Evaluation of Post-Larval Diets for Indoor Weaned Largemouth Bass (Micropterus salmoides)
Abstract
Simple Summary
Abstract
1. Introduction
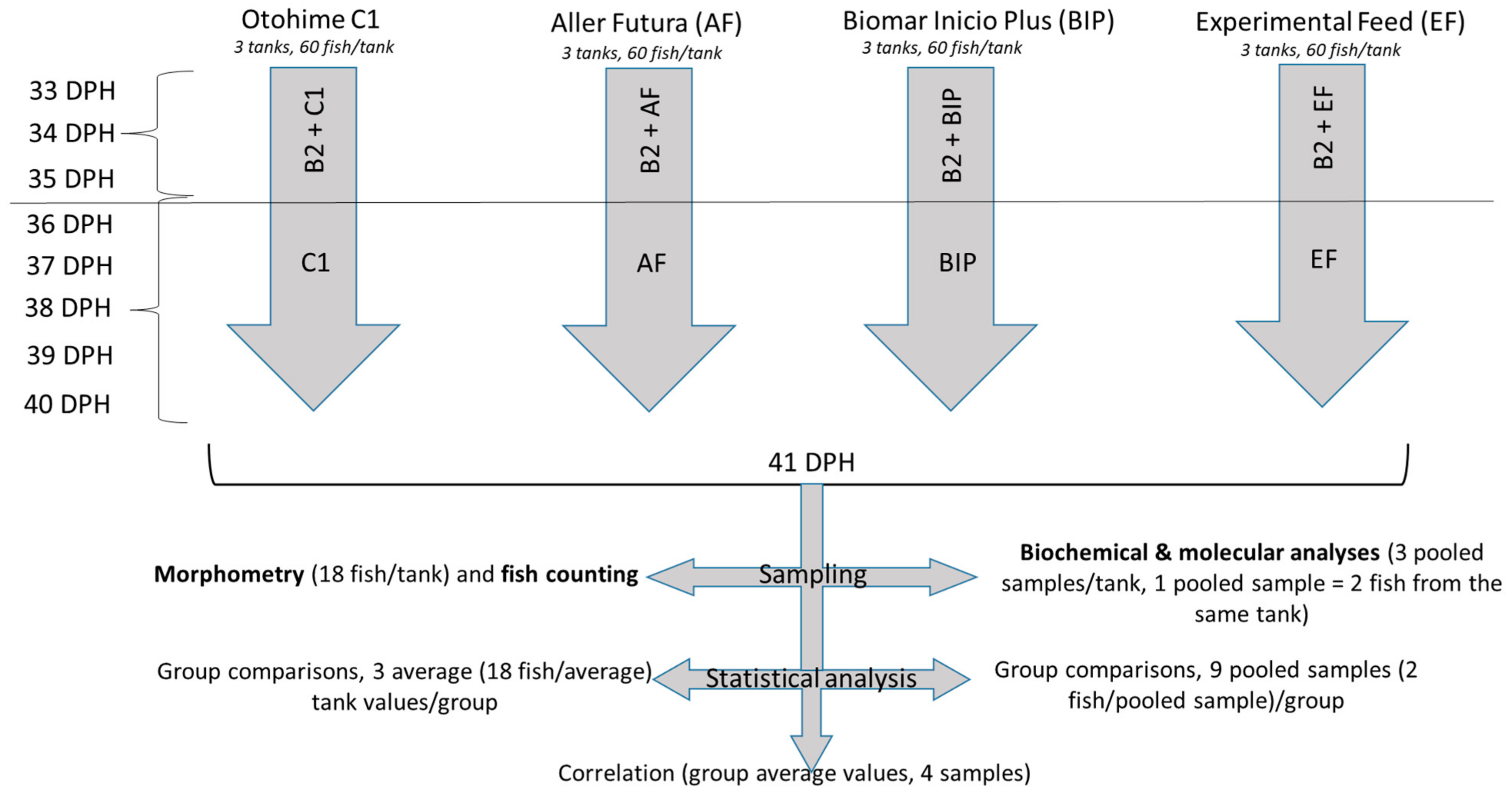
2. Materials and Methods
2.1. Feeds Used in the Study
2.2. Fish Origin
2.3. Study Design
2.4. Homogenization of Fish
2.5. Quantitative PCR (qPCR)
2.6. Biochemical Analyses
2.7. Economic Analysis
2.8. Data Analysis
3. Results
3.1. Feed Composition
3.2. Biometric Indices
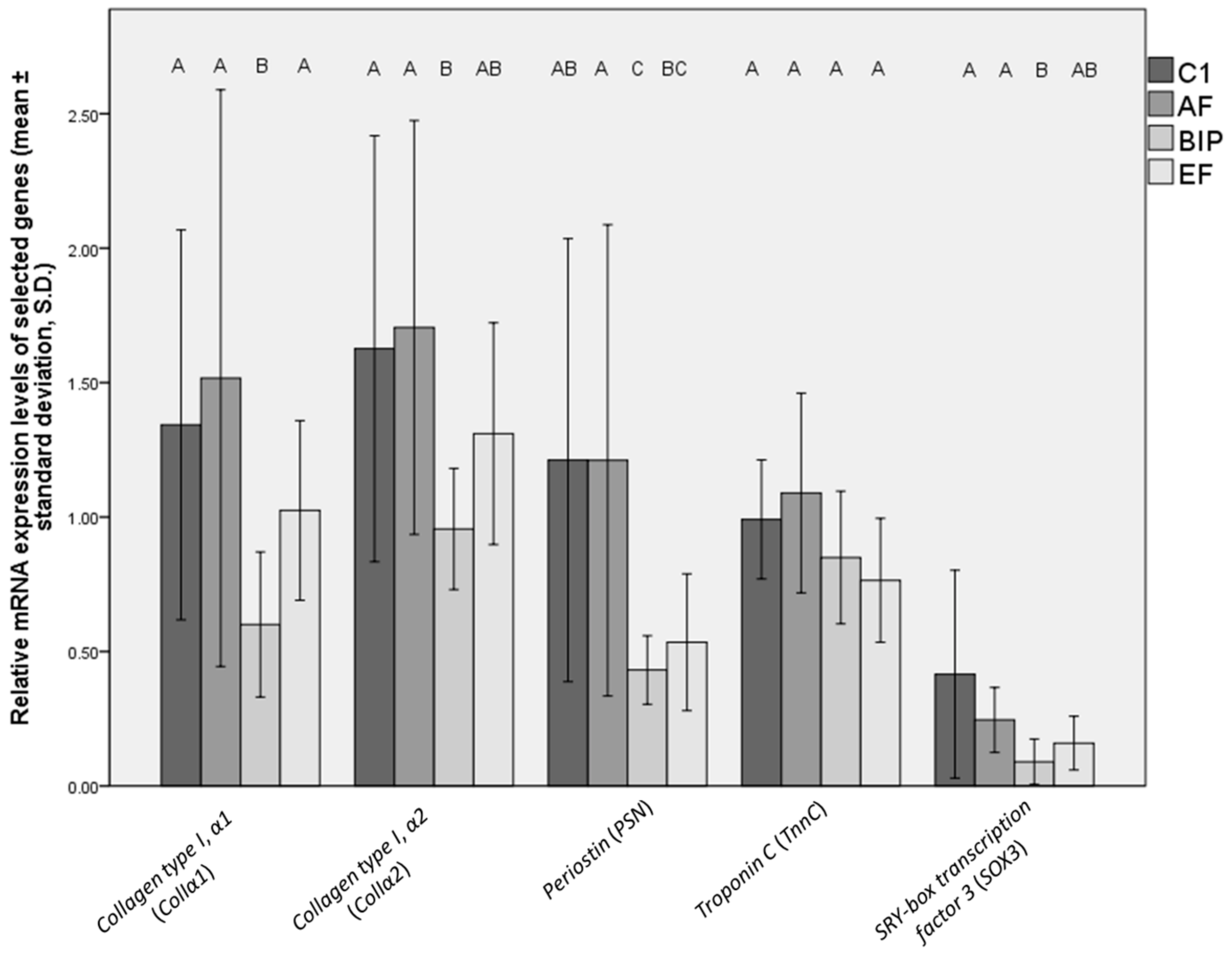
3.3. mRNA Expression
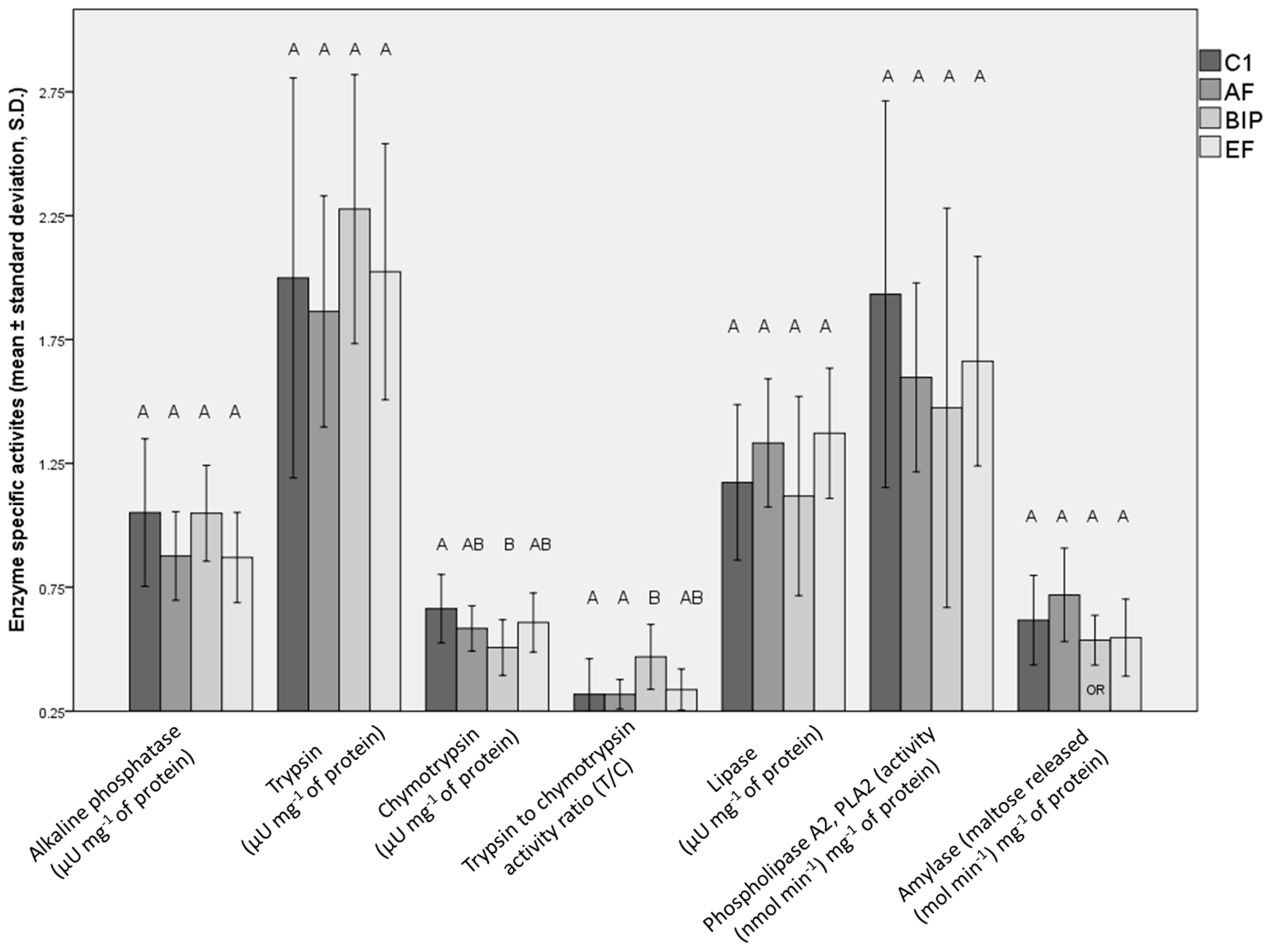
3.4. Digestive Enzyme Activity
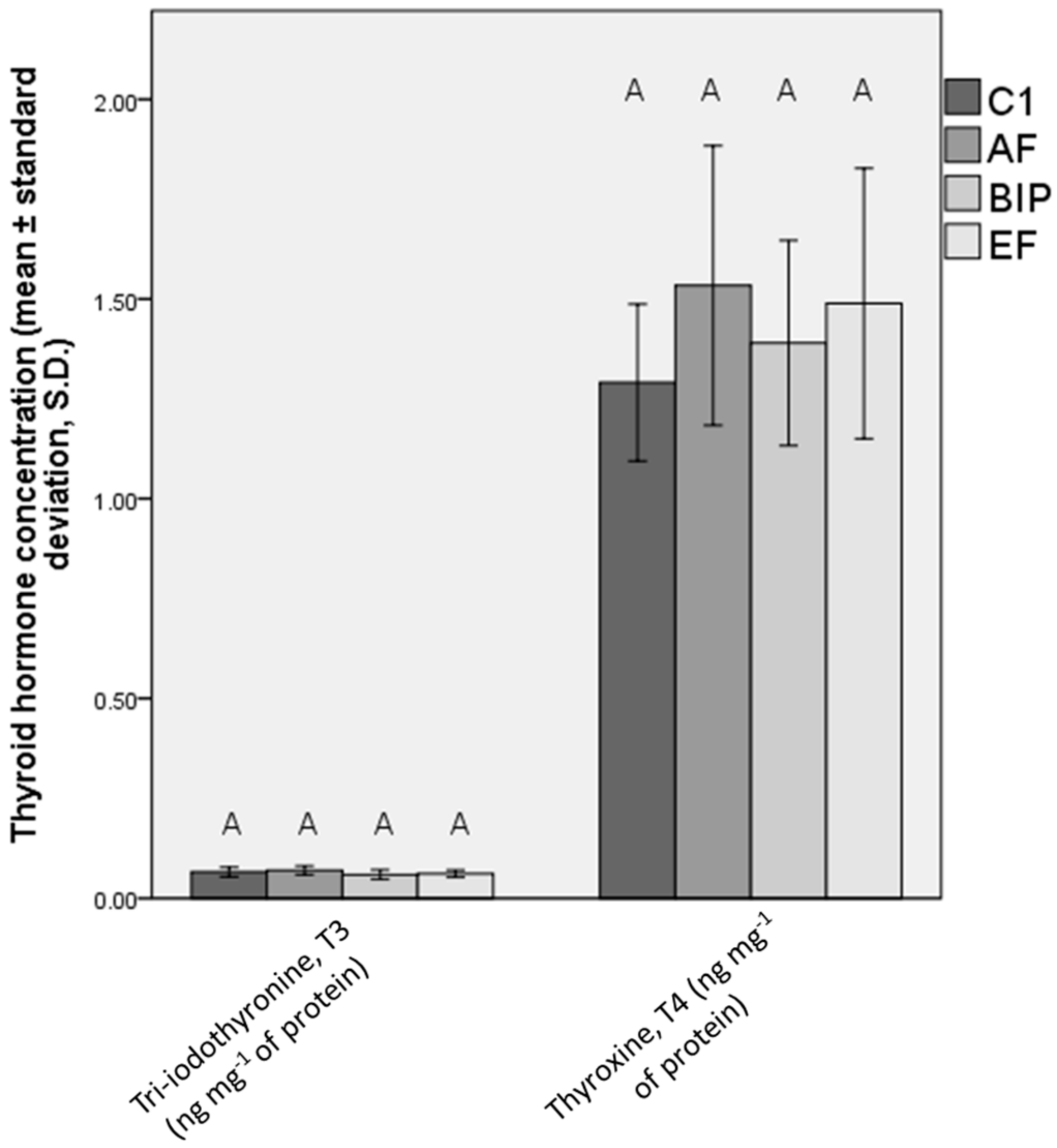
3.5. Hormonal Status
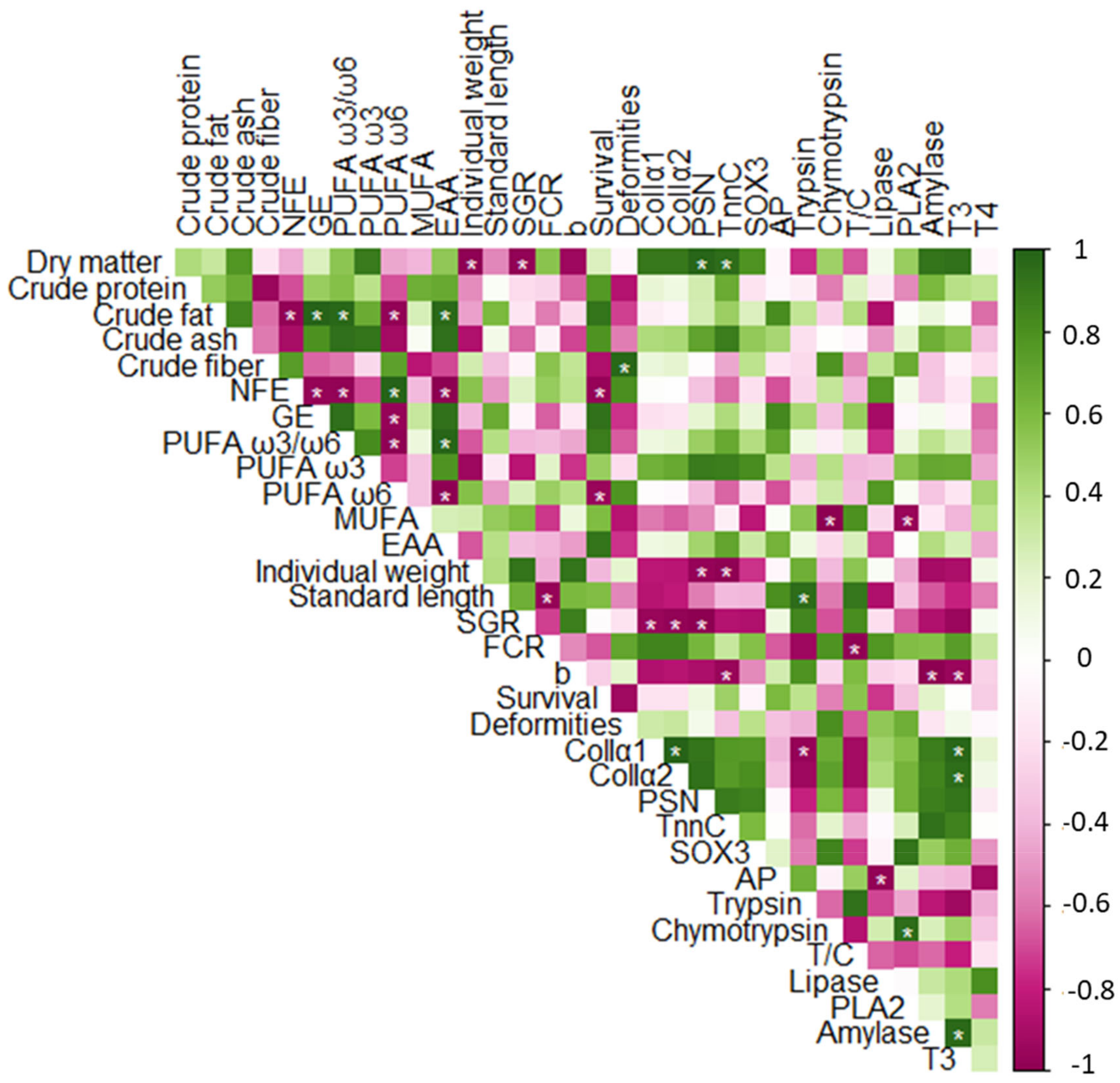
3.6. Correlation Analysis
3.7. Economic Calculations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, C.H.; Bie, H.Q.; Lu, Z.Y.; Ding, Y.; Guan, H.H.; Geng, L.H.; Ma, S.; Hu, Y.X.; Fan, Q.X.; Shen, Z.G. Out-of season spawning of largemouth bass in a controllable recirculating system. Front. Physiol. 2023, 14, 1175075. [Google Scholar] [CrossRef] [PubMed]
- Subhadra, B.; Lochmann, R.; Goodwin, A. Potential use of proximate and fatty acid composition to distinguish between cultured and wild largemouth bass (Micropterus salmoides). Open Agric. J. 2014, 8, 48–51. [Google Scholar] [CrossRef][Green Version]
- Sloane, M.B.; Lovshin, L.L. Feed training and intensive production of advanced largemouth bass fingerlings: A review. Rev. Fish. Sci. 1995, 3, 65–89. [Google Scholar] [CrossRef]
- Global SeaFood Alliance. Available online: https://www.globalseafood.org/advocate/feeds-feeding-methods-advance-largemouth-bass/ (accessed on 25 August 2023).
- Kentucky State University. Available online: https://www.ksuaquaculture.org/Species/Bass,Largemouth.htm (accessed on 25 August 2023).
- Péter, G.; Lukić, J.; Alvestad, R.; Horváth, Z.; Nagy, Z.; Rónyai, A.; Bársony, P.; Ljubobratović, U. Nursing of pike-perch (Sander lucioperca) in Recirculating Aquaculture System (RAS) provides growth advantage in juvenile growth phase. Animals 2023, 13, 347. [Google Scholar] [CrossRef]
- Matthews, M.D. Hatchery Techniques: Feed Training Juvenile Largemouth Bass, Micropterus salmoides floridanus; Texas A&M University: College Station, TX, USA, 2013; Available online: http://fisheries.tamu.edu/files/2013/09/Hatchery-Techniques-Feed-Training-Juvenile-Largemouth-Bass-Micropterus-salmoides-floridanus.pdf (accessed on 28 August 2023).
- Boahemaa-Kobil, E.E. Quality of Raw and Cooked Fillets of Atlantic Salmon Farmed in the Arctic: Effect of Dietary Oil and Thawing Temperature. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2020. [Google Scholar] [CrossRef]
- Liang, C.; Zhao, X.; Jiao, L.; Shen, Y.; Luo, J.; Zhu, T.; Zhao, W.; Gen, Z.; Zhou, Q.; Jin, M. Effects of different lipid sources on growth performance, fatty acids composition in tissue and expression of genes related to lipid metabolism in largemouth bass (Micropterus salmoides). Aquacult. Rep. 2022, 23, 101013. [Google Scholar] [CrossRef]
- Hungarian Standard MSZ ISO 6496:2001; Animal Feeding Stuffs. Determination of Moisture and Other Volatile Compounds.
- Hungarian Standard MSZ EN ISO 5983-2:2009; Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content. Available online: https://www.iso.org/standard/39145.html (accessed on 25 August 2023).
- 152/2009 EK III/H Determination of Crude Oils and Fats. Available online: https://www.legislation.gov.uk/eur/2009/152/annex/III/division/H (accessed on 25 August 2023).
- 152/2009 EK III/I Determination of Crude Fibre. Available online: https://www.legislation.gov.uk/eur/2009/152/annex/III/division/I (accessed on 25 August 2023).
- Hungarian Standard (Crude Ash MSZ 5984:1992_withdrawn). Available online: https://www.legislation.gov.uk/eur/2009/152/annex/III/division/M (accessed on 25 August 2023).
- Hungarian Standard MSZ EN ISO 13903:2005. Animal Feeding Stuffs—Determination of Amino Acids Content. Available online: https://www.iso.org/standard/37258.html (accessed on 25 August 2023).
- Hungarian Standard MSZ EN ISO 12966-2. Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters. Available online: https://www.iso.org/standard/72142.html (accessed on 25 August 2023).
- Ljubobratovic, U.; Kosanovic, D.; Vukotic, G.; Molnar, Z.; Stanisavljevic, N.; Ristovic, T.; Peter, G.; Lukic, J.; Jeney, G. Supplementation of lactobacilli improves growth, regulates microbiota composition and suppresses skeletal anomalies in juvenile pike-perch (Sander lucioperca) reared in recirculating aquaculture system (RAS): A pilot study. Res. Vet. Sci. 2017, 115, 451–462. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 21.0; IBM Corp.: Armonk, NY, USA, 2012. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 28 August 2023).
- Li, X.; Zheng, S.; Ma, X.; Cheng, K.; Wu, G. Effects of dietary protein and lipid levels on the growth performance, feed utilization, and liver histology of largemouth bass (Micropterus salmoides). Amino Acids 2020, 52, 1043–1061. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, S.; Ma, X.; Cheng, K.; Wu, G. Use of alternative protein sources for fishmeal replacement in the diet of largemouth bass (Micropterus salmoides). Part I: Effects of poultry by-product meal and soybean meal on growth, feed utilization, and health. Amino Acids 2021, 53, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H. Intake of arachidonic acid-containing lipids in adult humans: Dietary surveys and clinical trials. Lipids Health Dis. 2019, 18, 101. [Google Scholar] [CrossRef] [PubMed]
- Taşbozan, O.; Gökçe, M.A. Fatty Acids in Fish; InTech: Houston, TX, USA, 2017. [Google Scholar] [CrossRef]
- Zong, G.; Li, Y.; Sampson, L.; Dougherty, L.W.; Willett, W.C.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Hu, F.B.; Sun, Q. Monounsaturated fats from plant and animal sources in relation to risk of coronary heart disease among US men and women. Am. J. Clin. 2018, 107, 445–453. [Google Scholar] [CrossRef]
- Kertaoui, N.E.; Lund, I.; Betancor, M.B.; Carpentier, C.; Montero, D.; Kestemont, P. Dietary DHA and ARA level and ratio affect the occurrence of skeletal anomalies in pikeperch larvae (Sander lucioperca) through a regulation of immunity and stress related gene expression. Aquaculture 2021, 544, 737060. [Google Scholar] [CrossRef]
- Cahu, C.; Zambonino Infante, J.; Takeuchi, T. Nutritional components affecting skeletal development in fish larvae. Aquaculture 2003, 227, 245–258. [Google Scholar] [CrossRef]
- Bardon, A.; Vandeputte, M.; Dupont-Nivet, M.; Chavanne, H.; Haffray, P.; Vergnet, A.; Chatain, B. What is the heritable component of spinal deformities in the European sea bass (Dicentrarchus labrax)? Aquaculture 2009, 294, 194–201. [Google Scholar] [CrossRef][Green Version]
- Betancor, M.B.; Atalah, E.; Caballero, M.; Benítez-Santana, T.; Roo, J.D.; Montero, D.; Izquierdo, M. α-Tocopherol in weaning diets for European sea bass (Dicentrarchus labrax) improves survival and reduces tissue damage caused by excess dietary DHA contents. Aquac. Nutr. 2011, 17, 112–122. [Google Scholar] [CrossRef]
- Izquierdo, M.; Scolamacchia, M.; Betancor, M.; Roo, J.; Caballero, M.; Terova, G.; Witten, P. Effects of dietary DHA and α-tocopherol on bone development, early mineralisation and oxidative stress in Sparus aurata (Linnaeus, 1758) larvae. Br. J. Nutr. 2013, 109, 1796–1805. [Google Scholar] [CrossRef]
- Gibson, M.; Newsham, P. Meat: Food and science of the animal kingdom. In Food Science and the Culinary Arts; Gibson, M., Newsham, P., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 169–223. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Barja, G.; Battino, M.; Mataix, J.; Solfrizzi, V. Role of olive oil and monounsaturated fatty acids in mitochondrial oxidative stress and aging. Nutr. Rev. 2006, 64, S31–S39. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.W.; Qingyi Wei, Q.; Pu, H. Quantification and visualization of α-tocopherol in oil-in-water emulsionbased delivery systems by Raman microspectroscopy. LWT 2018, 96, 66–74. [Google Scholar] [CrossRef]
- Boglino, A.; Darias, M.J.; Andree, K.B.; Estévez, A.; Gisbert, E. The effects of dietary arachidonic acid on bone in flatfish larvae: The last but not the least of the essential fatty acids. J. Appl. Ichthyol. 2014, 30, 643–651. [Google Scholar] [CrossRef]
- De Vrieze, E.; Moren, M.; Metz, J.R.; Flik, G.; Lie, K.K. Arachidonic acid enhances turnover of the dermal skeleton: Studies on zebrafish scales. PLoS ONE 2014, 9, e89347. [Google Scholar] [CrossRef]
- Péter, G.; Lukić, J.; Milla, S.; Sándor, Z.J.; Brlás-Molnár, Z.; Ardó, L.; Bekefi, E.; Ljubobratović, U. Reproduction of hatchery-reared pike-perch (Sander lucioperca) fed diet with low-marine-ingredients: Role of dietary fatty acids. Fishes 2023, 8, 219. [Google Scholar] [CrossRef]
- Moah, A.; Coyle, S.D.; Webster, C.D.; Durborow, R.M.; Bright, L.A.; Tidwell, J.H. Effects of graded levels of carbohydrate on growth and survival of largemouth bass, Micropterus salmoides. J. World Aquac. Soc. 2008, 39, 397–405. [Google Scholar]
- Zamani, A.; Khajavi, M.; Abedian Kenari, A.; Haghbin Nazarpak, M.; Solouk, A.; Esmaeili, M.; Gisbert, E. Physicochemical and Biochemical Properties of Trypsin-like Enzyme from Two Sturgeon Species. Animals 2023, 13, 853. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S.; et al. Scientific Opinion on the assessment of the application for renewal of authorisation of Bactocell® (Pediococcus acidilactici CNCM I-4622) as a feed additive for all fish and shrimps and its extension of use for all crustaceans. EFSA J. 2019, 17, 5691. [Google Scholar] [CrossRef]
- Ljubobratovic, U.; Kosanovic, D.; Demény, F.Z.; Krajcsovics, A.; Vukotic, G.; Stanisavljevic, N.; Golic, N.; Jeney, G.; Lukic, J. The effect of live and inert feed treatment with lactobacilli on weaning success in intensively reared pike-perch larvae. Aquaculture 2020, 516, 734608. [Google Scholar] [CrossRef]
- Miller, W.; Reid, G. Human studies on probiotics and endogenous lactic acid bacteria in the urogenital tract. In Lactic Acid Bacteria, 4th ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Lamari, F.; Castex, M.; Larcher, T.; Ledevin, M.; Mazurais, D.; Bakhrouf, A.; Gatesoupe, F.J. Comparison of the effects of the dietary addition of two lactic acid bacteria on the development and conformation of sea bass larvae, Dicentrarchus labrax, and the influence on associated microbiota. Aquaculture 2013, 376, 137–145. [Google Scholar] [CrossRef]
- Wuertz, S.; Schroeder, A.; Wanka, K.M. Probiotics in fish nutrition—Long-standing household remedy or native nutraceuticals? Water 2021, 13, 1348. [Google Scholar] [CrossRef]
- Silva, T.S.D.C.; Santos, L.D.D.; Silva, L.C.R.D.; Michelato, M.; Furuya, V.R.B.; Furuya, W.M. Length-weight relationship and prediction equations of body composition for growing-finishing cage-farmed Nile tilapia. Rev. Bras. Zootec. 2015, 44, 133–137. [Google Scholar] [CrossRef]
- Baeverfjord, G.; Antony Jesu Prabhu, P.; Fjelldal, P.G.; Albrektsen, S.; Hatlen, B.; Denstadli, V.; Ytteborg, E.; Takle, H.; Lock, E.J.; Berntssen, M.H.G.; et al. Mineral nutrition and bone health in salmonids. Rev. Aquacult. 2019, 11, 740–765. [Google Scholar] [CrossRef]
- Costa, J.M.; Sartori, M.M.P.; Nascimento, N.F.D.; Kadri, S.M.; Ribolla, P.E.M.; Pinhal, D.; Pezzato, L.E. Inadequate dietary phosphorus levels cause skeletal anomalies and alter osteocalcin gene expression in zebrafish. Int. J. Mol. Sci. 2018, 19, 364. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, S.K.; Parichy, D.M. Metamorphosis in teleosts. Curr. Top. Dev. Biol. 2013, 103, 127–165. [Google Scholar] [CrossRef] [PubMed]
- Harikrishna, N.; Mahalakshmi, S.; Kiran Kumar, K.; Reddy, G. Fish scales as potential substrate for production of alkaline protease and amino acid rich aqua hydrolyzate by Bacillus altitudinis GVC11. Indian J. Microbiol. 2017, 57, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Gistelinck, C.; Gioia, R.; Gagliardi, A.; Tonelli, F.; Marchese, L.; Bianchi, L.; Landi, C.; Bini, L.; Huysseune, A.; Witten, P.E.; et al. Zebrafish collagen type I: Molecular and biochemical characterization of the major structural protein in bone and skin. Sci. Rep. 2016, 6, 21540. [Google Scholar] [CrossRef]
- Arendt, J.; Wilson, D.S.; Stark, E. Scale strength as a cost of rapid growth in sunfish. Oikos 2001, 93, 95–100. [Google Scholar] [CrossRef]
- Arendt, J.D.; Wilson, D.S. Population differences in the onset of cranial ossification in pumpkinseed (Lepomis gibbosus), a potential cost of rapid growth. Can. J. Fish. Aquat. Sci. 2011, 57, 351–356. [Google Scholar] [CrossRef]
- Dee, C.T.; Hirst, C.S.; Shih, Y.H.; Tripathi, V.B.; Patient, R.K.; Scotting, P.J. Sox3 regulates both neural fate and differentiation in the zebrafish ectoderm. Dev. Biol. 2008, 320, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Ali, Z.; Akhter, M.; Mahmood, K. Lipid Classes and Fatty Acids Comparison of Oscar and Dunkeld Variety of Canola Seeds. Asian J. Chem. 2013, 25, 4279. [Google Scholar] [CrossRef]
- Şen Özdemir, N.; Parrish, C.C.; Parzanini, C.; Mercier, A. Neutral and polar lipid fatty acids in five families of demersal and pelagic fish from the deep Northwest Atlantic. ICES J. Mar. Sci. 2019, 76, 1807–1815. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Hachem, M.; Ahmmed, F.; Rashidinejad, A.; Oz, F.; Bekhit, A.A.; Carne, A.; Bekhit, A.E.-D.A. Marine Fish-Derived Lysophosphatidylcholine: Properties, Extraction, Quantification, and Brain Health Application. Molecules 2023, 28, 3088. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Guillén, C.; Yúfera, M.; Perera, E. Biochemical features and modulation of digestive enzymes by environmental temperature in the greater amberjack, Seriola dumerili. Front. Mar. Sci. 2022, 9, 960746. [Google Scholar] [CrossRef]
- Rungruangsak-Torrissen, K.; Moss, R.; Andresen, L.H.; Berg, A.; Waagbø, R. Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 2006, 32, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Kolkovski, S. Digestive enzymes in fish larvae and juveniles—Implications and applications to formulated diets. Aquaculture 2011, 200, 181–201. [Google Scholar] [CrossRef]
- Moreno, M.; Lombardi, A.; Beneduce, L.; Silvestri, E.; Pinna, G.; Goglia, F.; Lanni, A. Are the effects of T3 on resting metabolic rate in euthyroid rats entirely caused by T3 itself? Endocrinology 2002, 143, 504–510. [Google Scholar] [CrossRef] [PubMed]
| C1, 0.58–0.84 mm https://www.mn-feed.com (accessed on 28 August 2023) | Fish-meal, krill-meal, squid-meal, wheat flour, potato starch, fish oil, brewer’s yeast, vitamin and mineral premix, inorganic calcium phosphate, soy lecithin, guar gum, betaine, Paracoccus bacterial cell (astaxanthin) powder, calcium carbonate |
| AF, 0.5–1 mm https://www.aller-aqua.com (accessed on 28 August 2023) | Fish-meal, fish oil, grain products, krill-meal, single-cell proteins, vegetable proteins, vitamins and minerals, undisclosed functional ingredients |
| BIP, 0.8 mm www.aqua-garant.com (accessed on 28 August 2023) | Fish-meal, wheat, fish oil, krill-meal, hydrolyzed fish proteins, soya concentrate, monocalcium phosphate, yeast extract, minerals, astaxanthin, Pediococcus acidilactici CNCM I-4622 (Bactocell®) |
| EF, 0.5–1 mm This study | Fish-meal, pre-digested fish-meal, krill-meal, squid-meal, egg powder, chicken protein concentrate, brewer’s yeast, wheat flour, egg protein, liver-meal |
| TnnC | Troponin C, skeletal muscle mRNA | GAACCTTCCCTGATCGCCTT | fw |
| Gene ID: 119906174, Product length: 99 bp, Slope: −3.0 | GAGGAGGTGGGGCTTAAGTG | rev | |
| Col1α2 | Collagen, type I, α 2 mRNA | TCTGAGAGGACTGAACGGACT | fw |
| Gene ID: 119906985, Product length: 88 bp, Slope: −2.8 | AACAAGGTGTTTTCCCGCGT | rev | |
| ColIα1 | Collagen, type I, α 1a mRNA | GCGGTGAGAGGAATGAAAGGA | fw |
| Gene ID: 119882269, Product length: 98 bp, Slope: −3.16 | TGGCTGTCAGTTTCACCGTT | rev | |
| PSN | Periostin, osteoblast specific factor b mRNA | ACCAAACCCAGCCGTTGTAA | fw |
| Gene ID: 119882985, Product length: 95 bp, Slope: −4.3 | TTTGTCAGTTATACCTATTGCAGGA | rev | |
| SOX3 | SRY-box transcription factor 3 mRNA | GAGAGGCTGGTGTGTTTCTGA | fw |
| Gene ID: 119888700, Product length: 97 bp, Slope: −2.9 | TTTGGACACAGTCGAGACAACT | rev | |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase mRNA | GCACTGTCACATCACCCACAT | fw |
| Gene ID: 119897446, Product length: 86 bp, Slope: −3.7 | TTCCTTCAGGCATCTAACAGGG | rev |
| C1 | AF | BIP | EF | |
|---|---|---|---|---|
| Dry Matter (%) | 93.2 | 93.5 | 91.6 | 91.6 |
| Crude Protein (%) | 52.0 | 55.9 | 54.3 | 50.9 |
| Crude Fat (%) | 17.6 | 14.9 | 18.4 | 8.3 |
| Crude Ash (%) | 12.8 | 12.9 | 11.8 | 9.0 |
| Crude Fiber (%) | 1.8 | 0.4 | 0.3 | 2.2 |
| * Nitrogen Free Extract (%) | 9.0 | 9.4 | 6.8 | 21.2 |
| ** Gross energy (MJ × kg−1) | 18.0 | 17.7 | 18.2 | 17.0 |
| C1 | AF | BIP | EF | |
|---|---|---|---|---|
| C12:0 | 0.09 | 0.04 | 0.05 | 0.20 |
| C14:0 | 6.77 | 4.13 | 5.54 | 5.65 |
| C14:l | 0.09 | 0.05 | 0.07 | 0.08 |
| Cl5:0 | 0.34 | 0.36 | 0.36 | 0.29 |
| C16:0 | 23.36 | 18.01 | 17.13 | 22.57 |
| Cl6:1 | 5.56 | 5.05 | 7.07 | 5.31 |
| C17:0 | 0.16 | 0.24 | 0.20 | 0.19 |
| C18:0 | 4.93 | 3.00 | 2.13 | 4.32 |
| Cl8:1 ω9, OA | 16.73 | 19.32 | 17.61 | 22.77 |
| C18:2 ω6, LA | 4.73 | 4.85 | 3.72 | 12.06 |
| C18:3 ω6 | 0.16 | 0.12 | 0.14 | 0.15 |
| C18:3 ω3, ALA | 2.27 | 1.51 | 1.23 | 1.47 |
| C20:0 | 0.15 | 0.23 | 0.22 | 0.26 |
| C20:1 | 2.71 | 10.49 | 17.06 | 3.36 |
| C20:2 | 0.18 | 0.39 | 0.29 | 0.26 |
| C20:3 ω6 | 0.09 | 0.09 | 0.09 | 0.12 |
| C20:4 ω6, ARA | 0.57 | 0.71 | 0.49 | 0.71 |
| C20:3 ω3 | 0.23 | 0.18 | 0.09 | 0.14 |
| C20:5 ω3, EPA | 14.74 | 10.79 | 9.53 | 9.03 |
| C22:0 | 0.18 | 0.20 | 0.14 | 0.23 |
| C22:1 | 1.11 | 1.49 | 2.15 | 0.80 |
| C22:5 ω3 | 1.52 | 0.82 | 0.89 | 0.76 |
| C24:0 | 0.13 | 0.1 | 0.09 | 0.17 |
| C22:6 ω3, DHA | 12.31 | 15.42 | 11.73 | 8.31 |
| C24:1 | 0.88 | 2.42 | 1.98 | 0.80 |
| SFA | 36.11 | 26.31 | 25.86 | 33.88 |
| MUFA | 27.08 | 38.82 | 45.94 | 33.12 |
| MUFA/SFA | 0.75 | 1.48 | 1.78 | 0.98 |
| PUFA ω3 | 31.07 | 28.72 | 23.47 | 19.71 |
| PUFA ω6 | 5.73 | 6.16 | 4.73 | 13.30 |
| PUFA ω3/ω6 | 5.42 | 4.66 | 4.96 | 1.48 |
| (DHA + EPA)/ARA | 47.5 | 36.9 | 43.4 | 24.4 |
| C1 | AF | BIP | EF | |
|---|---|---|---|---|
| Arginine | 3.31 | 3.22 | 3.31 | 2.82 |
| Histidine | 1.42 | 1.38 | 1.57 | 1.20 |
| Isoleucine | 2.41 | 2.77 | 2.32 | 2.38 |
| Leucine | 4.11 | 4.08 | 4.15 | 3.47 |
| Lysine | 4.22 | 3.93 | 4.21 | 4.31 |
| Methionine | 1.59 | 1.54 | 1.57 | 1.51 |
| Phenylalanine | 2.37 | 2.38 | 2.24 | 2.24 |
| Threonine | 2.25 | 2.34 | 2.28 | 2.04 |
| Valine | 2.78 | 2.73 | 2.85 | 2.33 |
| ƩEAA | 24.46 | 24.37 | 24.5 | 22.3 |
| Alanine | 3.49 | 3.20 | 3.41 | 2.95 |
| Aspartic acid | 5.32 | 5.31 | 5.26 | 4.52 |
| Cysteine | 0.49 | 0.41 | 0.45 | 0.44 |
| Glutamic acid | 9.86 | 8.09 | 9.01 | 8.91 |
| Glycine | 3.63 | 2.93 | 3.36 | 4.46 |
| Proline | 2.48 | 2.10 | 2.30 | 2.07 |
| Serine | 2.45 | 2.31 | 2.41 | 2.08 |
| Tyrosine | 1.78 | 1.91 | 1.79 | 1.56 |
| ƩNEAA | 29.50 | 26.26 | 27.99 | 26.99 |
| C1 | AF | BIP | EF | |
|---|---|---|---|---|
| Individual weight (mg) KW | 380 ± 25 A | 373 ± 42 A | 412 ± 19 A | 421 ± 63 A |
| Standard length (mm) KW | 33.09 ± 1.53 A | 32.65 ± 1.32 A | 33.72 ± 0.22 A | 32.83 ± 1.47 A |
| Specific growth rate (weight), SGR (% × day−1) KW | 6.96 ± 0.57 A | 6.94 ± 1.41 A | 8.31 ± 0.23 A | 7.95 ± 1.79 A |
| Relative condition factor, Kn KW | 1.02 ± 0.03 A | 1.03 ± 0.04 A | 1.01 ± 0.01 A | 1.01 ± 0.00 A |
| Feed conversion ratio, FCR KW | 6.30 ± 0.57 A | 6.50 ± 1.19 A | 4.90 ± 0.37 A | 6.45 ± 3.12 A |
| Allometric coefficient (b) KW | 2.62 ± 0.76 A | 2.14 ± 1.38 A | 3.04 ± 0.29 A | 3.05 ± 0.07 A |
| Survival (%) KW | 92.22 ± 2.55 AB | 93.33 ± 3.33 AB | 95.56 ± 0.96 B | 87.22 ± 5.85 AC |
| Deformities (%) KW | 61.11 ± 3.47 AB | 57.22 ± 5.36 AB | 55.00 ± 3.33 B | 63.33 ± 1.67 AC |
| C1 | AF | BIP | EF | |
|---|---|---|---|---|
| Nutrition costs (€ × juvenile−1) | 0.0889 | 0.0151 | 0.0144 | 0.0929 |
| Infrastructure rental fee * (€ × juvenile−1) | 0.1229 | 0.1233 | 0.1029 | 0.1076 |
| Total costs (€ × juvenile−1) | 0.2118 | 0.1383 | 0.1173 | 0.2005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukić, J.; Gyalog, G.; Horváth, Z.; Szűcs, A.A.; Ristović, T.; Terzić-Vidojević, A.; Sándor, Z.J.; Ljubobratović, U. Evaluation of Post-Larval Diets for Indoor Weaned Largemouth Bass (Micropterus salmoides). Animals 2023, 13, 3179. https://doi.org/10.3390/ani13203179
Lukić J, Gyalog G, Horváth Z, Szűcs AA, Ristović T, Terzić-Vidojević A, Sándor ZJ, Ljubobratović U. Evaluation of Post-Larval Diets for Indoor Weaned Largemouth Bass (Micropterus salmoides). Animals. 2023; 13(20):3179. https://doi.org/10.3390/ani13203179
Chicago/Turabian StyleLukić, Jovanka, Gergő Gyalog, Zoltán Horváth, Anita Annamária Szűcs, Tijana Ristović, Amarela Terzić-Vidojević, Zsuzsanna J. Sándor, and Uroš Ljubobratović. 2023. "Evaluation of Post-Larval Diets for Indoor Weaned Largemouth Bass (Micropterus salmoides)" Animals 13, no. 20: 3179. https://doi.org/10.3390/ani13203179
APA StyleLukić, J., Gyalog, G., Horváth, Z., Szűcs, A. A., Ristović, T., Terzić-Vidojević, A., Sándor, Z. J., & Ljubobratović, U. (2023). Evaluation of Post-Larval Diets for Indoor Weaned Largemouth Bass (Micropterus salmoides). Animals, 13(20), 3179. https://doi.org/10.3390/ani13203179








