Intestinal Microbiome in Dogs with Chronic Hepatobiliary Disease: Can We Talk about the Gut–Liver Axis?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Microbiome Analysis
2.3. Statistical Analysis
3. Results
3.1. Animals
3.2. Microbiome Analysis
3.2.1. Alpha Diversity
3.2.2. Multivariate Comparisons of Gut Microbiome Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
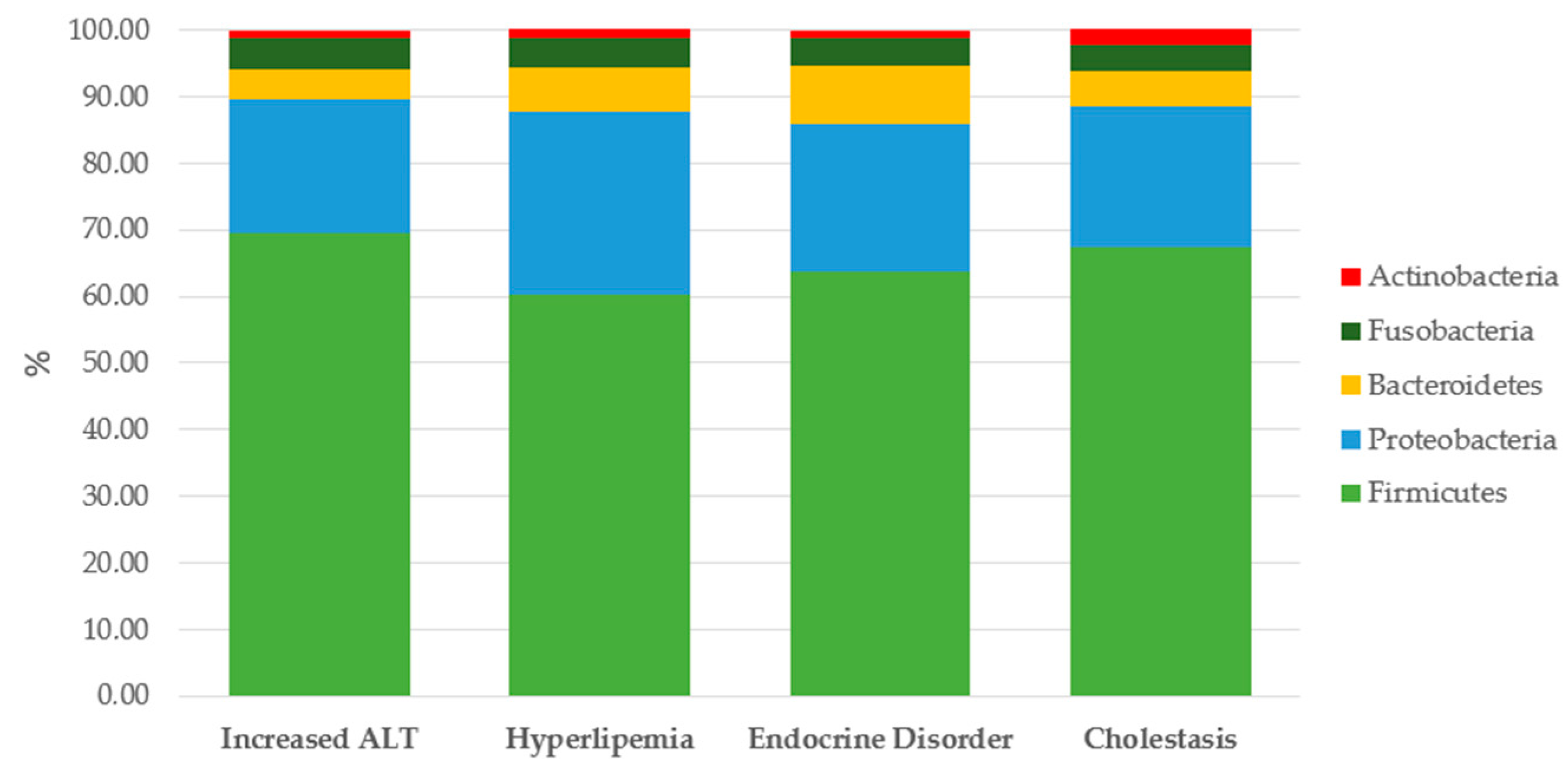
| Bacteria | ALT | HYPERLIPEMIA | ||||
|---|---|---|---|---|---|---|
| Normal (n = 22) | Increased (n = 43) | p-Value | Present (n = 41) | Absent (n = 24) | p-Value | |
| Phylum | ||||||
| Actinobacteria | 2.115 (0.01–27.29) | 1.030 (0.007–23.01) | 0.25 | 2.72 (0.01–27.29) | 1.045 (0.01–23.01) | 0.43 |
| Bacteroidetes | 9.58 (0.01–46.54) | 3.78 (0.01–57.34) | 3.455 (0.01–39.7) | 5.415 (0.01–57.34) | ||
| Firmicutes | 49.2 (8.72–91.85) | 57.4 (10.61–98.39) | 63.83 (11.67–92) | 48.85 (8.72–98.39) | ||
| Fusobacteria | 1.96 (0–33.29) | 3.88 (0.0006–26.5) | 3.13 (0–30.57) | 3.515 (0–33.29) | ||
| Proteobacteria | 10.03 (0.04–89.13) | 16.56 (0.02–85.62) | 3.77 (0.02–87.48) | 22.24 (0.04–89.13) | ||
| Family | ||||||
| Bacteroidaceae | 7686 (0–45,441) | 3224 (0–60,949) | 0.22 | 4921 (0–45,441) | 2962 (0–60,949) | 0.81 |
| Bifidobacteriaceae | 0 (0–229) | 0 (0–6746) | 0.46 | 0 (0–6746) | 0 (0–1112) | 0.44 |
| Clostridiaceae | 3543 (510–67,993) | 4860 (12–57,124) | 0.43 | 4210 (31–67,993) | 5750 (12–52,251) | 0.27 |
| Corynebacteriaceae | 0 (0–136) | 0 (0–182) | 0.49 | 0 (0–182) | 0 (0–11) | 0.22 |
| Enterobacteriaceae | 771 (0–150,487) | 14,633 (0–176,347) | 0.19 | 25,925 (0–176,347) | 1888 (0–134,116) | 0.07 |
| Enterococcaceae | 0 (0–2155) | 0 (0–63,111) | 0.99 | 0 (0–2155) | 0 (0–63,111) | 0.80 |
| Erysipelotrichaceae | 2442 (0–17,493) | 2211 (0–82,064) | 0.70 | 2549 (0–20,531) | 2075 (0–82,064) | 0.66 |
| Eubacteriaceae | 1720 (35–13,489) | 1966 (0–72,100) | 0.93 | 1720 (0–72,100) | 2134 (0–13,489) | 0.83 |
| Fusobacteriaceae | 1942 (0–80,154) | 5435 (0–66,183) | 0.35 | 2865 (0–66,183) | 4505 (0–80,154) | 0.96 |
| Lactobacillaceae | 0 (0–17,040) | 0 (0–112,621) | 0.39 | 0 (0–112,621) | 0 (0–50,550) | 0.63 |
| Ruminococcaceae | 1793 (0–29,161) | 639 (0–26,808) | 0.11 | 707 (0–3253) | 1438 (0–29,161) | 0.46 |
| Veillonellaceae | 1964 (0–102,369) | 434 (0–112,868) | 0.96 | 886 (0–112,868) | 1618 (0–60,369) | 0.75 |
| Genus | ||||||
| Acinetobacter | 0 (0–17) | 0 (0–20) | 0.99 | 0 (0–17) | 0 (0–20) | 0.76 |
| Actinomyces | 0 (0–672) | 0 (0–529) | 0.30 | 0 (0–529) | 0 (0–672) | 0.49 |
| Bacteroides | 3485 (0–41,349) | 701 (0–60,922) | 0.41 | 1354 (0–41,349) | 1458 (0–60,922) | 0.53 |
| Bifidobacterium | 0 (0–229) | 0 (0–6746) | 0.46 | 0 (0–6746) | 0 (0–1112) | 0.44 |
| Blautia | 5832 (0–64,285) | 4798 (0–42,730) | 0.24 | 4484 (0–51,345) | 6479 (0–64,285) | 0.30 |
| Campylobacter | 0 (0–1428) | 0 (0–21,646) | 0.25 | 0 (0–1428) | 0 (0–21,646) | 0.60 |
| Clostridium | 4786 (538–68,157) | 4047 (0–74,731) | 0.72 | 4101 (561–68,157) | 6446 (0–74,731) | 0.56 |
| Coprobacillus | 0 (0–68) | 0 (0–38) | 0.22 | 0 (0–68) | 0 (0–66) | 0.86 |
| Coprococcus | 0 (0–477) | 0 (0–48) | 0.99 | 0 (0–477) | 0 (0–48) | 0.99 |
| Corynebacterium | 0 (0–80) | 0 (0–182) | 0.57 | 0 (0–182) | 0 (0–11) | 0.22 |
| Cronobacter | 0 (0–41) | 0 (0–136) | 0.28 | 0 (0–136) | 0 (0–38) | 0.11 |
| Enterococcus | 0 (0–4.5) | 0 (0–14) | 0.78 | 0 (0–641) | 0 (0–62,340) | 0.39 |
| Escherichia | 0 (0–449) | 0 (0–8088) | 0.33 | 0 (0–506) | 0 (0–8088) | 0.02 |
| Faecalibacterium | 13 (0–18,000) | 0 (0–25,117) | 0.47 | 1293 (0–58,851) | 8 (0–25,117) | 0.62 |
| Fusobacterium | 1548 (0–49,887) | 3690 (0–58,851) | 0.49 | 0 (0–58,851) | 3195 (0–49,887) | 0.99 |
| Helicobacter | 0 (0–1071) | 0 (0–2234) | 0.92 | 0 (0–2234) | 0 (0–1117) | 0.57 |
| Klebsiella | 0 (0–0) | 0 (0–25,019) | 0.10 | 0 (0–25,019) | 0 (0–66) | 0.25 |
| Lactobacillus | 0 (0–17,025) | 0 (0–112,477) | 0.39 | 0 (0–112,477) | 0 (0–50,470) | 0.63 |
| Megamonas | 1907 (0–102,357) | 434 (0–112,780) | 0.98 | 886 (0–112,780) | 1574 (0–60,369) | 0.71 |
| Prevotella | 0 (0–39,549) | 0 (0–60,858) | 0.35 | 0 (0–60,858) | 0 (0–42,872) | 0.65 |
| Proteus | 0 (0–163) | 0 (0–415) | 0.95 | 0 (0–415) | 0 (0–163) | 0.88 |
| Pseudomonas | 0 (0–20) | 0 (0–734) | 0.59 | 0 (0–20) | 0 (0–734) | 0.08 |
| Ruminococcus | 4928 (0–40,941) | 3626 (0–48,001) | 0.64 | 2800 (0–48,001) | 6975 (0–40,941) | 0.17 |
| Salmonella | 0 (0–17) | 0 (0–152) | 0.29 | 0 (0–152) | 0 (0–0) | 0.03 |
| Serratia | 0 (0–20) | 0 (0–118) | 0.69 | 0 (0–118) | 0 (0–22) | 0.55 |
| Staphylococcus | 0 (0–12) | 0 (0–11) | 0.99 | 0 (0–10) | 0 (0–12) | 0.37 |
| Streptococcus | 0 (0–84,431) | 305 (0–21,959) | 0.03 | 0 (0–84,431) | 32 (0–17,430) | 0.38 |
| Turicibacter | 0 (0–1561) | 25 (0–12,604) | 0.15 | 0 (0–9496) | 7 (0–12,604) | 0.95 |
| Yersinia | 0 (0–0) | 0 (0–56) | 0.99 | 0 (0–56) | 0 (0–0) | 0.99 |
| Species | ||||||
| Clostridium difficile | 0 (0–747) | 0 (0–217) | 0.77 | 0 (0–747) | 0 (0–217) | 0.99 |
| Clostridium hiranonis | 323 (0–3406) | 440 (0–9876) | 0.66 | 323 (0–9872) | 451 (0–7827) | 0.73 |
| Clostridium perfringens | 156 (0–60,494) | 561 (0–48,468) | 0.44 | 298 (0–60,494) | 175 (0–48,468) | 0.87 |
| Enterococcus faecium | 0 (0–119) | 0 (0–9053) | 0.93 | 0 (0–119) | 0 (0–9053) | 0.03 |
| Escherichia coli | 0 (0–480) | 0 (0–480) | 0.29 | 14 (0–480) | 0 (0–166) | 0.02 |
| Escherichia shigella | 0 (0–2911) | 0 (0–9108) | 0.26 | 16 (0–2911) | 0 (0–9108) | 0.06 |
| Faecalibacterium prausnitzii | 0 (0–3482) | 0 (0–10,062) | 0.59 | 0 (0–3276) | 0 (0–10,062) | 0.92 |
| Helicobacter canis | 0 (0–93) | 0 (0–218) | 0.99 | 0 (0–93) | 0 (0–218) | 0.76 |
| Lactobacillus acidophilus | 0 (0–0) | 0 (0–15,127) | 0.39 | 0 (0–0) | 0 (0–40) | 0.13 |
| Ruminococcus faecis | 107 (0–2370) | 55 (0–5902) | 0.27 | 55 (0–2459) | 103 (0–5902) | 0.50 |
| Salmonella enterica | 0 (0–17) | 0 (0–152) | 0.29 | 0 (0–152) | 0 (0–0) | 0.03 |
| Bacteria | Endocrine Disorder | Cholestasis | ||||
|---|---|---|---|---|---|---|
| Present (n = 19) | Absent (n = 46) | p–Value | Present (n = 45) | Absent (n = 20) | p–Value | |
| Phylum | ||||||
| Actinobacteria | 0.88 (0.02–8) | 2.13 (0.01–27.29) | 0.23 | 1.9 (0.01–27.29) | 1.005 (0.01–11.6) | 0.9 |
| Bacteroidetes | 6.58 (0.01–57.34) | 2.67 (0.01–39.78) | 4.24 (0.01–57.3) | 13.5 (0.01–46.54) | ||
| Firmicutes | 49.42 (8.72–91.85) | 60.13 (10.61–98.39) | 53.95 (8.72–98) | 63.83 (11.67–92) | ||
| Fusobacteria | 3.36 (0–33.29) | 3.04 (0–30.57) | 3.13 (0–33.29) | 2.185 (0–17.55) | ||
| Proteobacteria | 17.21 (0.04–89.13) | 12.34 (0.02–87.48) | 16.93 (0.02–89) | 7.27 (0.21–69.28) | ||
| Family | ||||||
| Bacteroidaceae | 4602 (0–45,441) | 3855 (0–60,949) | 0.96 | 0 (0–60,949) | 4364 (0–28,431) | 0.001 |
| Bifidobacteriaceae | 0 (0–77) | 0 (0–6746) | 0.69 | 0 (0–861) | 0 (0–1112) | 0.99 |
| Clostridiaceae | 4210 (1029–52,251) | 4860 (12–67,993) | 0.48 | 4328 (0–57,124) | 5618 (12–67,993) | 0.12 |
| Corynebacteriaceae | 0 (0–136) | 0 (0–182) | 0.17 | 0 (0–16) | 0 (0–136) | 0.15 |
| Enterobacteriaceae | 12,671 (0–147,144) | 6669 (0–176,347) | 0.79 | 0 (0–129,390) | 5340 (0–71,302) | 0.001 |
| Enterococcaceae | 0 (0–2155) | 0 (0–63,111) | 0.54 | 0 (0–641) | 0 (0–63,111) | 0.24 |
| Erysipelotrichaceae | 2082 (0–7511) | 2431 (0–82,064) | 0.42 | 0 (0–82,064) | 2262 (0–15,060) | 0.0008 |
| Eubacteriaceae | 1261 (136–6053) | 2328 (666–72,100) | 0.36 | 0 (0–72,100) | 2299 (0–5247) | 0.0002 |
| Fusobacteriaceae | 3809 (0–66,183) | 3574 (0–80,154) | 0.62 | 0 (0–66,183) | 766.5 (0–32,662) | 0.011 |
| Lactobacillaceae | 0 (0–112,621) | 0 (0–50,550) | 0.68 | 0 (0–67,987) | 0 (0–23,094) | 0.23 |
| Ruminococcaceae | 873 (0–29,161) | 810 (0–26,808) | 0.40 | 0 (0–29,161) | 1561 (0–17,222) | 0.0001 |
| Veillonellaceae | 1110 (0–112,868) | 1234 (0–96,497) | 0.69 | 0 (0–102,369) | 3617 (0–60,369) | 0.001 |
| Genus | ||||||
| Acinetobacter | 0 (0–0) | 0 (0–20) | 0.59 | 0 (0–0) | 0 (0–20) | 0.09 |
| Actinomyces | 0 (0–0) | 0 (0–672) | 0.09 | 0 (0–672) | 0 (0–64) | 0.28 |
| Bacteroides | 1471 (0–11,884) | 790 (0–60,922) | 0.59 | 0 (0–60,922) | 0 (0–25,476) | 0.07 |
| Bifidobacterium | 0 (0–77) | 0 (0–6746) | 0.69 | 0 (0–861) | 0 (0–1112) | 0.80 |
| Blautia | 4075 (287–51,345) | 5668 (0–64,285) | 0.38 | 5328 (138–48,001) | 5353 (0–64,285) | 0.90 |
| Campylobacter | 0 (0–1428) | 0 (0–21,646) | 0.39 | 0 (0–240) | 0 (0–21,646) | 0.07 |
| Clostridium | 3915 (848–67,279) | 4720 (0–74,731) | 0.62 | 0 (0–63,427) | 4667 (0–74,731) | <0.0001 |
| Coprobacillus | 0 (0–66) | 0 (0–68) | 0.26 | 0 (0–177) | 0 (0–0) | 0.30 |
| Coprococcus | 0 (0–477) | 0 (0–48) | 0.12 | 0 (0–477) | 0 (0–48) | 0.79 |
| Corynebacterium | 0 (0–0) | 0 (0–182) | 0.17 | 0 (0–0) | 0 (0–34) | 0.09 |
| Cronobacter | 0 (0–92) | 0 (0–136) | 0.73 | 0 (0–92) | 0 (0–43) | 0.80 |
| Enterococcus | 0 (0–2155) | 0 (0–62,340) | 0.35 | 0 (0–641) | 0 (0–62,340) | 0.39 |
| Escherichia | 8 (0–340) | 0 (0–8088) | 0.95 | 0 (0–6557) | 0 (0–8088) | 0.0002 |
| Faecalibacterium | 20 (0–18,000) | 0 (0–25,117) | 0.31 | 0 (0–25,117) | 0 (0–14,314) | 0.75 |
| Fusobacterium | 3135 (0–58,851) | 1654 (0–49,887) | 0.75 | 0 (0–58,851) | 597.5 (0–28,558) | 0.016 |
| Helicobacter | 0 (0–2234) | 0 (0–1117) | 0.43 | 0 (0–2234) | 0 (0–1117) | 0.26 |
| Klebsiella | 0 (0–25,019) | 0 (0–563) | 0.021 | 0 (0–152) | 0 (0–0) | 0.18 |
| Lactobacillus | 0 (0–112,477) | 0 (0–50,470) | 0.68 | 0 (0–67,797) | 0 (0–22,927) | 0.82 |
| Megamonas | 938 (0–112,477) | 1234 (0–96,463) | 0.67 | 0 (0–102,357) | 2456 (0–60,369) | 0.002 |
| Prevotella | 0 (0–60,858) | 0 (0–42,870) | 0.022 | 125 (0–39,549) | 0 (0–42,872) | 0.21 |
| Proteus | 0 (0–415) | 0 (0–163) | 0.82 | 0 (0–415) | 0 (0–162) | 0.25 |
| Pseudomonas | 0 (0–0) | 0 (0–734) | 0.32 | 0 (0–28) | 0 (0–734) | 0.34 |
| Ruminococcus | 4187 (208–26,797) | 5024 (0–48,001) | 0.94 | 0 (0–26,797) | 5106 (0–40,941) | <0.0001 |
| Salmonella | 0 (0–152) | 0 (0–94) | 0.06 | 0 (0–796) | 0 (0–0) | 0.16 |
| Serratia | 0 (0–118) | 0 (0–22) | 0.70 | 0 (0–48,468) | 0 (0–22) | 0.005 |
| Staphylococcus | 0 (0–0) | 0 (0–12) | 0.55 | 0 (0–0) | 0 (0–11) | 0.30 |
| Streptococcus | 22 (0–18,261) | 232 (0–84,431) | 0.17 | 0 (0–18,261) | 381.5 (0–84,431) | 0.001 |
| Turicibacter | 31 (0–2736) | 0 (0–12,604) | 0.79 | 0 (0–6137) | 7 (0–12,604) | 0.032 |
| Yersinia | 0 (0–56) | 0 (0–0) | 0.27 | 0 (0–367) | 0 (0–11) | 0.99 |
| Species | ||||||
| Clostridium difficile | 0 (0–747) | 0 (0–217) | 0.79 | 0 (0–8707) | 0 (0–217) | 0.99 |
| Clostridium hiranonis | 659 (0–5714) | 261 (0–9876) | 0.44 | 0 (0–3995) | 534.5 (0–7827) | 0.002 |
| Clostridium perfringens | 612 (0–48,468) | 101 (0–60,494) | 0.32 | 1492 (0–41,349) | 93 (0–60,494) | 0.44 |
| Escherichia coli | 8 (0–303) | 0 (0–480) | 0.78 | 0 (0–392) | 0 (0–174) | 0.72 |
| Escherichia shigella | 0 (0–47) | 0 (0–9108) | 0.70 | 16 (0–9108) | 0 (0–636) | 0.005 |
| Faecalibacterium prausnitzii | 0 (0–3482) | 0 (0–10,062) | 0.34 | 0 (0–10,062) | 0 (0–2472) | 0.84 |
| Helicobacter canis | 0 (0–0) | 0 (0–218) | 0.59 | 0 (0–205) | 0 (0–218) | 0.80 |
| Lactobacillus acidophilus | 0 (0–15,127) | 0 (0–40) | 0.70 | 0 (0–15,127) | 0 (0–40) | 0.89 |
| Ruminococcus faecis | 56 (0–2370) | 96 (0–5902) | 0.65 | 0 (0–2370) | 129 (0–5902) | <0.0001 |
| Salmonella enterica | 0 (0–152) | 0 (0–72) | 0.06 | 0 (0–30) | 0 (0–0) | 0.20 |
| Bacteria | Diabetic | Hepatic | Gastrointestinal | Maintenance | p |
|---|---|---|---|---|---|
| Phylum | |||||
| Actinobacteria | 1.72 (0.02–8) | 0.37 (0.01–12.26) | 1.07 (0.01–27.29) | 2.47 (0.02–23.01) | 0.6 |
| Bacteroidetes | 25.74 (1.06–38.34) | 0.14 (0.01–12.29) | 2.43 (0.01–46.54) | 7.54 (0.01–57.34) | |
| Firmicutes | 48.88 (29.89–87.69) | 59.35 (13.9–91.7) | 66.83 (10.61–98.39) | 52.71 (8.72–92.12) | |
| Fusobacteria | 9.25 (0.01–33.29) | 0.08 (0–20.80) | 3.04 (0–26.5) | 3.8 (0–30.57) | |
| Proteobacteria | 4.49 (1.95–23.32) | 39.3 (0.02–85.62) | 10.47 (0.04–87.48) | 20.47 (0.16–89.13) | |
| Family | |||||
| Bacteroidaceae | 7630 (245–45,441) | 390 (0–28,438) | 3395 (0–44,830) | 8138 (0–60,949) | 0.13 |
| Bifidobacteriaceae | 0 (0–0) | 0 (0–6746) | 0 (0–861) | 0 (0–1137) | 0.78 |
| Clostridiaceae | 6701 (2402–16,423) | 2791 (638–67,993) | 4479 (519–64,861) | 4803 (12–40,690) | 0.41 |
| Corynebacteriaceae | 0 (0–0) | 0 (0–182) | 0 (0–136) | 0 (0–27) | 0.39 |
| Enterobacteriaceae | 537 (0–33,341) | 8171 (0–149,132) | 6513 (0–150,487) | 16,024 (0–176,347) | 0.42 |
| Enterococcaceae | 0 (0–160) | 0 (0–8123) | 0 (0–745) | 0 (0–63,111) | 0.95 |
| Erysipelotrichaceae | 1856 (44–7511) | 1372 (0–82,064) | 2327 (0–16,972) | 2331 (0–17,493) | 0.93 |
| Eubacteriaceae | 2351 (136–3193) | 560 (35–22,397) | 2353 (0–72,100) | 1818 (0–15,460) | 0.56 |
| Fusobacteriaceae | 22,480 (0–50,374) | 63 (0–38,350) | 1446 (0–43,551) | 8378 (0–80,154) | 0.34 |
| Lactobacillaceae | 0 (0–27) | 0 (0–3000) | 0 (0–50,550) | 0 (0–112,621) | 0.53 |
| Ruminococcaceae | 455 (68–29,161) | 182 (0–639) | 1749 (0–22,925) | 1066 (13–26,808) | 0.03 |
| Veillonellaceae | 881 (0–12,997) | 108 (0–112,868) | 434 (0–96,497) | 1339 (0–102,369) | 0.92 |
| Genus | |||||
| Acinetobacter | 0 (0–0) | 0 (0–0) | 0 (0–17) | 0 (0–20) | 0.86 |
| Actinomyces | 0 (0–0) | 0 (0–119) | 0 (0–250) | 0 (0–672) | 0.67 |
| Bacteroides | 1171 (0–4923) | 0 (0–27,902) | 619 (0–41,349) | 3652 (0–60,922) | 0.14 |
| Bifidobacterium | 0 (0–0) | 0 (0–6746) | 0 (0–861) | 0 (0–1137) | 0.78 |
| Blautia | 3011 (287–10,860) | 1266 (0–35,913) | 5668 (138–51,345) | 6173 (0–40,508) | 0.12 |
| Campylobacter | 0 (0–41) | 0 (0–0) | 0 (0–1428) | 0 (0–21,646) | 0.50 |
| Clostridium | 7044 (2570–16,473) | 3020 (561–74,731) | 3937 (538–64,285) | 4912 (0–43,602) | 0.91 |
| Coprobacillus | 0 (0–66) | 0 (0–0) | 0 (0–38) | 0 (0–68) | 0.13 |
| Coprococcus | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–477) | 0.36 |
| Corynebacterium | 0 (0–0) | 0 (0–182) | 0 (0–80) | 0 (0–27) | 0.38 |
| Cronobacter | 0 (0–0) | 0 (0–136) | 0 (0–51) | 0 (0–92) | 0.72 |
| Enterococcus | 0 (0–160) | 0 (0–8112) | 0 (0–745) | 0 (0–62,340) | 0.96 |
| Escherichia | 0 (0–69) | 0 (0–506) | 0 (0–235) | 7 (0–8088) | 0.60 |
| Faecalibacterium | 0 (0–18,000) | 0 (0–52) | 121 (0–18,796) | 0 (0–25,117) | 0.31 |
| Fusobacterium | 18,567 (0–38,777) | 50 (0–31,680) | 1144 (0–35,805) | 7082 (0–60,026) | 0.47 |
| Helicobacter | 222 (0–1071) | 0 (0–123) | 0 (0–780) | 0 (0–2234) | 0.035 |
| Klebsiella | 0 (0–4784) | 0 (0–25,019) | 0 (0–0) | 0 (0–152) | 0.18 |
| Lactobacillus | 0 (0–27) | 0 (0–2984) | 0 (0–50,470) | 0 (0–112,477) | 0.53 |
| Megamonas | 538 (0–8463) | 86 (0–112,780) | 434 (0–96,463) | 1574 (0–102,357) | 0.92 |
| Prevotella | 653 (0–39,549) | 0 (0–122) | 0 (0–36,829) | 0 (0–60,858) | 0.04 |
| Proteus | 0 (0–0) | 0 (0–51) | 0 (0–0) | 0 (0–415) | 0.19 |
| Pseudomonas | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–734) | 0.22 |
| Ruminococcus | 4748 (1161–13,410) | 1462 (0–48,001) | 5024 (92–33,236) | 6975 (0–40,941) | 0.59 |
| Salmonella | 0 (0–0) | 0 (0–152) | 0 (0–17) | 0 (0–18) | 0.06 |
| Serratia | 0 (0–10) | 0 (0–118) | 0 (0–0) | 0 (0–22) | 0.37 |
| Staphylococcus | 0 (0–0) | 0 (0–11) | 0 (0–12) | 0 (0–0) | 0.05 |
| Streptococcus | 0 (0–98) | 305 (0–21,959) | 36 (0–84,431) | 30 (0–18,261) | 0.32 |
| Turicibacter | 0 (0–1454) | 25 (0–2558) | 82 (0–12,604) | 0 (0–9496) | 0.80 |
| Yersinia | 0 (0–0) | 0 (0–56) | 0 (0–0) | 0 (0–0) | 0.10 |
| Species | |||||
| Clostridium difficile | 0 (0–0) | 0 (0–217) | 0 (0–0) | 0 (0–747) | 0.45 |
| Clostridium hiranonis | 687 (21–3182) | 159 (0–5714) | 483 (0–3995) | 285 (0–9872) | 0.88 |
| Clostridium perfringens | 227 (33–16,369) | 0 (0–60,494) | 298 (0–57,416) | 358 (0–28,548) | 0.90 |
| Enterococcus faecium | 0 (0–15) | 0 (0–515) | 0 (0–143) | 0 (0–9053) | 0.82 |
| Escherichia coli | 0 (0–34) | 0 (0–480) | 0 (0–180) | 7 (0–449) | 0.56 |
| Escherichia shigella | 0 (0–0) | 0 (0–490) | 16 (0–2911) | 0 (0–9108) | 0.30 |
| Faecalibacterium prausnitzii | 0 (0–3482) | 0 (0–52) | 0 (0–4588) | 0 (0–10,062) | 0.38 |
| Helicobacter canis | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–218) | 0.55 |
| Lactobacillus acidophilus | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–15,127) | 0.36 |
| Ruminococcus faecis | 169 (38–2370) | 0 (0–158) | 97 (0–2119) | 76 (0–5902) | 0.06 |
| Salmonella enterica | 0 (0–0) | 0 (0–152) | 0 (0–17) | 0 (0–18) | 0.07 |
References
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Deng, P.; Swanson, K.S. Gut microbiota of humans. dogs and cats: Current knowledge and future opportunities and challenges. Br. J. Nutr. 2015, 113, S6–S17. [Google Scholar] [CrossRef]
- Guard, B.C.; Suchodolski, J.S. Canine intestinal microbiology and metagenomics: From phylogeny to function. J. Anim. Sci. 2016, 94, 2247–2261. [Google Scholar] [CrossRef]
- Holmes, E.; Li, J.V.; Marchesi, J.R.; Nicholson, J.K. Gut Microbiota Composition and Activity in Relation to Host Metabolic Phenotype and Disease Risk. Cell Metab. 2012, 16, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, R.; Leung, P.; Gershwin, M.E.; Ma, X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun. Rev. 2017, 16, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Henao-Mejia, J.; Elinav, E.; Thaiss, C.A. Licona-Limon, P.; Flavell, R.A. Role of the intestinal microbiome in liver disease. J. Autoimmun. 2013, 46, 66–73. [Google Scholar] [CrossRef]
- Dhillon, A.K.; Kummen, M.; Trøseid, M.; Åkra, S.; Liaskou, E.; Moum, B.; Vesterhus, M.; Karlsen, T.H.; Seljeflot, I.; Hov, J.R. Circulating Markers of Gut Barrier Function Associated with Disease Severity in Primary Sclerosing Cholangitis. Liver Int. 2019, 39, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Balmer, M.L.; Slack, E.; De Gottardi, A.; Lawson, M.A.E.; Hapfelmeier, S.; Miele, L.; Grieco, A.; Van Vlierberghe, H.; Fahrner, R.; Patuto, N.; et al. The Liver May Act as a Firewall Mediating Mutualism between the Host and Its Gut Commensal Microbiota. Sci. Transl. Med. 2014, 6, 237–266. [Google Scholar] [CrossRef] [PubMed]
- Dubinkina, V.B.; Tyakht, A.V.; Odintsova, V.Y.; Yarygin, K.S.; Kovarsky, B.A.; Pavlenko, A.V.; Ischenko, D.S.; Popenko, A.S.; Alexeev, D.G.; Taraskina, A.Y.; et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 2017, 5, 141. [Google Scholar] [CrossRef]
- Rai, R.; Saraswat, V.A.; Dhiman, R.K. Gut microbiota: Its role in hepatic encephalopathy. J. Clin. Exp. Hepatol. 2015, 5, S29–S36. [Google Scholar] [CrossRef]
- Betrapally, N.S.; Gillevet, P.M.; Bajaj, J.S. Gut microbiome and liver disease. Transl. Res. 2017, 179, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Pengo, G.; Caldin, M.; Palumbo Piccionello, A.; Steiner, J.M.; Cohen, N.D.; Jergens, A.E.; Suchodolski, J.S. Comparison of microbiological. histological. and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS ONE 2014, 9, e94699. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; White, M.B.; Monteith, P.; Noble, N.A.; Unser, A.B.; Daita, K.; Fisher, A.R.; et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J. Hepatol. 2014, 60, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Kummen, M.; Hov, J.R. The gut microbial influence on cholestatic liver disease. Liver Int. 2019, 39, 1186–1196. [Google Scholar] [CrossRef]
- Trebicka, J.; Bork, P.; Krag, A.; Arumugam, M. Utilizing the gut microbiome in decompensated cirrhosis and acute-on-chronic liver failure. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 167–180. [Google Scholar] [CrossRef]
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules 2021, 12, 56. [Google Scholar] [CrossRef]
- AlShawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Libury, J.A.; Steiner, J.M. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93, fix136. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Xenoulis, P.G.; Paddock, C.G.; Steiner, J.M.; Jergens, A.E. Molecular analysis of the bacterial microbiota in duodenal biopsies from dogs with idiopathic inflammatory bowel disease. Vet. Microbiol. 2010, 142, 394–400. [Google Scholar] [CrossRef]
- Minamoto, Y.; Otoni, C.C.; Steelman, S.M.; Büyükleblebici, O.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes. 2015, 6, 33–47. [Google Scholar] [CrossRef]
- Honneffer, J.B.; Minamoto, Y.; Suchodolski, J.S. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. World J. Gastroenterol. 2014, 20, 16489–16497. [Google Scholar] [CrossRef]
- Handl, S.; German, A.J.; Holden, S.L.; Dowd, S.E.; Steiner, J.M.; Heilmann, R.M.; Grant, R.W.; Swanson, K.S.; Suchodolski, J.S. Faecal microbiota in lean and obese dogs. FEMS Microbiol. Ecol. 2013, 84, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Suchodolski, J.S. The Gut Microbiome of Dogs and Cats, and the Influence of Diet. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Rhimi, S.; Kriaa, A.; Mariaule, V.; Saidi, A.; Drut, A.; Jablaoui, A.; Akermi, N.; Maguin, E.; Hernandez, J.; Rhimi, M. The Nexus of Diet, Gut Microbiota and Inflammatory Bowel Diseases in Dogs. Metabolites 2022, 12, 1176. [Google Scholar] [CrossRef] [PubMed]
- Squire, N.; Lux, C.; Tolbert, K.; Lidbury, J.; Sun, X.; Suchodolski, J.S. Characterization of the Fecal Microbiome in Dogs Receiving Medical Management for Congenital Portosystemic Shunts. Front. Vet. Sci. 2022, 9, 897760. [Google Scholar] [CrossRef] [PubMed]
- Hang, J.; Desai, V.; Zavaljevski, N.; Yang, Y.; Lin, X.; Satya, R.V.; Martinez, L.J.; Blaylock, J.M.; Jarman, R.G.; Thomas, S.J.; et al. 16S RRNA Gene Pyrosequencing of Reference and Clinical Samples and Investigation of the Temperature Stability of Microbiome Profiles. Microbiome 2014, 2, 31. [Google Scholar] [CrossRef]
- Tanprasertsuk, J.; Jha, A.R.; Shmalberg, J.; Jones, R.B.; Perry, L.M.; Maughan, H.; Honaker, R.W. The microbiota of healthy dogs demonstrates individualized responses to synbiotic supplementation in a randomized controlled trial. Anim. Microbiome 2021, 10, 36. [Google Scholar] [CrossRef]
- You, I.; Kim, M.J. Comparison of Gut Microbiota of 96 Healthy Dogs by Individual Traits: Breed, Age, and Body Condition Score. Animals 2021, 11, 2432. [Google Scholar] [CrossRef]
- Choi, J.; Joseph, L.; Pilote, L. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 232–244. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M.; et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e51907. [Google Scholar] [CrossRef]
- Moon, C.; Young, W.; Maclean, P.; Cookson, A.; Bermingham, E. Metagenomic insights into the roles of Proteobacteria in the gastrointestinal microbiomes of healthy dogs and cats. MicrobiologyOpen 2018, 7, e677. [Google Scholar] [CrossRef]
- Kil, D.Y.; Swanson, K.S. Companion animals symposium: Role of microbes in canine and feline health. JAS 2011, 89, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio 2019, 10, e02566-18. [Google Scholar] [CrossRef]
- Zhang, W.; Mackay, C.R.; Gershwin, M.E. Immunomodulatory Effects of Microbiota-Derived Short-Chain Fatty Acids in Autoimmune Liver Diseases. J. Immunol. Res. 2023, 210, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wu, Y.; Pham, Q.; Li, R.W.; Yu, L.; Chen, M.; Boue, S.M.; Yokoyama, W.; Li, B.; Wang, T.T.Y. Effects of differences in resistant starch content of rice on intestinal microbial composition. J. Agric. Food Chem. 2021, 69, 8017–8027. [Google Scholar] [CrossRef]
- Rau, M.; Rehman, A.; Dittrich, M.; Groen, A.K.; Hermanns, H.M.; Seyfried, F.; Beyersdorf, N.; Dandekar, T.; Rosenstiel, P.; Geier, A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United Eur. Gastroenterol. J. 2018, 6, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Dowd, S.E.; Wilke, V.; Steiner, J.M.; Jergens, A.E. 16S RRNA Gene Pyrosequencing Reveals Bacterial Dysbiosis in the Duodenum of Dogs with Idiopathic Inflammatory Bowel Disease. PLoS ONE 2012, 7, e39333. [Google Scholar] [CrossRef]
- Giaretta, P.R.; Suchodolski, J.S.; Jergens, A.E.; Steiner, J.M.; Lidbury, J.A.; Cook, A.K.; Hanifeh, M.; Spillmann, T.; Kilpinen, S.; Syrjä, P.; et al. Bacterial Biogeography of the Colon in Dogs With Chronic Inflammatory Enteropathy. Vet. Pathol. 2020, 57, 258–265. [Google Scholar] [CrossRef]
- Baltazar-Díaz, T.A.; González-Hernández, L.A.; Aldana-Ledesma, J.M.; Peña-Rodríguez, M.; Vega-Magaña, A.N.; Zepeda-Morales, A.S.M.; López-Roa, R.I.; Del Toro-Arreola, S.; Martínez-López, E.; Salazar-Montes, A.M.; et al. Escherichia/Shigella, SCFAs, and Metabolic Pathways—The Triad That Orchestrates Intestinal Dysbiosis in Patients with Decompensated Alcoholic Cirrhosis from Western Mexico. Microorganisms 2022, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Baeza, Y.; Hyde, E.R.; Suchodolski, J.S.; Knight, R. Dog and Human Inflammatory Bowel Disease Rely on Overlapping yet Distinct Dysbiosis Networks. Nat. Microbiol. 2016, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Greve, J.W.; Gouma, D.J.; Buurman, W.A. Bile Acids Inhibit Endotoxin-induced Release of Tumor Necrosis Factor by Monocytes: An in Vitro Study. Hepatology 1989, 10, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Mustafi, R.; Cerda, S.; Chumsangsri, A.; Xia, Y.R.; Li, Y.C.; Bissonnette, M. Lithocholic acid down-regulation of NFκB activity through vitamin D receptor in colonic cancer cells. J. Steroid Biochem. Mol. Biol. 2008, 111, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.X.; Fang, D.Q.; Shi, D.; Chen, D.Y.; Yan, R.; Zhu, Y.X.; Chen, Y.F.; Shao, L.; Guo, F.F.; Wu, W.R.; et al. Alterations and Correlations of the Gut Microbiome, Metabolism and Immunity in Patients with Primary Biliary Cirrhosis. Environ. Microbiol. 2016, 18, 2272–2286. [Google Scholar] [CrossRef]
- Torres, J.; Palmela, C.; Brito, H.; Bao, X.; Ruiqi, H.; Moura-Santos, P.; Pereira da Silva, J.; Oliveira, A.; Vieira, C.; Perez, K.; et al. The gut microbiota, bile acids and their correlation in primary sclerosing cholangitis associated with inflammatory bowel disease. United Eur. Gastroenterol. J. 2018, 6, 112–122. [Google Scholar] [CrossRef]
- Zhang, S.M.; Huang, S.L. The Commensal Anaerobe Veillonella dispar Reprograms Its Lactate Metabolism and Short-Chain Fatty Acid Production during the Stationary Phase. Microbiol. Spectr. 2023, 11, e0355822. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut Microbiota-Derived Short Chain Fatty Acids Are Potential Mediators in Gut Inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Nahidi, L.; Leach, S.T.; Mitchell, H.M.; Kaakoush, N.O.; Lemberg, D.A.; Munday, J.S.; Huinao, K.; Day, A.S. Inflammatory Bowel Disease Therapies and Gut Function in a Colitis Mouse Model. Biomed. Res. Int. 2013, 2023, 909613. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Dai, Z.; Wan, P.; Ye, H.; Zeng, X.; Sun, Y. Kudingcha and Fuzhuan Brick Tea Prevent Obesity and Modulate Gut Microbiota in High-Fat Diet Fed Mice. Mol. Nutr. Food Res. 2018, 62, e1700485. [Google Scholar] [CrossRef] [PubMed]
- Maslennikov, R.; Ivashkin, V.; Alieva, A.; Poluektova, E.; Kudryavtseva, A.; Krasnov, G.; Zharkova, M.; Zharikov, Y. Gut Dysbiosis and Body Composition in Cirrhosis. World J. Hepatol. 2022, 14, 1210–1225. [Google Scholar] [CrossRef]
- Martínez, I.; Perdicaro, D.J.; Brown, A.W.; Hammons, S.; Carden, T.J.; Carr, T.P.; Eskridge, K.M.; Walter, J. Diet-Induced Alterations of Host Cholesterol Metabolism Are Likely to Affect the Gut Microbiota Composition in Hamsters. Appl. Environ. Microbiol. 2013, 79, 516–524. [Google Scholar] [CrossRef]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Karr, S. Epidemiology and Management of Hyperlipidemia. Am. J. Manag. Care 2017, 23, S139–S148. [Google Scholar]
- Xenoulis, P.G.; Steiner, J.M. Lipid Metabolism and Hyperlipidemia in Dogs. Vet. J. 2010, 183, 12–21. [Google Scholar] [CrossRef]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat—The Relationship between Diet, Microbiota, and Metabolic Disorders—A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef] [PubMed]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2021, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhao, Y.; Li, Y.; Ma, S.; Wang, Z. Gut Dysbiosis Is Associated with Primary Hypothyroidism with Interaction on Gut-Thyroid Axis. Clin. Sci. 2020, 134, 1521–1535. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, Y.; Meng, C.; Ding, X.; Huang, J.; Luo, X.; Cao, Y.; Gao, F.; Zou, M. The Role of the Microbiome in Diabetes Mellitus. Diabetes Res. Clin. Pract. 2021, 172, 108645. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; de Pasquale, C.; Ekinci, E.I.; Coughlan, M.T. Gut Microbiome, Prebiotics, Intestinal Permeability and Diabetes Complications. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101507. [Google Scholar] [CrossRef]
- Ramezani, M.; Sajadi Hezaveh, Z. The Effect of Synbiotic Supplementation on Thyroid Hormones, Blood Pressure, Depression and Quality of Life in Hypothyroid Patients: A Study Protocol for a Randomized Double-Blind Placebo Controlled Clinical Trial. Clin. Nutr. ESPEN 2022, 48, 472–478. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lauber, C.L.; Czarnecki-Maulden, G.; Pan, Y.; Hannah, S.S. Effects of the Dietary Protein and Carbohydrate Ratio on Gut Microbiomes in Dogs of Different Body Conditions. mBio 2017, 8, e01703-16. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut Microbiota: Role in Pathogen Colonization, Immune Responses, and Inflammatory Disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Reimschuessel, R.; Grabenstein, M.; Guag, J.; Nemser, S.M.; Song, K.; Qiu, J.; Clothier, K.A.; Byrne, B.A.; Marks, S.L.; Cadmus, K.; et al. Multilaboratory Survey to Evaluate Salmonella Prevalence in Diarrheic and Nondiarrheic Dogs and Cats in the United States between 2012 and 2014. J. Clin. Microbiol. 2017, 55, 1350–1368. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, C.; Cui, J.; Lu, J.; Yan, C.; Wei, X.; Zhao, X.; Li, N.N.; Li, S.; Xue, G.; et al. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella Pneumoniae. Cell Metab. 2019, 30, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Cui, P.; Jiang, J.; Ning, C.; Liang, B.; Zhou, J.; Tian, L.; Zhang, Y.; Lei, T.; Zuo, T.; et al. Streptococcus, the Predominant Bacterium to Predict the Severity of Liver Injury in Alcoholic Liver Disease. Front. Cell. Infect. Microbiol. 2021, 11, 649060. [Google Scholar] [CrossRef] [PubMed]
- Manchester, A.C.; Webb, C.B.; Blake, A.B.; Sarwar, F.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Long-Term Impact of Tylosin on Fecal Microbiota and Fecal Bile Acids of Healthy Dogs. J. Vet. Intern. Med. 2019, 33, 2605–2617. [Google Scholar] [CrossRef]

| CHD Dogs | Reference Range | |
|---|---|---|
| ALP | 512 (43–8907) | 45–250 U/L |
| GGT | 3.4 (1.1–375) | 2–11 U/L |
| AST | 44 (18–1493) | 15–40 U/L |
| ALT | 113 (25–2652) | 20–70 U/L |
| Tot Bil | 0.22 (0.07–19.3) | 0.07–0.3 mg/dL |
| ALT | Hyperlipemia | |||||
| Normal (n = 22) | Increased (n = 43) | p-Value | Present (n = 41) | Absent (n = 24) | p-Value | |
| N° Species | 17,223 ± 6270 | 16,640 ± 4208 | 0.65 | 16,737 ± 4205 | 17,008 ± 6135 | 0.83 |
| Alpha diversity | 2.217 ± 0.76 | 2.246 ± 0.62 | 0.87 | 2.190 ± 0.66 | 2.314 ± 0.68 | 0.47 |
| Endocrine Disorder | Cholestasis | |||||
| Present (n = 19) | Absent (n = 46) | p-Value | Present (n = 45) | Absent (n = 20) | p-Value | |
| N° Species | 17,032 ± 5213 | 16,757 ± 4908 | 0.84 | 16,698 ± 4921 | 17,150 ± 5160 | 0.73 |
| Alpha diversity | 2.293 ± 0.74 | 2.212 ± 0.64 | 0.66 | 2.168 ± 0.64 | 2.388 ± 0.7 | 0.22 |
| Diet Category | ||||||
| Diabetic (n = 5) | Hepatic (n = 9) | Gastrointestinal (n = 19) | Maintenance (n = 32) | p-Value | ||
| N° Species | 20,000 (10,000–29,000) | 14,000 (8000–25,000) | 14,000 (7000–23,000) | 18,000 (11,500–30,000) | 0.12 | |
| Alpha diversity | 3.02 (1.34–3.24) | 1.57 (0.93–3.14) | 2.3 (1.01–3.14) | 2.54 (0.83–3.16) | 0.02 | |
| Bacteria | Values |
|---|---|
| Phylum | |
| Actinobacteria | 1.72 (0.01–27.29) |
| Bacteroidetes | 4.72 (0.01–57.34) |
| Firmicutes | 56.96 (8.72–98.39) |
| Fusobacteria | 3.13 (0–33.29) |
| Proteobacteria | 12.34 (0.02–89.13) |
| Family | |
| Bacteroidaceae | 14 (0–60,949) |
| Bifidobacteriaceae | 0 (0–1112) |
| Clostridiaceae | 4774 (0–67,993) |
| Corynebacteriaceae | 0 (0–136) |
| Enterobacteriaceae | 14 (0–129,390) |
| Enterococcaceae | 0 (0–63,111) |
| Erysipelotrichaceae | 57 (0–82,064) |
| Eubacteriaceae | 666 (0–72,100) |
| Fusobacteriaceae | 23 (0–66,183) |
| Lactobacillaceae | 0 (0–67,987) |
| Ruminococcaceae | 47 (0–29,161) |
| Veillonellaceae | 0 (0–102,369) |
| Genus | |
| Acinetobacter | 0 (0–20) |
| Actinomyces | 0 (0–672) |
| Bacteroides | 0 (0–60,922) |
| Bifidobacterium | 0 (0–1112) |
| Blautia | 5328 (0–64,285) |
| Campylobacter | 0 (0–21,646) |
| Clostridium | 1685 (0–74,731) |
| Coprobacillus | 0 (0–177) |
| Coprococcus | 0 (0–477) |
| Corynebacterium | 0 (0–34) |
| Cronobacter | 0 (0–92) |
| Enterococcus | 0 (0–62,340) |
| Escherichia | 76 (0–8088) |
| Faecalibacterium | 0 (0–25,117) |
| Fusobacterium | 0 (0–58,851) |
| Helicobacter | 0 (0–2234) |
| Klebsiella | 0 (0–152) |
| Lactobacillus | 0 (0–67,797) |
| Megamonas | 0 (0–102,357) |
| Prevotella | 42 (0–42,872) |
| Proteus | 0 (0–415) |
| Pseudomonas | 0 (0–734) |
| Ruminococcus | 657 (0–40,941) |
| Salmonella | 0 (0–796) |
| Serratia | 0 (0–48,468) |
| Staphylococcus | 0 (0–11) |
| Streptococcus | 0 (0–84,431) |
| Turicibacter | 0 (0–12,604) |
| Yersinia | 0 (0–367) |
| Species | |
| Clostridium difficile | 0 (0–8707) |
| Clostridium hiranonis | 63 (0–7827) |
| Clostridium perfringens | 642 (0–60,494) |
| Enterococcus faecium | 0 (0–9053) |
| Escherichia coli | 0 (0–392) |
| Escherichia shigella | 0 (0–9108) |
| Faecalibacterium prausnitzii | 0 (0–10,062) |
| Helicobacter canis | 0 (0–218) |
| Lactobacillus acidophilus | 0 (0–15,127) |
| Ruminococcus faecis | 0 (0–5902) |
| Salmonella enterica | 0 (0–30) |
| Increased ALT (n = 43) | p | Hyperlipemia (n = 41) | p | Endocrine Disorder (n = 19) | p | Cholestasis (n = 45) | p | |
|---|---|---|---|---|---|---|---|---|
| Bacteroidaceae | ↓ | 0.001 | ||||||
| Enterobacteriaceae | ↓ | 0.001 | ||||||
| Erysipelotrichaceae | ↓ | 0.0008 | ||||||
| Eubacteriaceae | ↓ | 0.0002 | ||||||
| Fusobacteriaceae | ↓ | 0.0115 | ||||||
| Ruminococcaceae | ↓ | 0.0001 | ||||||
| Veillonellaceae | ↓ | 0.001 | ||||||
| Clostridium | ↓ | <0.0001 | ||||||
| Escherichia | ↓ | 0.02 | ↓ | 0.0002 | ||||
| Fusobacterium | ↓ | 0.01 | ||||||
| Helicobacter | ||||||||
| Klebsiella | ↑ | 0.021 | ||||||
| Megamonas | ↓ | 0.002 | ||||||
| Prevotella | ↑ | 0.022 | ||||||
| Ruminococcus | ↓ | <0.0001 | ||||||
| Salmonella | ↑ | 0.03 | ||||||
| Serratia | ↑ | 0.005 | ||||||
| Streptococcus | ↑ | 0.03 | ↓ | 0.001 | ||||
| Turicibacter | ↓ | 0.03 | ||||||
| Clostridium hiranonis | ↓ | 0.002 | ||||||
| Enterococcus faecium | ↓ | 0.03 | ||||||
| Escherichia coli | ↑ | 0.02 | ||||||
| Escherichia shigella | ↑ | 0.005 | ||||||
| Ruminococcus faecis | ↓ | <0.0001 | ||||||
| Salmonella enterica | ↑ | 0.03 |
| Gastointestinal (n = 19) | Maintenance (n = 32) | Diabetic (n = 5) | Hepatic (n = 9) | p | |
|---|---|---|---|---|---|
| Alpha Diversity (Shannon) | 2.3 (1.01–3.14) d | 2.54 (0.83–3.16) d | 3.02 (1.34–3.24) d | 1.57 (0.93–3.14) a,b,c | 0.02 |
| Ruminococcaceae | 1749 (0–22,925) d | 1066 (13–26,808) | 455 (68–29,161) | 182 (0–638) a | 0.03 |
| Helicobacter | 0 (0–780) c | 0 (0–2234) | 222 (0–1071) a,d | 0 (0–123) c | 0.03 |
| Prevotella | 0 (0–36,829) c | 0 (0–60,858) | 653 (0–39,549) a,d | 0 (0–122) c | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habermaass, V.; Olivero, D.; Gori, E.; Mariti, C.; Longhi, E.; Marchetti, V. Intestinal Microbiome in Dogs with Chronic Hepatobiliary Disease: Can We Talk about the Gut–Liver Axis? Animals 2023, 13, 3174. https://doi.org/10.3390/ani13203174
Habermaass V, Olivero D, Gori E, Mariti C, Longhi E, Marchetti V. Intestinal Microbiome in Dogs with Chronic Hepatobiliary Disease: Can We Talk about the Gut–Liver Axis? Animals. 2023; 13(20):3174. https://doi.org/10.3390/ani13203174
Chicago/Turabian StyleHabermaass, Verena, Daniela Olivero, Eleonora Gori, Chiara Mariti, Erika Longhi, and Veronica Marchetti. 2023. "Intestinal Microbiome in Dogs with Chronic Hepatobiliary Disease: Can We Talk about the Gut–Liver Axis?" Animals 13, no. 20: 3174. https://doi.org/10.3390/ani13203174
APA StyleHabermaass, V., Olivero, D., Gori, E., Mariti, C., Longhi, E., & Marchetti, V. (2023). Intestinal Microbiome in Dogs with Chronic Hepatobiliary Disease: Can We Talk about the Gut–Liver Axis? Animals, 13(20), 3174. https://doi.org/10.3390/ani13203174







