Effects of Tributyltin-Contaminated Aquatic Environments and Remediated Water on Early Development of Sea Urchin (Hemisentrotus pulcherrimus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sea Urchin and Study Conditions
2.2. Experimental Design
2.3. TBT Concentration Analysis
2.4. Statistical Analysis
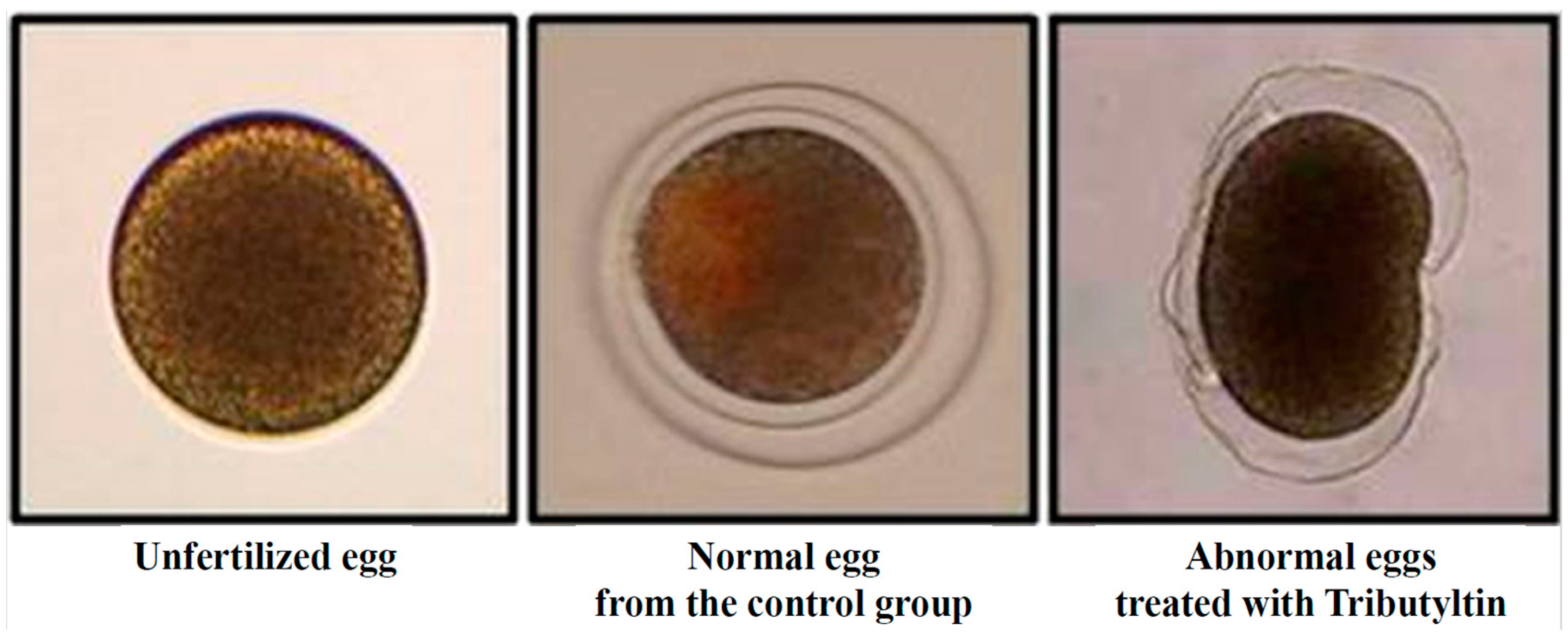
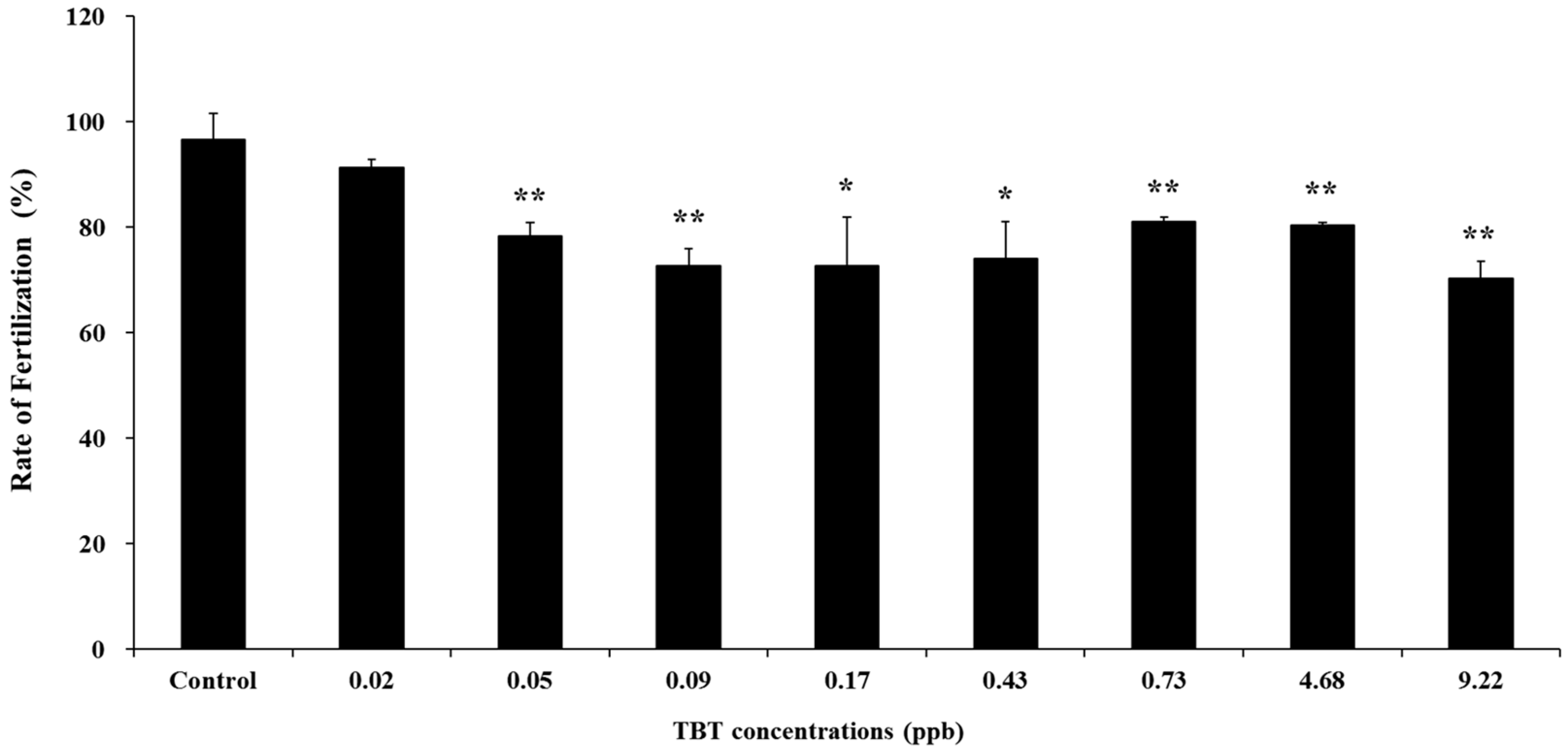
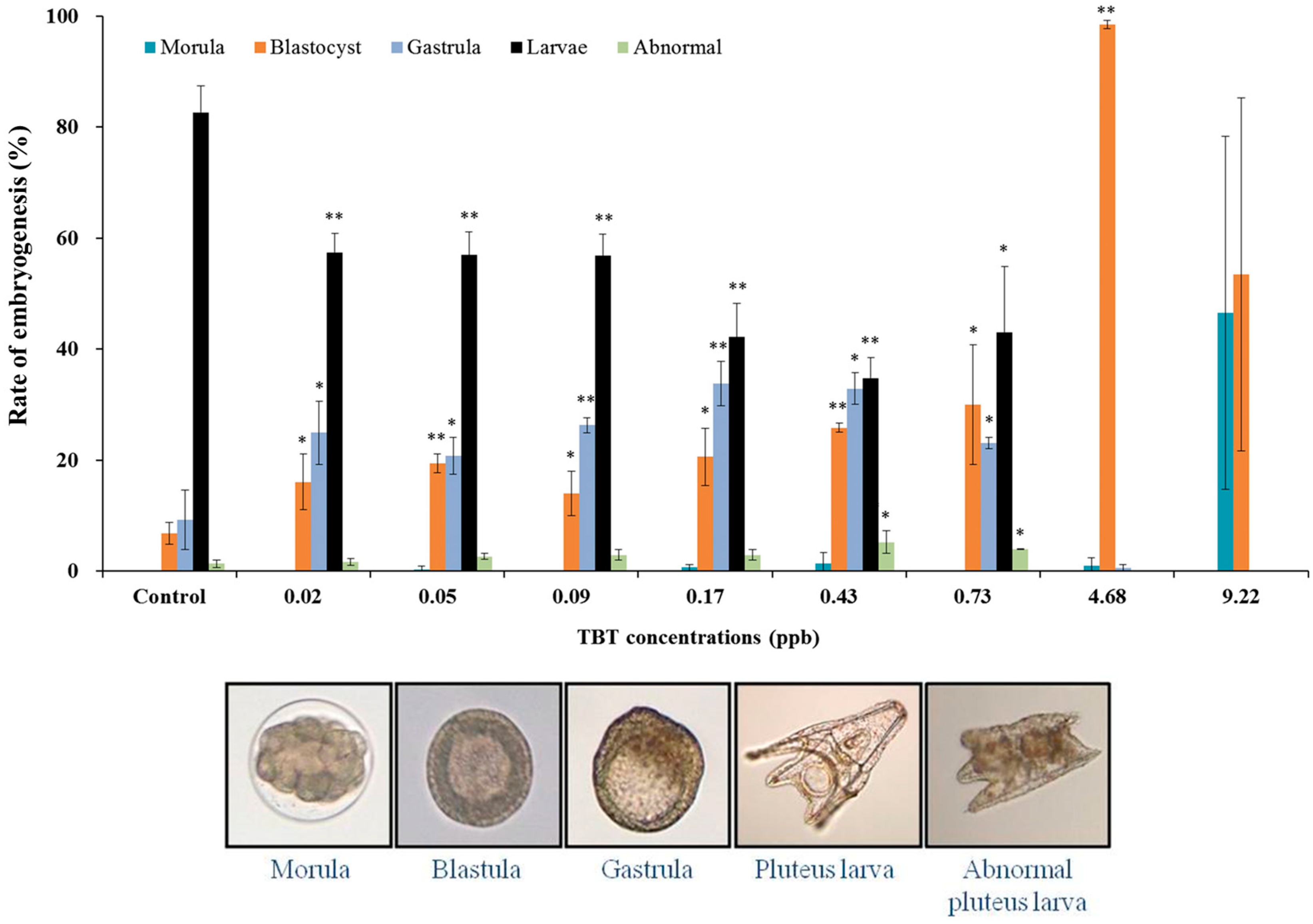
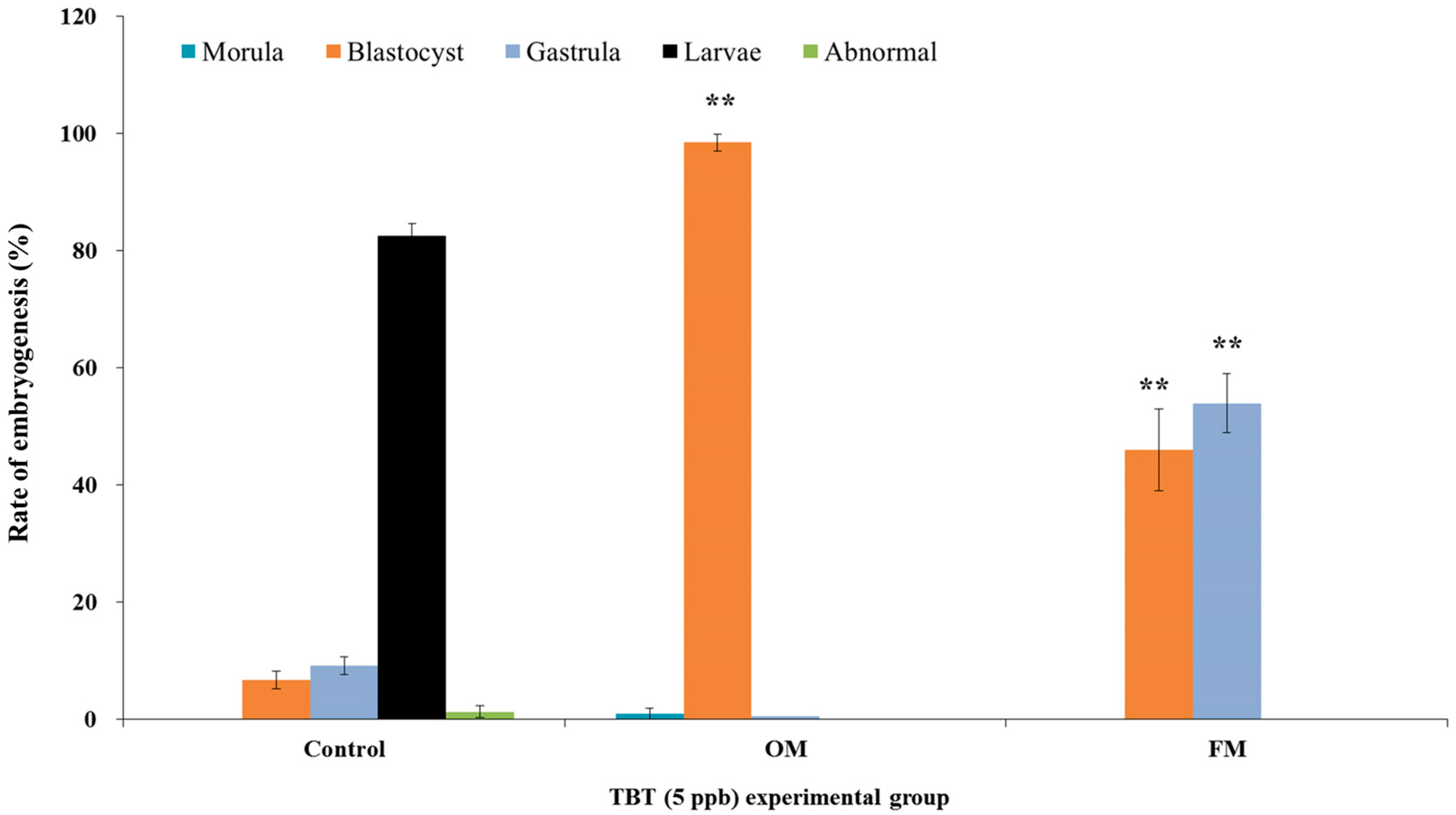
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, V.; Sharma, N.; Sharma, P.; Pasrija, R.; Kaur, K.; Umesh, M.; Thazeem, B. Toxicity analysis of endocrine disrupting pesticides on non-target organisms: A critical analysis on toxicity mechanisms. Toxicol. Appl. Pharmacol. 2023, 474, 116623. [Google Scholar] [CrossRef] [PubMed]
- Tabb, M.; Blumberg, B. New modes of action for endocrine-disrupting chemicals. Mol. Endocrinol. 2006, 20, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Thacharodi, A.; Hassan, S.; Hegde, T.A.; Thacharodi, D.D.; Brindgadevi, K.; Pugazhendhi, A. Water a major source of endocrine-disrupting chemicals: An overview on the occurrence, implications on human health and bioremediation strategies. Environ. Res. 2023, 231, 116097. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.; Jeung, E.B. Endocrine-disrupting chemicals and disease endpoints. Int. J. Mol. Sci. 2023, 24, 5342. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xiao, Q.; Zhang, M.; Li, D.; Wang, X. Toxic effects and mechanisms of polybrominated diphenyl ethers. Int. J. Mol. Sci. 2023, 24, 13487. [Google Scholar] [CrossRef]
- Beyer, J.; Song, Y.; Tollefsen, K.E.; Berge, J.A.; Tveiten, L.; Helland, A.; Øxnevad, S.; Schøyen, M. The ecotoxicology of marine tributyltin (TBT) hotspots: A review. Mar. Environ. Res. 2022, 179, 105689. [Google Scholar] [CrossRef]
- Andrade, M.N.; Melo-Paiva, F.D.; Teixeira, M.P.; Lima-Junior, N.C.; Soares, P.; Graceli, J.B.; Carvalho, D.P.; Morris, E.A.R.; Ferreira, A.C.F.; Miranda-Alves, L. Environmentally relevant dose of the endocrine disruptor tributyltin disturbs redox balance in female thyroid gland. Mol. Cell Endocrinol. 2022, 1, 111689. [Google Scholar] [CrossRef]
- He, S.; Li, P.; Li, Z.H. Review on endocrine disrupting toxicity of triphenyltin from the perspective of species evolution: Aquatic, amphibious and mammalian. Chemosphere 2021, 269, 128711. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, Z.; Han, Q.; Peng, R.; Shi, H.; Jiang, X. Embryonic exposure to environmentally relevant levels of tributyltin affects embryonic tributyltin bioaccumulation and the physiological responses of juveniles in cuttlefish (Sepia pharaonis). Ecotoxicol. Environ. Saf. 2023, 256, 114894. [Google Scholar] [CrossRef]
- Guillette, L.J., Jr.; Iguchi, T. Life in a contaminated world. Science 2012, 337, 1614–1615. [Google Scholar] [CrossRef]
- Chapman, R.W.; Guillette, L.J., Jr. Contaminants and imposex: Transcriptomics of contaminant induced sex change. Mol. Ecol. 2013, 22, 1485–1487. [Google Scholar] [CrossRef] [PubMed]
- Castro, Í.B.; Iannacone, J.; Santos, S.; Fillmann, G. TBT is still a matter of concern in Peru. Chemosphere 2018, 205, 253–259. [Google Scholar] [CrossRef]
- Warford, L.; Mason, C.; Lonsdale, J.; Bersuder, P.; Blake, S.; Evans, N.; Thomas, B.; James, D. A reassessment of TBT action levels for determining the fate of dredged sediments in the United Kingdom. Mar. Pollut. Bull. 2022, 176, 113439. [Google Scholar] [CrossRef]
- Mil-Homens, M.; Almeida, C.M.R.; Dias, S.; Soares, W.; van Gaever, P.; de Stigter, H.; Santos, M.M.; Santana, A.; Freitas, M.; Abrantes, F.; et al. Spatial distribution and temporal trends of butyltin compounds (TBT, DBT & MBT) in short sediment cores of the SW Portuguese Shelf (western Iberian Margin, NE Atlantic). Sci. Total Environ. 2023, 900, 165872. [Google Scholar] [PubMed]
- Ueno, D.; Inoue, S.; Takahashi, S.; Ikeda, K.; Tanaka, H.; Subaramanian, A.N.; Fillmann, G.; Zheng, J.; Muchtar, M.; Prudente, M.; et al. Global pollution monitoring of butyltin compounds using skipjack tuna as a bioindicator. Environ. Pollut. 2004, 127, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Choi, H.G.; Moon, H.B.; Kim, G.Y. Spatial and temporal distribution of tributyltin (TBT) in seawater, sediments and bivalves from coastal areas of Korea during 2001–2005. Environ. Monit. Assess. 2009, 151, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Alzieu, C. Tributyltin: Case study of a chronic contaminant in the coastal environment. Ocean Coast. Manag. 1998, 40, 23–36. [Google Scholar] [CrossRef]
- Alzieu, C. Environmental impact of TBT: The French experience. Sci. Total Environ. 2000, 258, 99–102. [Google Scholar] [CrossRef]
- Shimasaki, Y.; Kitano, T.; Oshima, Y.; Inoue, S.; Imada, N.; Tadokoro, H.; Honjo, T. Tributyltin causes masculinization in fish. Environ. Toxicol. Chem. 2003, 22, 141–144. [Google Scholar] [CrossRef]
- Nakayama, K.; Oshima, Y.; Yamaguchi, T.; Tsuruda, Y.; Kang, I.J.; Kobayashi, M.; Imada, N.; Honjo, T. Fertilization success and sexual behavior in male medaka, Oryzias latipes, exposed to tributyltin. Chemosphere 2004, 55, 1331–1337. [Google Scholar] [CrossRef]
- Stange, D.; Sieratowicz, A.; Oehlmann, J. Imposex development in Nucella lapillus evidence for the involvement of retinoid X receptor and androgen signalling pathways in vivo. Aquat. Toxicol. 2012, 106–107, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Alzieu, C. Impact of tributyltin on marine invertebrates. Ecotoxicology 2000, 9, 71–76. [Google Scholar] [CrossRef]
- Bryan, G.W.; Gibbs, P.E.; Hummerstone, L.G.; Burt, G.R. The decline of the gastropod Nucella lapillus around south-west england: Evidence for the effect of tributyltin from antifouling paints. J. Mar. Biol. Assoc. UK 2009, 66, 611–640. [Google Scholar] [CrossRef]
- Inadera, H.; Shimomura, A. Environmental chemical tributyltin augments adipocyte differentiation. Toxicol. Lett. 2005, 159, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Grűn, F.; Watanabe, H.; Zamanian, Z.; Maeda, L.; Arima, K.; Chubacha, R.; Gardiner, D.M.; Iguchi, T.; Kanno, J.; Blumberg, B. Endocrine disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol. Endocrinol. 2006, 20, 2141–2155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zuo, Z.; He, C.; Cai, J.; Wang, Y.; Chen, Y.; Wang, C. Effect of tributyltin on testicular development in Sebastiscus marmoratus and the mechanism involved. Environ. Toxicol. Chem. 2009, 28, 1528–1535. [Google Scholar] [CrossRef]
- Di Natale, M.; Bennici, C.; Biondo, G.; Masullo, T.; Monastero, C.; Tagliavia, M.; Torri, M.; Costa, S.; Ragusam, M.A.; Cuttitta, A.; et al. Aberrant gene expression profiles in Mediterranean Sea urchin reproductive tissues after metal exposures. Chemophere 2019, 216, 48–58. [Google Scholar] [CrossRef]
- Masullo, T.; Biondo, G.; Di Natale, M.; Tagiliavia, M.; Bennici, C.D.; Musco, M.; Ragusa, M.A.; Costa, S.; Cuttitta, A.; Nicosia, A. Gene expression changes after parental exposure to metals in the sea urchin affect timing of genetic programme of embryo development. Biology 2021, 10, 103. [Google Scholar] [CrossRef]
- Migliaccio, O.; Castellano, I.; Cirino, P.; Romano, G.; Palumbo, A. Maternal Exposure to Cadmium and Manganese Impairs Reproduction and Progeny Fitness in the Sea Urchin Paracentrotus lividus. PLoS ONE 2015, 10, e0131815. [Google Scholar] [CrossRef]
- Hwang, U.K.; Kim, D.H.; Ryu, H.M.; Lee, J.W.; Park, S.Y.; Kang, H.S. Effect of bisphenol A on early embryonic development and the expression of glutathione s-transferase (GST) in the sea urchin (Hemicentrotus pulcherrimus). Korea J. Environ. Biol. 2016, 32, 234–242. [Google Scholar] [CrossRef]
- Rato, L.; Sousa, A.C.A. The Impact of Endocrine-Disrupting Chemicals in Male Fertility: Focus on the Action of Obesogens. J. Xenobiot. 2021, 11, 163–196. [Google Scholar] [CrossRef] [PubMed]
- Langlois, M.R.; Oorlynck, L.; Vandekerckhove, F.; Criel, A.; Bernard, D.; Blaton, V. Discrepancy between sperm acrosin activity and sperm morphology: Significance for fertilization in vitro. Clin. Chim. Acta 2005, 351, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Chen, Y.; Zuo, Z.; Chen, Y.; Yang, Z.; Wang, C. Effects of tributyltin on epididymal function and sperm maturation in mice. Environ. Toxicol. Pharmacol. 2009, 28, 19–24. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H.; Zhang, M.; Zhang, S.; Hu, J.; Wu, G.; Yang, J. Taurine increases spermatozoa quality and function in asthenospermia rats impaired by ornidazole. Taurine 2019, 11, 507–520. [Google Scholar]
- Marin, M.G.; Moschino, V.; Cima, F.; Celli, C. Embryotoxicity of butyltin compounds to the sea urchin Paracentrotus lividus. Mar. Environ. Res. 2000, 50, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Perina, F.C.; Abessa, D.M.; Pinho, G.L.; Fillmann, G. Comparative toxicity of antifouling compounds on the development of sea urchin. Ecotoxicology 2011, 20, 1870–1880. [Google Scholar] [CrossRef]
- Cima, F.; Varello, R. Effects of Exposure to Trade Antifouling Paints and Biocides on Larval Settlement and Metamorphosis of the Compound Ascidian Botryllus schlosseri. J. Mar. Sci. Eng. 2022, 10, 123. [Google Scholar] [CrossRef]
- Mai, H.; Morin, B.; Pardon, P.; Gonzalez, P.; Budzinski, H.; Cachot, J. Environmental concentrations of irgarol, diuron and S-metolachlor induce deleterious effects on gametes and embryos of the Pacific oyster, Crassostrea gigas. Mar. Environ. Res. 2013, 89, 1–8. [Google Scholar] [CrossRef]
- Jeferey, D.G.; James, M.M.; William, J.S. The effects of methoxychlor on early sea urchin development. Environ. Res. 1997, 72, 56–64. [Google Scholar]
- Hwang, J.; Suh, S.S.; Chang, M.; Park, S.Y.; Ryu, T.K.; Lee, S.; Lee, T.K. Effects of triclosan on reproductive parameters and embryonic development of sea urchin, Strongylocentrotus nudus. Ecotoxicol. Environ. Saf. 2014, 100, 148–152. [Google Scholar] [CrossRef]

| Test Parameters | Condition |
|---|---|
| Culture type | Static non-renewal 10 min—64 h toxicity test |
| Photoperiod | Ambient light condition and 8L:16D period |
| Temperature | 16 ± 0.5 °C |
| pH | 7.8–8.2 |
| Salinity | 32 ± 1.0 psu |
| Culture dish | 6-well plate culture dish |
| Solution | Filtered (0.45 μm) and sterilized seawater |
| Solution exchange | None |
| Experiment period | 10 min–64 h |
| Investigation item | Fertilization, larval development rates |
| Acceptability criterion | >90% fertilized eggs and pluteus larvae in control |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-C.; Lee, J.-W.; Hwang, U.-K.; Jeon, H.-J.; Oh, S.-Y.; Kim, C.-W.; Kang, H.-S. Effects of Tributyltin-Contaminated Aquatic Environments and Remediated Water on Early Development of Sea Urchin (Hemisentrotus pulcherrimus). Animals 2023, 13, 3078. https://doi.org/10.3390/ani13193078
Choi H-C, Lee J-W, Hwang U-K, Jeon H-J, Oh S-Y, Kim C-W, Kang H-S. Effects of Tributyltin-Contaminated Aquatic Environments and Remediated Water on Early Development of Sea Urchin (Hemisentrotus pulcherrimus). Animals. 2023; 13(19):3078. https://doi.org/10.3390/ani13193078
Chicago/Turabian StyleChoi, Hee-Chan, Ju-Wook Lee, Un-Ki Hwang, Ha-Jeong Jeon, Sung-Yong Oh, Chul-Won Kim, and Han-Seung Kang. 2023. "Effects of Tributyltin-Contaminated Aquatic Environments and Remediated Water on Early Development of Sea Urchin (Hemisentrotus pulcherrimus)" Animals 13, no. 19: 3078. https://doi.org/10.3390/ani13193078
APA StyleChoi, H.-C., Lee, J.-W., Hwang, U.-K., Jeon, H.-J., Oh, S.-Y., Kim, C.-W., & Kang, H.-S. (2023). Effects of Tributyltin-Contaminated Aquatic Environments and Remediated Water on Early Development of Sea Urchin (Hemisentrotus pulcherrimus). Animals, 13(19), 3078. https://doi.org/10.3390/ani13193078






