Simple Summary
Vaccination can prevent infection by opportunistic bacteria that affect fish. We have developed and analyzed a bivalent vaccine against two of the main pathogens that affect fish. We found that the vaccine was safe and effective in laboratory tests and in large-scale tests, with better survival and feed conversion in immunized animals. These results indicate the need for field tests to confirm real protection. This developed vaccine could allow fish farmers greater protection for commercial fish production.
Abstract
One of the main factors limiting tilapia’s production is the occurrence of infections caused by Aeromonas and Streptococcus species. This work intended to evaluate a bivalent vaccine against A. hydrophila and S. agalactiae by intraperitoneal (i.p) administration in Nile tilapia (Oreochromis niloticus) in Brazil. The study was carried out in two phases: one in the laboratory, on a small scale, and from the results obtained, the study was expanded to a large scale in a production system in cages. The vaccine proved to be safe and effective in laboratory tests, with a vaccine efficacy (VE) of 93.66%. However, in large-scale tests with 12,000 tilapias, the VE was 59.14%, with a better food conversion ratio (1.54 kg) in the vaccinated group compared to the control group (1.27 kg). These results corroborate the efficiency of this tested vaccine; however, they indicate the need for field tests to attest to real protection.
1. Introduction
Nile tilapia (Oreochromis niloticus) has a wide global distribution, present in more than 120 countries, and is one of the main fish species produced in the world [1]. This species is currently the most important fish farmed in Brazil, accounting for 63.93% (550,060 tons) of national fish farming [2]. The intensive production of Tilapia in cages presents advantages such as rapid implantation and investment return, high productivity, population control, and greater ease of handling [3]. However, the intensification of these production systems at high densities can alter water quality and the nutritional status of fish, contributing to chronic stress and immunosuppression of fish [4,5].
One of the main factors that limit tilapia production is the occurrence of opportunistic bacteria present in the water and in the fish microbiota. This can trigger diseases when the host is susceptible, with such bacteria being important pathogens with a significant economic impact on commercial fish production [6]. Aeromonas spp. and Streptococcus spp. are some of the main pathogens that affect fish. They can be diagnosed simultaneously, highlighting an association between these diseases [6,7,8].
The pathogenicity of Aeromonas spp. is associated with several virulence factors that aid in infection, allowing the invasion and colonization of other bacteria and causing damage [9]. In fish, Aeromonas spp. It mainly affects the liver, spleen, kidneys, brain, gills, and skeletal muscles, presenting hemorrhagic and ulcerative lesions, lack of appetite, lethargy, and hemorrhages, among other clinical signs, leading to death [7,9,10,11].
Streptococcus agalactiae (Lancefield’s group B Streptococcus) causes great losses in aquaculture in Brazil, especially serotype Ib, although strains of serotype Ia and III have been isolated in outbreaks [12], and is considered the main sanitary risk for commercial fish in the country [6]. Classic clinical signs of S. agalactiae infection in fish are anorexia, skin darkening; nervous symptoms associated with erratic swimming; lethargy; exophthalmia; corneal opacity; diffuse hemorrhages in the body, at the base of the fins, and at the opercula; ulceration of the epidermis; gill congestion; hepatomegaly; splenomegaly; and death [7,13,14]. There is also the occurrence of atypical clinical streptococcosis, where lethargic fish and outbreaks of deaths without external clinical signs are observed [15].
Currently, the main therapeutic measure used in bacterial outbreaks is the use of antibiotics, which, when used indiscriminately, cause important environmental impacts. Furthermore, there is the possibility of the selection and dissemination of resistant strains, as well as the presence of antimicrobial residues in fish and the environment [16,17,18,19].
An alternative to antibiotics in infection prevention is fish vaccination, which induces specific antibodies, conferring protection and safety to the immunized host [20]. Widely used due to economic benefits, inactivated cell vaccines are produced to protect fish against various bacterial diseases [21]. This work intended to evaluate the immunoprotective capacity of a bivalent vaccine against Aeromonas hydrophila and Streptococcus agalactiae, by intraperitoneal (i.p.) administration, in Nile tilapia (Oreochromis niloticus) from production tanks in Brazil.
2. Materials and Methods
2.1. Bacterial Strains
Aeromonas hydrophila and Streptococcus agalactiae were isolated from tilapias with clinical signs of bacteriosis from tanks in Foz do Iguaçu, Paraná, Brazil. For the isolation of vaccine and challenge strains, samples of the kidney, liver, spleen, heart, and brain were collected, inoculated in 5% sheep blood agar plate, and incubated at 30 °C for 48 h. After colony growth, these were identified through the Catalase [22] and Gram staining [23] tests and hemolytic activity [24] as in previous studies.
2.2. Biochemical Identification of Bacterial Strains
Automated bacterial identification was performed using VITEK® 2 Compact equipment (bioMérieux, Inc., Foz do Iguaçu, Brazil) according to the manufacturer’s instructions, and for the identification of the strains, the GN card (card for the identification of Gram-negative bacteria) and the GP card (card for the identification of Gram-positive bacteria) were used [25]. Streptococcus agalactiae were subjected to serum agglutination analysis to determine the serotype with the commercial kit Strep B Latex Ib (Statens Serum Institut, Copenhagen, Denmark) lot LBIb-P1 and the serotype III with the commercial kit Strep B Latex III (Statens Serum Institut, Copenhagen, Denmark) lot LSBIII-1-8, according to the manufacturer’s recommendations [26]. Analyses were carried out by the Aquatic Animal Disease Laboratory at the Federal University of Minas Gerais. After identification, the strains were inoculated in a brain and heart infusion (BHI) and incubated in a shaker incubator at 30 °C for 48 h.
2.3. Molecular Identification of Bacterial Strains with Loop-Amplification-Mediated Purification (LAMP)
For genomic material extraction, bacteria were cultivated for 12 h in a yeast extract medium (Kasvi, São José dos Pinhais, Brazil) and submitted to the extraction protocol of the Blood and Tissue Genomic DNA Miniprep System kit (Viogene Biotek, Taipei, Taiwan), according to the manufacturer’s instructions [27]. The obtained material was stored at −80 °C.
The LAMP reactions for S. agalactiae contained 1.6 μM of each primer FIP and BIP, 0.2 μM of each primer F3 and B3, 0.4 μM of each primer LF and LB, 1× reaction buffer termopol (20 mM Tris-HCl pH 8.8, 0.1 M KCl, 10 mM (NH4)2SO4, 2 mM MgSO4) (Cellco, São Carlos, SP, Brazil), 0.8 M of betaine (Sigma-Aldrich, St. Louis, MO, USA), 6 mM MgSO4, 2 mM dNTPs mix (Sigma-Aldrich, USA), 8 U Bst DNA polymerase (Cellco, São Carlos, Brazil) and 100 ng of DNA, in a total volume of 25 μL. The reaction was incubated for 60 min at 60 °C and then stopped at 90 °C for 2 min.
A. hydrophila analyses were carried out in a mix of 25 μL containing 1.6 μM of each primer FIP and BIP, 0.2 μM of each primer F3 and B3, and 0.4 μM of each primer LF and LB, 1× reaction buffer termopol (20 mM Tris-HCl pH 8.8, 0.1 M KCl, 10 mM (NH4)2SO4, 2 mM MgSO4) (Cellco, São Carlos, Brazil), 1.6 M of betaine (Sigma-Aldrich, USA), 6 mM MgSO4, 2 mM dNTPs mix (Sigma-Aldrich, USA), 8 U Bst DNA polimerase (Cellco, São Carlos, Brazil), and 100 ng of DNA. The reaction was incubated for 60 min at a temperature of 63 °C and then stopped at 90 °C for 2 min. The specific primers used for the detection of A. hydrophila (Cai, Y. et al. 2016 [28]) and S. agalactiae (Zhou, Q. et al., 2020 [29]) are shown in Table 1.

Table 1.
Primers for detection of A. hydrophila and S. agalactiae in molecular identification.
2.4. Development of the Bivalent Vaccine
For vaccine preparation, the cultures were inactivated by the addition of 10% buffered formalin, to a final concentration of 3%, in a shaker incubator at 100 RPM (rotations per minute) for 24 h at 25 °C. An aliquot of each culture was seeded in blood agar to confirm cell inactivation. Inactivated cultures were centrifuged at 6000 RPM for 30 min at 4 °C. The culture pellets were resuspended in sterile saline (NaCl 0.9%), and cells were counted in Neubauer chambers (Olen K5-0111, Kasvi Brazil), corresponding to a final concentration of 2 × 108 cells/mL for S. agalactiae and 9 × 108 cells/mL for A. hydrophila. For the vaccine dose, the concentration of each strain was adjusted to 1 × 107 cells/dose, which was mixed with water-in-oil emulsions for intraperitoneal injection adjuvant (Montanide™ ISA 763 A VG, SEPPIC, Puteaux, France) [30]. After vaccine homogenization, it had its pH adjusted and was refrigerated for 48 h for stability analysis. The non-phase separation of the vaccine was stable. The stability analyses were followed for 12 months at temperatures between 2 °C and 8 °C.
2.5. Pre-Experimental Period
For initial analyses, in aquariums, the experiment was conducted at the Vaccine Production Technology Laboratory of the Federal University of Latin American Integration—UNILA—Brazil. Simulating high-stocking-density production, a total of 72 male fish (Oreochromis niloticus) with an average weight of 50 g of commercial origin from the cities of Toledo and Foz do Iguaçu, Paraná, were distributed in two aquaria of 200 L each, with a controlled temperature of 30 ± 2 °C [31,32,33]. The fish were fed twice a day with commercial food (36% gross protein, extruded) in a proportion of 3% live weight per day [34]. The aquariums contained 200 L of dechlorinated water, with a flow of 1000 L/h, continuous aeration, and cleaning performed daily by suction. Aquarium water was analyzed and corrected daily for ideal quality parameters such as pH, ammonia, nitrate, and dissolved oxygen.
2.6. Laboratory Experiment Design
For vaccination and challenge, 36 individuals in the control group (non-vaccinated) and 36 individuals in the treatment group (vaccinated) were used, totaling 72 animals. In both procedures, the fish were anesthetized with eugenol (175 mg L−1) [35] and weighed. In the treatment group, each fish received, via i.p. administration, 50 μL of the vaccine. In the control group, each fish received, via i.p. administration, 50 μL of sterile saline (NaCl 0.9%). Induction of the experimental infection was performed after 30 days of vaccination; the concentration of both bacteria was adjusted to 1 × 108 cells/dose, and doses of 50 μL were applied via i.p. administration. An agglutination test was conducted 30 days after the vaccination of the aquarium fish. Slide agglutination tests were conducted by mixing a drop of each antigen suspension of both bacteria, A. hydrophila and S. agalactiae, separately with a drop of the antiserum (NaCl 0.9% at 1:10, 1:100, and 1:1000 dilutions) of 3 fish from each laboratory experiment group on a glass slide [36]. Microscope-visible agglutinations were recorded as positive. Evaluation of clinical signs and fish mortality was performed daily in the post-vaccine and post-challenge periods by observing the first appearance of macroscopic external changes, behavioral changes, and deaths, which were noted in a specific form. Skin fragments were collected from the intracoelomic region of 5 fish from each experimental group one week after vaccination (T1), from 3 fish from each group two weeks after vaccination (T2), and from 2 fish from each group three weeks after vaccination (T3). At the end of the experiment, all surviving fish were euthanized by deepening the anesthetic plane and necropsied, and liver and spleen fragments were collected from 4 fish from each experimental group. The samples were placed in paraformaldehyde and refrigerated until the time of histopathological analysis. The samples were sent to the Federal University of Espírito Santo (UFES) and processed for histological sections of 5 μm, stained with hematoxylin and eosin. The slides were analyzed for structural and cellular alterations and classified according to the intensity of the lesion: (0) absent, (1) slight, (2) moderate, and (3) intense or severe.
2.7. Field Experiment Design
For field analyses, 12,000 fish were selected, divided into two groups, a control group (6000) and a vaccinated group (6000), and distributed in 10 m3 cages with a population density of 150 animals/m3. The animals had an initial weight between 80 g and 120 g. Feeding was performed twice a day using commercial feed. The vaccine evaluation in the field was carried out by submitting the animals to field conditions at high density without experimental challenge, only natural infection. For vaccination, the animals were anesthetized with eugenol (175 mg L−1) [35]. The vaccine was administered via intraperitoneal injection, applying 50 μL per fish. The control group received no treatment. The biometrics were performed within a 30-day interval to estimate the animals’ biomass. Fifty animals per cage were collected and weighed to assess the average mass of the animals. In addition, all animals present in each tank were weighed to assess the biomass of each group throughout the study.
2.8. Statistical Analysis
To verify the statistical significance between the vaccinated and control groups in the laboratory and field experiments, the Yates-corrected chi-square test (p < 0.05) was used. The relative risk (RR) (CI = 95%) was calculated to verify the strength of the association between exposure to the vaccine and its protective effect. To evaluate the weights of the control and vaccinated groups in the field, a Welch’s test (p < 0.05) was applied. The Kaplan–Meier curve was used to analyze the effect of vaccination and for the graphic representation of the probability of survival. Once the proportionality of the risks was verified, the Cox semi-parametric model was used to compare the experimental groups. To evaluate the equality of the survival functions of the groups, the log-rank test (p < 0.01) was used. For the calculations mentioned, the GraphPad Prism 10 and the PAST (4.03) software were used. Vaccine efficacy was calculated from the formula VE = (1 − OR) × 100, with OR being the odds ratio value.
3. Results
3.1. Bacterial Identification of Aeromonas hydrophila and Streptococcus agalactiae
The biochemical identification obtained a 99% probability for A. hydrophila and a 98% probability for S. agalactiae. Serotyping analysis showed that the isolated S. agalactiae refers to serotype III.
The LAMP products were detected by adding SYBR green fluorescence dye. The tubes containing A. hydrophila samples (A1 and A2) and the tubes containing S. agalactiae samples (S1 and S2) produced positive reactions that appeared yellow, while the negative reaction solution remained orange. The LAMP products were visualized using two percent agarose gel electrophoresis (Figure 1).
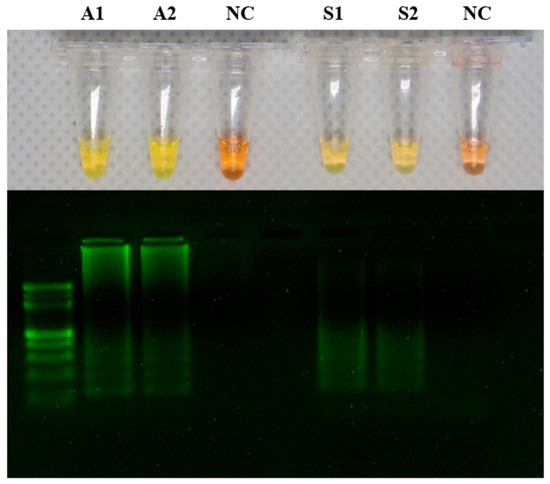
Figure 1.
Detection of DNA amplification in the loop-mediated isothermal amplification (LAMP) assay by a change in color from orange to yellow. LAMP results in addition of SYBR green dye showing A1 and A2 as positive for Aeromonas hydrophila, S1 and S2 as positive for Streptococcus agalactiae, and NC (negative control) as a negative reaction. The LAMP reaction product was analyzed on a 2% agarose gel.
3.2. Vaccinated Group Fish Antiserum Presented Antigen Agglutination after Vaccination in Laboratory Experiment
The agglutination tests showed strong agglutination with vaccinated fish sera for the antigens of both bacteria in the three dilutions tested (1:10, 1:100, and 1:1000) after 30 days of vaccination by i.p. administration. Fish sera from the control group showed no agglutination for the antigens of both bacteria (Figure 2).
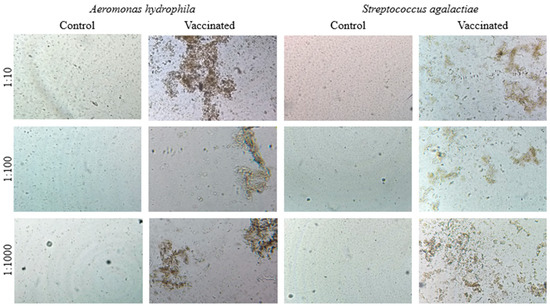
Figure 2.
Photomicroscopy of the direct agglutination test with Nile tilapia sera from the control and vaccinated groups at dilutions of 1:10, 1:100, and 1:1000 associated with inactivated Aeromonas hydrophila and Streptococcus agalactiae antigens.
3.3. Histopathological Analysis of Liver, Spleen, and Tissue from the Vaccine Injection Region
Histopathological analysis of tilapia livers from both groups showed congestion, diffuse or multifocal microgoticular degeneration, and diffuse or multifocal mononuclear inflammatory infiltrate. Analysis of the spleen of tilapia from both groups revealed white pulp hyperplasia (vaccinated group), red pulp hyperplasia (control group), and hemosiderosis. There was no statistical difference between the vaccinated and control groups. (Figure 3).
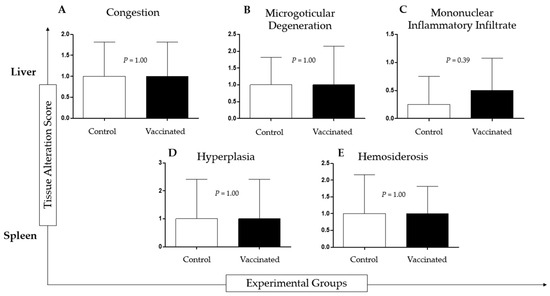
Figure 3.
Histopathological analysis of liver and spleen of vaccinated and non-vaccinated (control) Nile tilapia after intraperitoneal infection with 1 × 108 cells/dose of Aeromonas hydrophila and 1 × 108 cells/dose of Streptococcus agalactiae during the experimental period of 30 days. The groups did not show significant differences.
Tissue histological analysis of the intraperitoneal region of tilapia in the vaccinated group showed collagen deposition perpendicular to the musculature, focal areas of cartilage formation, hyperkeratosis and hyperplasia in T2, and subepithelial melanocytes in T3. The control group presented inflammatory infiltrate in T1 and hyperplasia, microgoticular muscle degeneration, and autolysis in T3.
3.4. Bivalent Vaccine against Aeromonas hydrophila and Streptococcus agalactiae Efficacy in Laboratory and Field Experiments
In the laboratory experiment, the bivalent vaccine against A. hydrophila and S. agalactiae inoculated via i.p. administration in tilapia obtained a chi-square test result with p = 0.0042, RR = 0.13 (95% CI: 0.02–0.75), and VE = 93.66%. In the field experiment, the bivalent vaccine obtained a chi-square test result with p = 0.0001, RR = 0.43 (95% CI: 0.36–0.50), and VE = 59.14% (Table 2).

Table 2.
Efficacy of a bivalent inactivated vaccine against Aeromonas hydrophila and Streptococcus agalactiae inoculated via i.p. administration in Nile tilapia (Oreochromis niloticus). VE = Vaccine Efficacy.
3.5. Bivalent Vaccine against Aeromonas hydrophila and Streptococcus agalactiae Protects Tilapia against Clinical Signs of Both Diseases
In the post-vaccinated period in the laboratory experiment, fish of both groups presented low food intake, which returned to normal on the next day. At the end of the experiment, the vaccinated group of fish that were necropsied did not show macroscopic external and internal clinical signs. External clinical signs of control group fish were hemorrhages; deterioration and hemorrhage of fins; ulcerative lesions; anorexia; altered skin color; gills with excess mucus and presence of necrotic areas (yellowish or brown); lethargy; erratic swimming (indicating central nervous system involvement); and opaque eyes. Internal clinical signs of fish in the control group were yellow or bloody fluid present in the visceral cavity; a decayed and hemorrhagic liver; a dark gallbladder; and a pale color of organs (Table S1) (Figure 4).
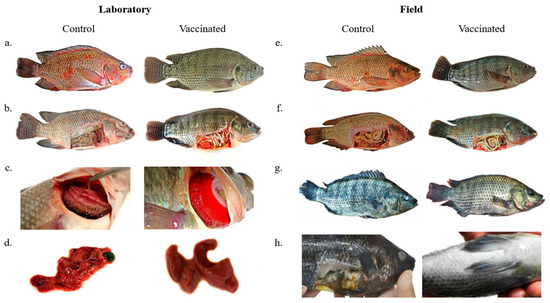
Figure 4.
(a) Control group fish after experimental infection with A. hydrophila and S. agalactiae presenting body and fin hemorrhages and ulcerative lesions. Fish from the vaccinated group showing no external clinical signs after experimental infection. (b) Control group fish after experimental infection with A. hydrophila and S. agalactiae presenting skin pallor, yellowish fluid in the visceral cavity, and organ deterioration. Fish from the vaccinated group showing no internal clinical signs after experimental infection. (c) Control group fish after experimental infection with A. hydrophila and S. agalactiae presenting excess mucus, necrosis, and pallor of the gills. Fish gills of the vaccinated group with normal appearance and color after experimental infection. (d) Fish liver from the control group after experimental infection with A. hydrophila and S. agalactiae showing deterioration and hemorrhage. Fish liver of the vaccinated group showing normal appearance and color after experimental infection. (e) Control group fish presenting body and fin hemorrhages. Vaccinated group fish showing no external clinical signs. (f) Control group fish presenting skin pallor, yellowish fluid in the visceral cavity, and organ deterioration. Vaccinated group fish showing no internal clinical signs. (g) Control group fish presenting tail rot and corneal opacity. Vaccinated group fish showing weight gain and no clinical signs. (h) Control group fish showing skin darkening, corneal opacity, and internal organ deterioration.
3.6. Mortality Was Lower in the Vaccinated Tilapia Groups after Laboratory Experiment Challenge and Lower in the Field Experiment without Challenge
Mortality in the vaccinated group of the laboratory experiment was 01 (2.94%) and in the control group was 11 (32.35%). In the vaccinated group, the fish died on the 18th day after experimental infection but did not present clinical signs of the diseases (only signs of fight). In the control group, the 11 fish died on days 12, 15, 21, 22, and 30 after the experimental infection. Survival curves of the control and vaccinated groups in the laboratory experiment had a significant difference (p = 0.001), and Cox’s analysis showed a significant risk of event (death) between groups, with a reduction in the risk of death in the vaccinated group: HR = 0.06056 (95% CI: 0.003256–0.3252) (Figure 5).
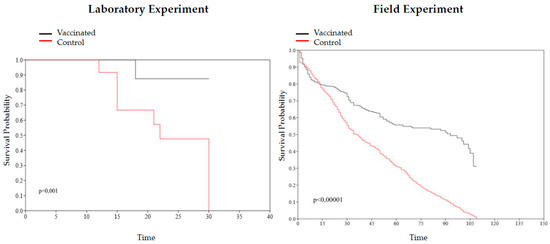
Figure 5.
Kaplan–Meier survival curve and log-rank test comparing the vaccinated and control groups from laboratory (p = 0.001) and field (p < 0.00001) experiments.
In the field, the vaccine was able to reduce mortality; control and vaccinated groups had a mortality rate of 7.78% and 3.33%, respectively. In addition, the daily mortality rate was reduced to less than half in the vaccinated cages. Survival curves of the control and vaccinated groups in the laboratory experiment had a significant difference (p < 0.00001), and Cox’s analysis showed a significant difference, with a reduction in the risk of death in the vaccinated group: HR = 0.1713 (95% CI: 0.1281–0.2244) (Figure 5).
3.7. Effect of Vaccination on Weight Gain and Feed Conversion
In the laboratory experiment, on the vaccination day, fish of the control and vaccinated groups had an average weight of 50.2 ± 17.2 g and 50.0 ± 19.2 g, respectively, with no significant difference (p = 0.7133). Thirty days after vaccination and before experimental infection, the control and vaccinated fish had an average weight of 119.3 ± 27.9 g and 103.3 ± 23.7 g, respectively, with a significant difference (p = 0.0092). Sixty days after vaccination and thirty days after experimental infection, the control and vaccinated fish had an average weight of 150.0 ± 50.3 g and 164.2 ± 33.7 g, respectively, presenting no significant difference (p = 0.2197).
In field experiments, vaccinated animals had greater growth than the control animals, as shown in Table 3. Both groups showed a similar pattern of fattening during the first months; however, at the end, the vaccinated group had approximately 22% more body mass per animal compared to the control. Added to this, a greater homogeneity was also observed in the final average weight of the animals in the vaccinated cages. The feed conversion ratio (FCR) considers the amount of food given to the animals and the final biomass. The analysis of this parameter showed that the immunized animals had better use of the food compared to the non-immunized ones, being able to transform the food more efficiently into body mass (Table 3).

Table 3.
Final Average Weight, Food Conversion Ratio (FCR), and Average daily mortality of Control and vaccinated groups in field experiment.
4. Discussion
Inactivated whole-cell vaccines correspond to the most used type for veterinary vaccines, as they do not represent the risk of virulence reversion [37,38,39]. The intraperitoneal administration system presents better results compared to other systems, such as by immersion bath, via spray, or orally, since these systems, despite being easier to handle, do not effectively stimulate antibody production [38,39,40,41,42].
Studies showed a greater agglutination titer after fish vaccination against Streptococcus spp. and Aeromonas spp. [43,44,45,46]. In the present study, agglutination was shown to be increased for A. hydrophila and S. agalactiae in the three dilutions tested after 30 days of vaccination in the laboratory experiment, confirming that the vaccine induced the production of antibodies to both bacteria.
With the aim of mimicking the occurrence of bacterial outbreaks in production tanks where bacteria would replicate in large quantities, especially at high temperatures, the fish were challenged with a high dose, showing that the vaccine was able to protect the vaccinated group. However, despite mimicking high-density production in the laboratory, in addition to thermal stress, large-scale tests showed different effectiveness. However, when compared with the control group in the field, the vaccinated group showed an improvement in survival and feed conversion.
Another important factor in the use of intraperitoneal vaccines is cost-effectiveness, where the effectiveness is sufficiently high in relation to the costs of the vaccine and the labor required for individual vaccination of fish. The application of more than one vaccine dose can increase protection in fish [47,48]. However, the investment required to repeat the dose may not be attractive to the producer due to logistical problems and the cost of commercial production. The bivalent vaccine was able to provide protection to tilapia vaccinated with just one dose, representing a good cost–benefit ratio.
Used in the field, the bivalent vaccine proved to be effective in reducing mortality in tilapia, and the VE obtained was 59.14%. Pasnik (2005) [49] showed that the immune response generated is effective 180 days after vaccination, being able to protect animals throughout the fattening period with a single dose. This study managed to keep the mortality rate reduced over 118 days, showing that vaccination with one dose protects the animals during the entire fattening period. It is noteworthy that the above analyses were conducted with the experimental infection of the animals. In this work, the animals were submitted to conditions of high production density to intensify the natural infection rate.
The bivalent vaccine incorporated with the adjuvant proved to be non-toxic to fish in the vaccinated group, proving its safety. Histopathological analysis of the skin, liver, and spleen of both vaccinated and control groups showed no significant differences. Steckert et al. (2018) [50] concluded that alterations such as hyperplasia may be present in non-diseased animals, suggesting an adaptation of fish to the confinement environment. The skin of fish in the control group showed a mononuclear inflammatory infiltrate, characterized by a large amount of leukocytes accumulated in an inflammatory response site in the first week after the inoculation of sterile saline solution, hyperplasia, and macro- and microgoticular degeneration after three weeks. The histological findings of the fish skin of the vaccinated group showed that 2 to 3 weeks after immunization, the vaccine inoculation site underwent a healing process. This factor is extremely important, since a vaccine candidate should not leave lesions in the inoculum region except in the first days after vaccination due to the expected inflammatory process.
In large-scale trials, vaccination was shown to help increase productivity and improve feed conversion. Immunized fish, after 118 days of the experiment, weighed about 0.792 kg (±0.016) compared to the 0.657 kg (±0.10) in control. This indicated an increase in biomass and greater homogeneity due to immunization. The better use of the feed can be related to the reduction in stress inherent to the presence of infections. In addition, most of the fish in the control group had several lesions on the scales, and when analyzing practical and legal issues under production conditions in the country, fish with skin lesions cannot be marketed [51].
5. Conclusions
In general terms, immunization with a bivalent vaccine against A. hydrophila and S. agalactiae administered via i.p. administration demonstrated to be safe and effective in reducing mortality in fish raised in high-density production tanks. Furthermore, the benefits of vaccination also led to an improvement in the animals’ feed conversion, representing direct gains in fish productivity.
6. Patents
Patent of invention titled “Bivalent Vaccine for Tilapia” (BR1020190263644), deposited in Instituto Nacional de Propriedade Intelectual (INPI).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13213338/s1, Table S1: External and internal physical characteristics and behavior characteristics analyzed in the post-vaccination and post-infection periods and in necropsy, in control and vaccinated groups. dpv = days post-vaccination; dpi = days post-infection; C = control group; V = vaccinated group.
Author Contributions
Conceptualization, K.F.V. and A.V.R.; methodology, A.V.R., A.G.V.d.S., A.B.d.S., G.B.J., G.F.d.S. and E.M.d.S.; resources, K.F.V. and E.M.d.S.; data curation, A.V.R., A.G.V.d.S. and A.B.d.S.; writing—original draft preparation, A.V.R. and A.G.V.d.S.; writing—review and editing, K.F.V.; supervision, K.F.V.; project administration, K.F.V.; histopathological analysis, L.d.C.N.; funding acquisition, K.F.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Federal University of Latin American Integration and Fundação Araucária (02/2018).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of the Federal University of Latin American Integration (CEUA UNILA N° 001/2018—21 April 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. The State of World Fisheries and Aquaculture. Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- PEIXEBR. Anuário Brasileiro da Piscicultura, PEIXE BR 2023. São Paulo: Associação Brasileira da Piscicultura. 2023. Available online: https://www.aen.pr.gov.br/sites/default/arquivos_restritos/files/documento/2023-03/anuariopeixebr2023.pdf (accessed on 15 January 2023).
- Ayroza, D.M.M.R.; Carmo, F.J.; Ayroza, L.M.S. Panorama da Piscicultura no Brasil—Destaque para o Potencial do Estado de São Paulo; Casa da Agricultura: São Paulo, Brazil, 2011; pp. 9–10.
- Hirsch, D.; Pereira Júnior, D.J.; Logato, P.V.R.; Piccoli, R.H.; Figueiredo, H.C.P. Identificação e resistência a antimicrobianos de espécies de Aeromonas móveis isoladas de peixes e ambientes aquáticos. Ciênc. Agrotec. 2006, 30, 1211–1217. [Google Scholar] [CrossRef]
- Xu, D.H.; Shoemaker, C.A.; Martins, M.L.; Pridgeon, J.W.; Klesius, P.H. Enhanced susceptibility of channel catfish to the bacterium Edwardsiella ictaluri after parasitism by Ichthyophthirius multifiliis. Vet. Microbiol. 2012, 158, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Leira, M.H.; de Assis Lago, A.; Viana, J.A.; da Cunha, L.T.; Mendonça, F.G.; de Freitas, R.T.F. As principais doenças na criação de tilápias no Brasil: Revisão de literatura. Nutr. Time 2017, 14, 4982–4996. [Google Scholar]
- Schering-Plough. Principais Doenças Bacterianas em Criações Comerciais de Peixes no Brasil. Boletim Técnico, Cotia, SP, 2007. 8p. Available online: https://www.snatural.com.br/wp-content/uploads/2017/05/Doencas-Peixes-Tratamento.pdf (accessed on 15 June 2022).
- Kubitza, F. Tilápias na mira dos patógenos. Rev. Panor. Aquicultura 2008, 18, 28–37. [Google Scholar]
- Batra, P.; Mathur, P.; Misra, M.C. Aeromonas spp.: An emerging nosocomial pathogen. J. Lab. Physicians 2016, 8, 1. [Google Scholar] [CrossRef]
- Figueiredo, H.C.P.; Castro, G.A.C.; Leal, C.A.G.; Lopes, C.O. Sanidade aquícola: Quem tem medo de Aeromonas? Rev. Panor. Aquicultura 2008, 108, 26–31. [Google Scholar]
- Sime-Ngando, T. Aeromonas; Caister Academic Press: Norfolk, UK, 2015. [Google Scholar]
- Delphino, M.K.; Barone, R.S.; Leal, C.A.; Figueiredo, H.C.; Gardner, I.A.; Gonçalves, V.S. Economic appraisal of vaccination against Streptoccocus agalactiae in Nile tilapia farms in Brazil. Prev. Vet. Med. 2019, 162, 131–135. [Google Scholar] [CrossRef]
- Salvador, R.; Muller, E.E.; Freitas, J.C.D.; Leonhadt, J.H.; Pretto-Giordano, L.G.; Dias, J.A. Isolation and characterization of Streptococcus spp. group B in Nile tilapias (Oreochromis niloticus) reared in hapas nets and earth nurseries in the northern region of Parana State, Brazil. Cienc. Rural. 2005, 35, 1374–1378. [Google Scholar] [CrossRef]
- Marcusso, P.F.; Eto, S.F.; Claudiano, G.D.S.; Vieira, F.C.F.; Salvador, R.; Moraes, J.R.E.D.; Moraes, F.R.D. Isolamento de Streptococcus agalactiae em diferentes órgãos de tilápias-do-nilo (Oreochromis niloticus) criadas em tanques-rede. Biosci. J. 2015, 31, 549–554. [Google Scholar] [CrossRef]
- Leal, C.A.G. Estreptococose clínica em tilápia: Passado e presente. Rev. Panor. Aquicultura 2018, 169, 28–35. [Google Scholar]
- Smith, P.; Hiney, M.P.; Samuelsen, O.B. Bacterial resistance to antimicrobial agents used in fish farming: A critical evaluation of method and meaning. Annu. Rev. Fish Dis. 1994, 4, 273–313. [Google Scholar] [CrossRef]
- Abutbul, S.; Golan-Goldhirsh, A.; Barazani, O.; Zilberg, D. Use of Rosmarinus officinalis as a treatment against Streptococcus iniae in tilapia (Oreochromis sp.). Aquaculture 2004, 238, 97–105. [Google Scholar] [CrossRef]
- Burridge, L.; Weis, J.S.; Cabello, F.; Pizarro, J.; Bostick, K. Chemical use in salmon aquaculture: A review of current practices and possible environmental effects. Aquaculture 2010, 306, 7–23. [Google Scholar] [CrossRef]
- Pádua, S.B.; Menezes Filho, R.N. Antibióticos na aquicultura e os critérios para o uso racional. Rev. Panor. Aquicultura 2014, 145, 31. [Google Scholar]
- Chakravarti, D.N.; Fiske, M.J.; Fletcher, L.D.; Zagursky, R.J. Application of genomics and proteomics for identification of bacterial gene products as potential vaccine candidates. Vaccine 2000, 19, 601–612. [Google Scholar] [CrossRef]
- Dumrongphol, Y.; Hirota, T.; Kondo, H.; Aoki, T.; Hirono, I. Identification of novel genes in Japanese flounder (Paralichthys olivaceus) head kidney up-regulated after vaccination with Streptococcus iniae formalin-killed cells. Fish Shellfish Immunol. 2009, 26, 197–200. [Google Scholar] [CrossRef]
- Reiner, K. Catalase Test Protocol; American Society for Microbiology: Washington, DC, USA, 2010; pp. 1–9. Available online: https://asm.org/getattachment/72a871fc-ba92-4128-a194-6f1bab5c3ab7/Catalase-Test-Protocol.pdf (accessed on 15 June 2022).
- Barile, M.F. Gram Staining Technique. In Methods in Mycoplasmology V1: Mycoplasma Characterization; Razin, S., Ed.; Academic Press: New York, NY, USA, 2012; Volume 1, p. 39. [Google Scholar]
- Buxton, R. Blood Agar Plates and Hemolysis Protocols; American Society for Microbiology: Washington, DC, USA, 2005; pp. 1–9. Available online: https://asm.org/getattachment/7ec0de2b-bb16-4f6e-ba07-2aea25a43e76/protocol-2885.pdf (accessed on 15 June 2022).
- BioMérieux. VITEK 2 Compact. Available online: https://www.biomerieux.com.br/produto/vitekr-2-compact (accessed on 15 June 2022).
- Slotved, H.C.; Elliott, J.; Thompson, T.; Konradsen, H.B. Latex assay for serotyping of group B Streptococcus isolates. J. Clin. Microbiol. 2003, 41, 4445–4447. [Google Scholar] [CrossRef]
- Viogene Biotek, Blood and Tissue Genomic DNA Miniprep System, Taiwan. Available online: https://www.viogene.com/uploads/product/protocol/20/GG1002.pdf (accessed on 15 January 2023).
- Cai, Y.; Zhou, Q.J.; Chen, J. Establishment of loop-mediated isothermal amplification method combined with a lateral flow dipstick for rapid detection of Aeromonas hydrophila. J. Vet. Sci. Technol. 2016, 36, 256–264. [Google Scholar] [CrossRef]
- Zhou, Q.J.; Lu, J.F.; Su, X.R.; Jin, J.L.; Li, S.Y.; Zhou, Y.; Wang, L.; Shao, X.-B.; Wang, Y.H.; Chen, J.; et al. Simultaneous detection of multiple bacterial and viral aquatic pathogens using a fluorogenic loop-mediated isothermal amplification-based dual-sample microfluidic chip. J. Fish Dis. 2021, 44, 401–413. [Google Scholar] [CrossRef]
- SEPPIC. Animal Species and Veterinary Vaccines; SEPPIC: Courbevoie, France; Available online: https://www.seppic.com/en/animal-health/animal-species (accessed on 25 September 2023).
- Popma, T.J.; Green, B.W. Reversão sexual de tilápias em tanques de terra. In Manual de Produção em Aquacultura; University Aurburn: Aurburn, FL, USA, 1990; p. 52. [Google Scholar]
- Kubitza, F.; Kubitza, L.M.M. Tilápias: Qualidade da água, sistemas de cultivo, planejamento da produção, manejo nutricional e alimentar e sanidade–Parte 2. Rev. Panor. Aqüicultura 2000, 60, 31–53. [Google Scholar]
- Marengoni, N.G. Produção de tilápia-do-Nilo Oreochromis niloticus (linhagem chitralada), cultivada em tanques-rede, sob diferentes densidades de estocagem. Arch. Zootec. 2006, 55, 127–138. [Google Scholar]
- Kubitza, F. Manejo nutricional e alimentar de tilápias. Rev. Panor. Aquicultura 2000, 10, 31–36. [Google Scholar]
- Rotili, D.A.; Devens, M.A.; Diemer, O.; Lorenz, E.K.; Lazzari, R.; Boscolo, W.R. Uso de eugenol como anestésico em pacu. Pesq. Agropec. Trop. 2012, 42, 288–294. [Google Scholar] [CrossRef]
- Sørensen, U.B.; Larsen, J.L. Serotyping of Vibrio anguillarum. Appl. Environ. Microbiol. 1986, 51, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Melo, C.C.V.; Bruhn, F.R.P.; Ascari, I.J.; Leira, M.H.; Zangeronimo, M.G.; Pereira, L.J.; Mian, G.F. A eficácia das vacinas contra Streptococcus agalactiae em tilápias: Uma revisão sistemática. Rev. Científica Eletrônica Med. Veterinária 2015, 13, 1–15. [Google Scholar]
- Liu, G.; Zhu, J.; Chen, K.; Gao, T.; Yao, H.; Liu, Y.; Zhang, W.; Lu, C. Development of Streptococcus agalactiae vaccines for tilapia. Dis. Aquat. Org. 2016, 122, 163–170. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Paul, J.; Evensen, Ø. An overview of vaccination strategies and antigen delivery systems for Streptococcus agalactiae vaccines in Nile tilapia (Oreochromis niloticus). Vaccines 2016, 4, 48. [Google Scholar] [CrossRef]
- Lamers, C.H.J.; De Hass, M.J.H.; Van Muiswinkel, W.B. Humoral response and memory formation in carp after injection of Aeromonas hydrophila bacterin. Dev. Comp. Immunol. 1985, 9, 65–75. [Google Scholar] [CrossRef]
- Evans, J.J.; Klesius, P.H.; Shoemaker, C.A. Efficacy of Streptococcus agalactiae (group B) vaccine in tilapia (Oreochromis niloticus) by intraperitoneal and bath immersion administration. Vaccin 2004, 22, 3769–3773. [Google Scholar] [CrossRef]
- Longhi, E.; Pretto-Giordano, L.G.; Müller, E.E. Effectiveness of homologous inactivated Streptococcus agalactiae vaccine by immersion bath in Nile tilapia (Oreochromis niloticus). Semin. Cienc. Agrar. 2013, 33 (Suppl. 2), 3191–3200. [Google Scholar] [CrossRef]
- Klesius, P.H.; Shoemaker, C.A.; Evans, J.J. Efficacy of single and combined Streptococcus iniae isolate vaccine administered by intraperitoneal and intramuscular routes in tilapia (Oreochromis niloticus). Aquaculture 2000, 188, 237–246. [Google Scholar] [CrossRef]
- Sevaraj, V.; Sampath, K.; Sekar, V. Extraction and characterization of lipopolysaccharide from Aeromonas hydrophila and its effects on survival and hematology of the carp, Cyprinus carpio. Asian Fish. Sci. 2004, 17, 163–173. [Google Scholar]
- Khoshbavar-Rostami, H.A.; Soltani, M.; Hassan, H.M.D. Immune responses of great sturgeon Huso huso to Aeromonas hydrophila bacterin. J. Fish Biol. 2007, 70, 1931–1938. [Google Scholar] [CrossRef]
- Silva, B.C.; Martins, M.L.; Jatobá, A.; Buglione Neto, C.C.; Vieira, F.N.; Pereira, G.V.; Jerônimo, G.T.; Seiffert, W.Q.; Mouriño, J.L.P. Resposta hematológica e imunológica de tilápia do Nilo após administração de vacina polivalente por diferentes vias. Pesq. Vet. Bras. 2009, 29, 874–880. [Google Scholar] [CrossRef]
- Eldar, A.; Horovitcz, A.; Bercovier, H. Development and efficacy of a vaccine against Streptococcus iniae infection in farmed rainbow trout. Vet. Immunol. Immunopathol. 1997, 56, 175–183. [Google Scholar] [CrossRef]
- Pretto-Giordano, L.G.; Müller, E.E.; Klesius, P.; Da Silva, V.G. Efficacy of an experimentally inactivated Streptococcus agalactiae vaccine in Nile tilapia (Oreochromis niloticus) reared in Brazil. Aquac. Res. 2010, 41, 1539–1544. [Google Scholar] [CrossRef]
- Pasnik, D.J.; Evans, J.J.; Panangala, V.S.; Klesius, P.H.; Shelby, R.A.; Shoemaker, C.A. Antigenicity of Streptococcus agalactiae extracellular products and vaccine efficacy. J. Fish Dis. 2005, 28, 205–212. [Google Scholar] [CrossRef]
- Steckert, L.D.; Cardoso, L.; Jerônimo, G.T.; de Pádua, S.B.; Martins, M.L. Investigation of farmed Nile tilapia health through histopathology. Aquaculture 2018, 486, 161–169. [Google Scholar] [CrossRef]
- Ministério da Agricultura e Pecuária. Decreto Nº 9.013, de 29 de Março de 2017. Regulamenta a Lei nº 1.283, de 18 de Dezembro de 1950, e a Lei nº 7.889, de 23 de Novembro de 1989; Diário Oficial da União; Poder Executivo; Ministério da Agricultura e Pecuária: Brasília, Brasil, 2017.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).