadaptation and Redox Regulation. EMBO J. 1997, 16, 2179–2187. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, D. Toward Glycerol Biorefinery: Metabolic Engineering for the Production of Biofuels and Chemicals from Glycerol. Biotechnol. Biofuels 2016, 9, 205. [Google Scholar] [CrossRef]
- Casazza, J.P.; Frietas, J.; Stambuk, D.; Morgan, M.Y.; Veech, R.L. The Measurement of 1, 2-Propanediol, D, L-2, 3-Butanediol and Meso-2, 3-Butanediol in Controls and Alcoholic Cirrhotics. Alcohol Alcohol Suppl. 1987, 1, 607–609. [Google Scholar]
- Montgomery, J.A.; Jetté, M.; Brunengraber, H. Assay of Physiological Levels of 2, 3-Butanediol Diastereomers in Blood and Urine by Gas Chromatography-Mass Spectrometry. Anal. Biochem. 1990, 185, 71–76. [Google Scholar] [CrossRef]
- Heer, K.R.; Althaus, U.; Mettler, D.; Schilt, W.; Thoelen, H. 2,3-Butanediol in Experimental Myocardial Ischaemia in Pigs. Eur. Heart J. 1991, 12, 654–656. [Google Scholar] [CrossRef]
- Ji, X.-J.; Huang, H.; Ouyang, P.-K. Microbial 2,3-Butanediol Production: A State-of-the-Art Review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef]
- Fago, A. New Insights into Survival Strategies to Oxygen Deprivation in Anoxia-tolerant Vertebrates. Acta Physiol. 2022, 235, e13841. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic Accumulation of Succinate Controls Reperfusion Injury through Mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Chinopoulos, C. Succinate in Ischemia: Where Does It Come From? Int. J. Biochem. Cell Biol. 2019, 115, 105580. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, A.; Ruhr, I.M.; Fago, A.; Galli, G.L.J. Metabolic Adaptations to Anoxia and Reoxygenation: New Lessons from Freshwater Turtles and Crucian Carp. Curr. Opin. Endocr. Metab. Res. 2020, 11, 55–64. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef]
- Kula-Alwar, D.; Prag, H.A.; Krieg, T. Targeting Succinate Metabolism in Ischemia/Reperfusion Injury. Circulation 2019, 140, 1968–1970. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shekhovtsov, S.V.; Bulakhova, N.A.; Tsentalovich, Y.P.; Zelentsova, E.A.; Osik, N.A.; Meshcheryakova, E.N.; Poluboyarova, T.V.; Berman, D.I. Metabolomic Profiling Reveals Differences in Hypoxia Response between Far Eastern and Siberian Frogs. Animals 2023, 13, 3349. https://doi.org/10.3390/ani13213349
Shekhovtsov SV, Bulakhova NA, Tsentalovich YP, Zelentsova EA, Osik NA, Meshcheryakova EN, Poluboyarova TV, Berman DI. Metabolomic Profiling Reveals Differences in Hypoxia Response between Far Eastern and Siberian Frogs. Animals. 2023; 13(21):3349. https://doi.org/10.3390/ani13213349
Chicago/Turabian StyleShekhovtsov, Sergei V., Nina A. Bulakhova, Yuri P. Tsentalovich, Ekaterina A. Zelentsova, Nataliya A. Osik, Ekaterina N. Meshcheryakova, Tatiana V. Poluboyarova, and Daniil I. Berman. 2023. "Metabolomic Profiling Reveals Differences in Hypoxia Response between Far Eastern and Siberian Frogs" Animals 13, no. 21: 3349. https://doi.org/10.3390/ani13213349
APA StyleShekhovtsov, S. V., Bulakhova, N. A., Tsentalovich, Y. P., Zelentsova, E. A., Osik, N. A., Meshcheryakova, E. N., Poluboyarova, T. V., & Berman, D. I. (2023). Metabolomic Profiling Reveals Differences in Hypoxia Response between Far Eastern and Siberian Frogs. Animals, 13(21), 3349. https://doi.org/10.3390/ani13213349





