Primary Feline Tauopathy: Clinical, Morphological, Immunohistochemical, and Genetic Studies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Control Samples
2.2. Magnetic Resonance Imaging (MRI)
2.3. Histology and Immunohistochemistry (IHC)
2.4. Ultrastructural Study
2.5. Sequencing, Read Mapping, and Variant Calling
3. Results
3.1. Clinical Alterations Related to Tau Pathology
3.2. Tau Pathology in Cat Brains and Control Samples
3.3. Primary Feline Tauopathy
3.3.1. Clinical History
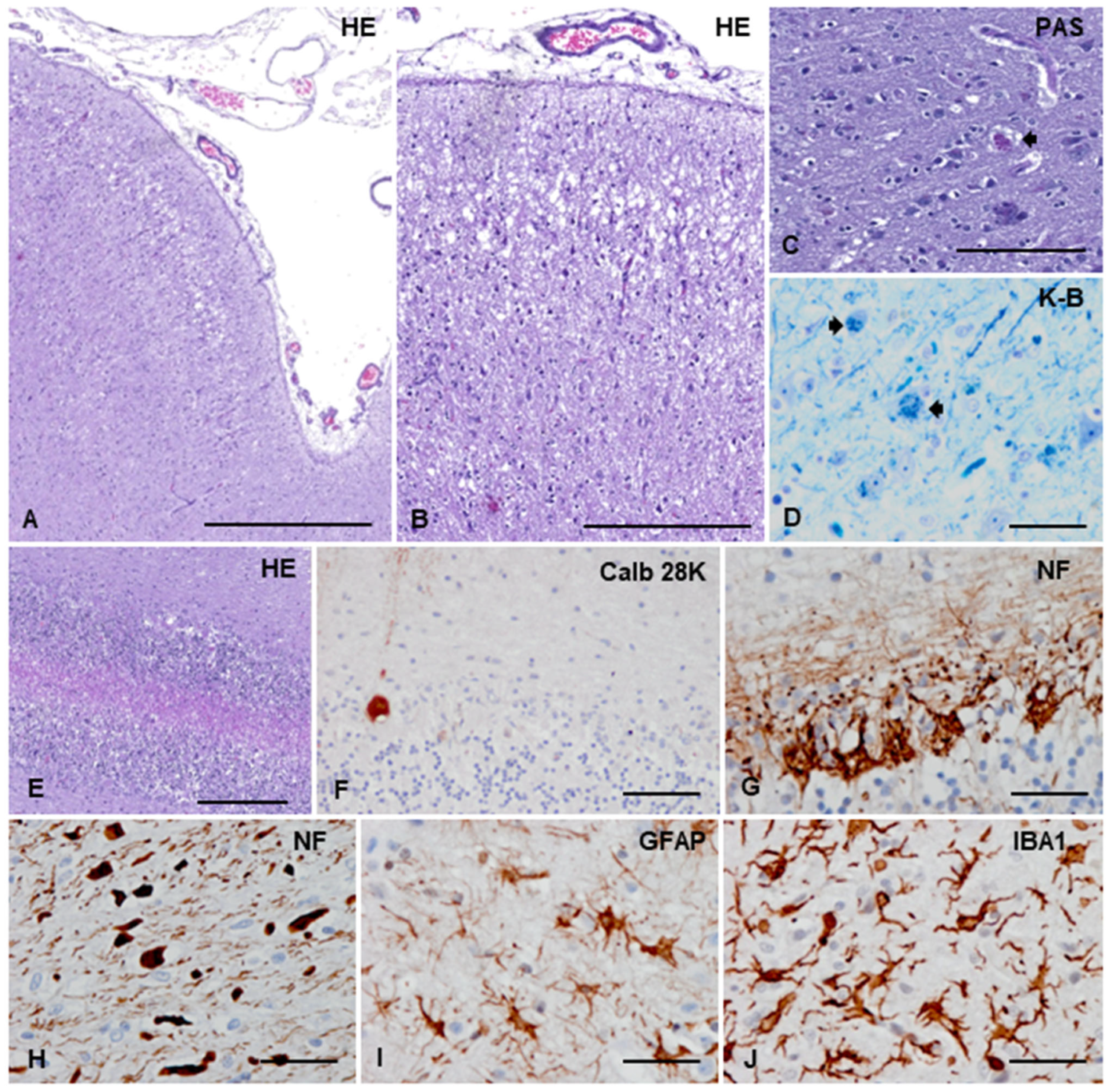
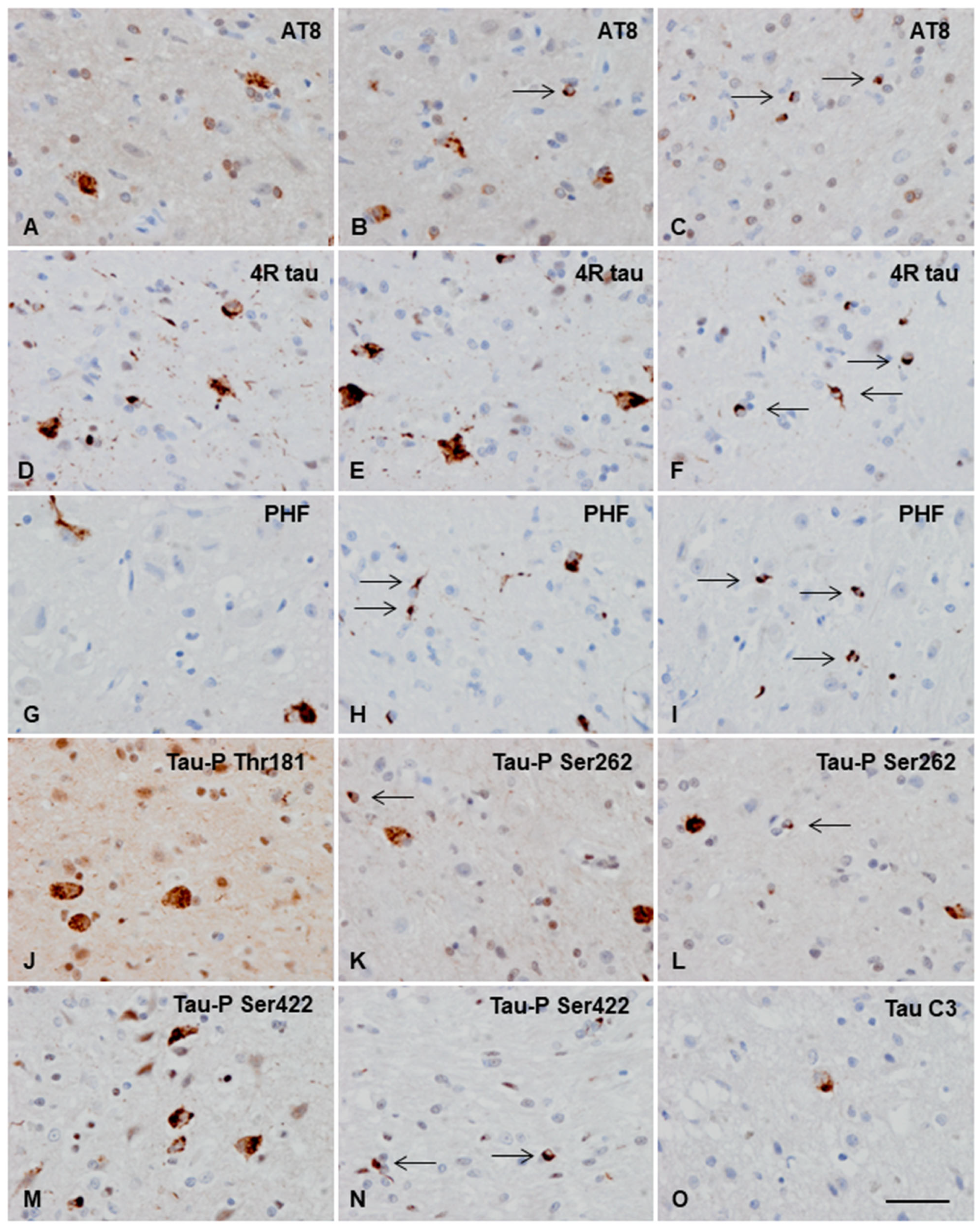
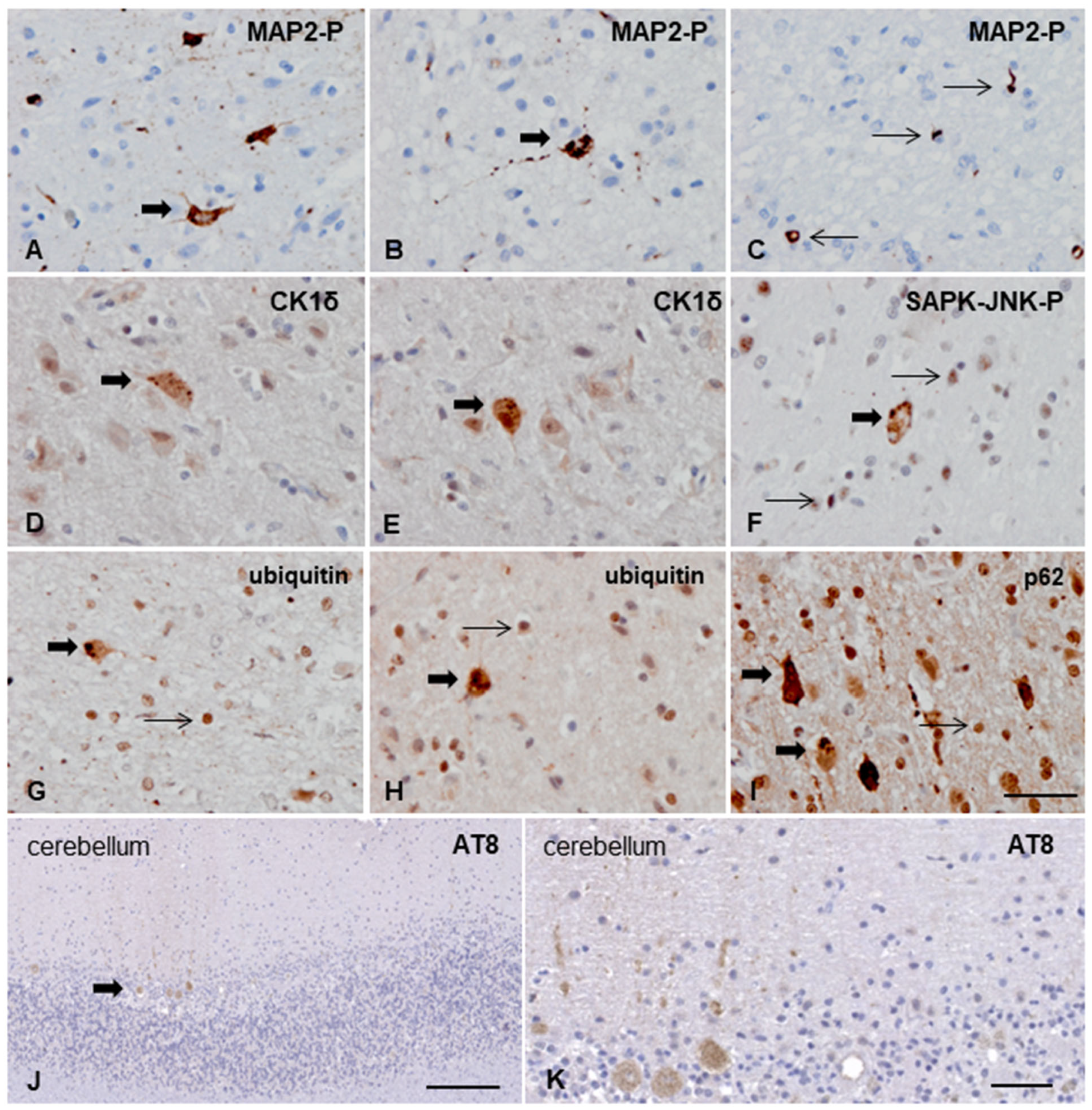
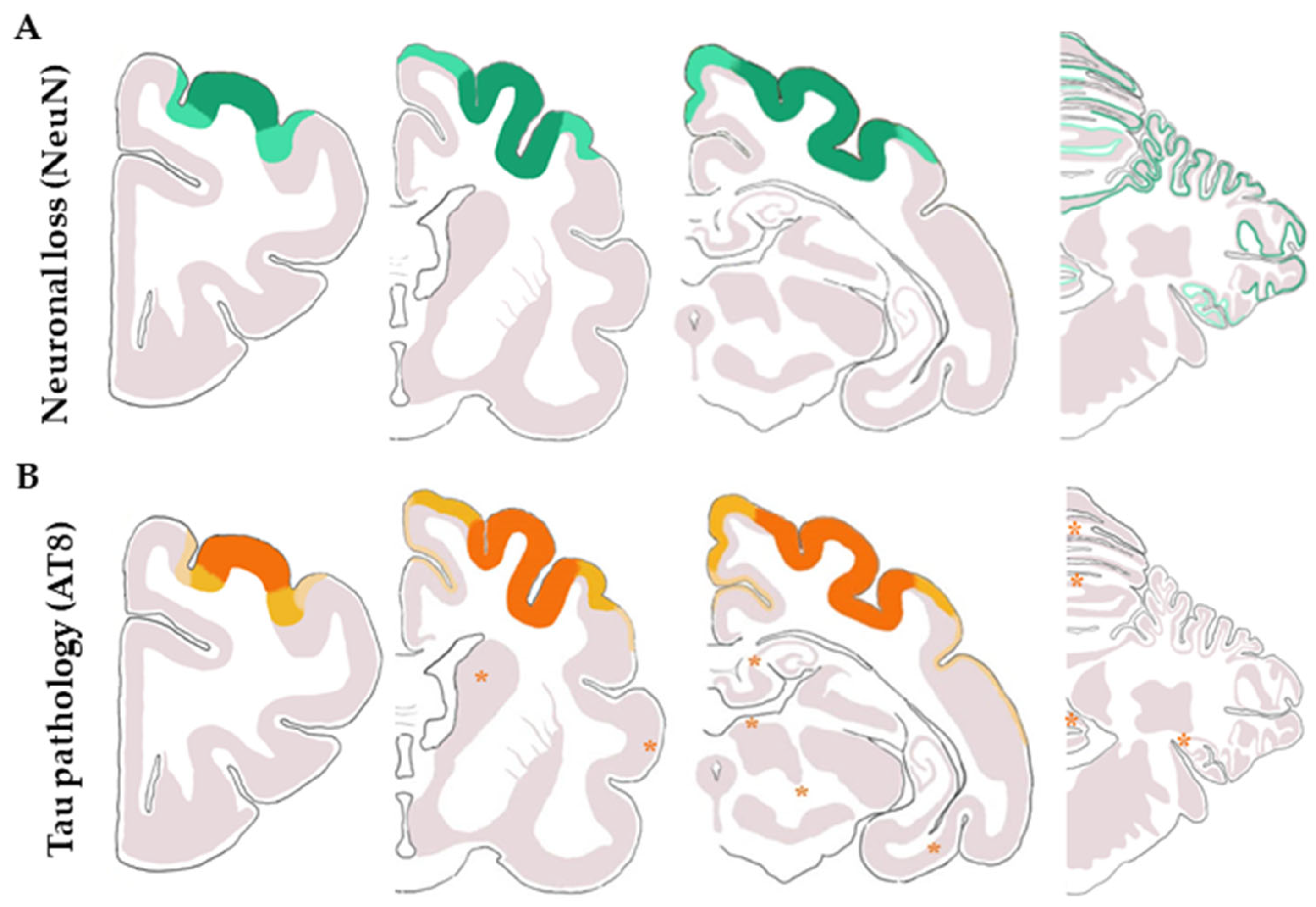
3.3.2. Macroscopic, Microscopic, and Immunohistochemical Examination
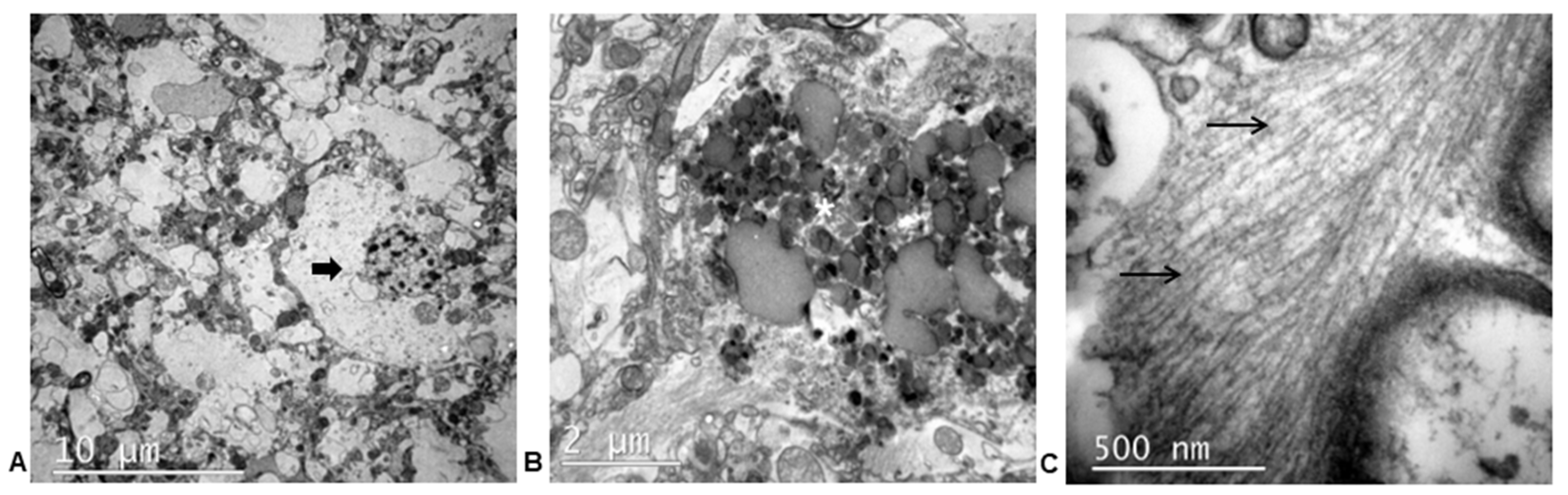
3.3.3. Ultrastructural Study
3.3.4. Genetics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dujardin, S.; Colin, M.; Buée, L. Invited Review: Animal Models of Tauopathies and Their Implications for Research/Translation into the Clinic. Neuropathol. Appl. Neurobiol. 2015, 41, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Arendt, T.; Stieler, J.T.; Holzer, M. Tau and Tauopathies. Brain Res. Bull. 2016, 126, 238–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mandelkow, E. Tau in Physiology and Pathology. Nat. Rev. Neurosci. 2016, 17, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Leugers, C.J. Tau and Tauopathies; Elsevier: Amsterdam, The Netherlands, 2012; Volume 107, ISBN 9780123858832. [Google Scholar]
- Kovacs, G.G. Invited Review: Neuropathology of Tauopathies: Principles and Practice. Neuropathol. Appl. Neurobiol. 2015, 41, 3–23. [Google Scholar] [CrossRef]
- Götz, J.; Halliday, G.; Nisbet, R.M. Molecular Pathogenesis of the Tauopathies. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 239–261. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, W.; Yang, Y.; Murzin, A.G.; Falcon, B.; Kotecha, A.; van Beers, M.; Tarutani, A.; Kametani, F.; Garringer, H.J.; et al. Structure-Based Classification of Tauopathies. Nature 2021, 598, 359–363. [Google Scholar] [CrossRef]
- Chung, D.; Eun, C.; Roemer, S.; Petrucelli, L.; Dickson, D.W. Cellular and Pathological Heterogeneity of Primary Tauopathies. Mol. Neurodegener. 2021, 16, 57. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Goedert, M. Tau Pathology and Neurodegeneration. Lancet Neurol. 2013, 12, 609–622. [Google Scholar] [CrossRef]
- Arakhamia, T.; Lee, C.E.; Carlomagno, Y.; Duong, D.M.; Kundinger, S.R.; Wang, K.; Williams, D.; DeTure, M.; Dickson, D.W.; Cook, C.N.; et al. Posttranslational Modifications Mediate the Structural Diversity of Tauopathy Strains. Cell 2020, 180, 633–644. [Google Scholar] [CrossRef]
- Kametani, F.; Yoshida, M.; Matsubara, T.; Murayama, S.; Saito, Y.; Kawakami, I.; Onaya, M.; Tanaka, H.; Kakita, A.; Robinson, A.C.; et al. Comparison of Common and Disease-Specific Post-Translational Modifications of Pathological Tau Associated With a Wide Range of Tauopathies. Front. Neurosci. 2020, 14, 581936. [Google Scholar] [CrossRef]
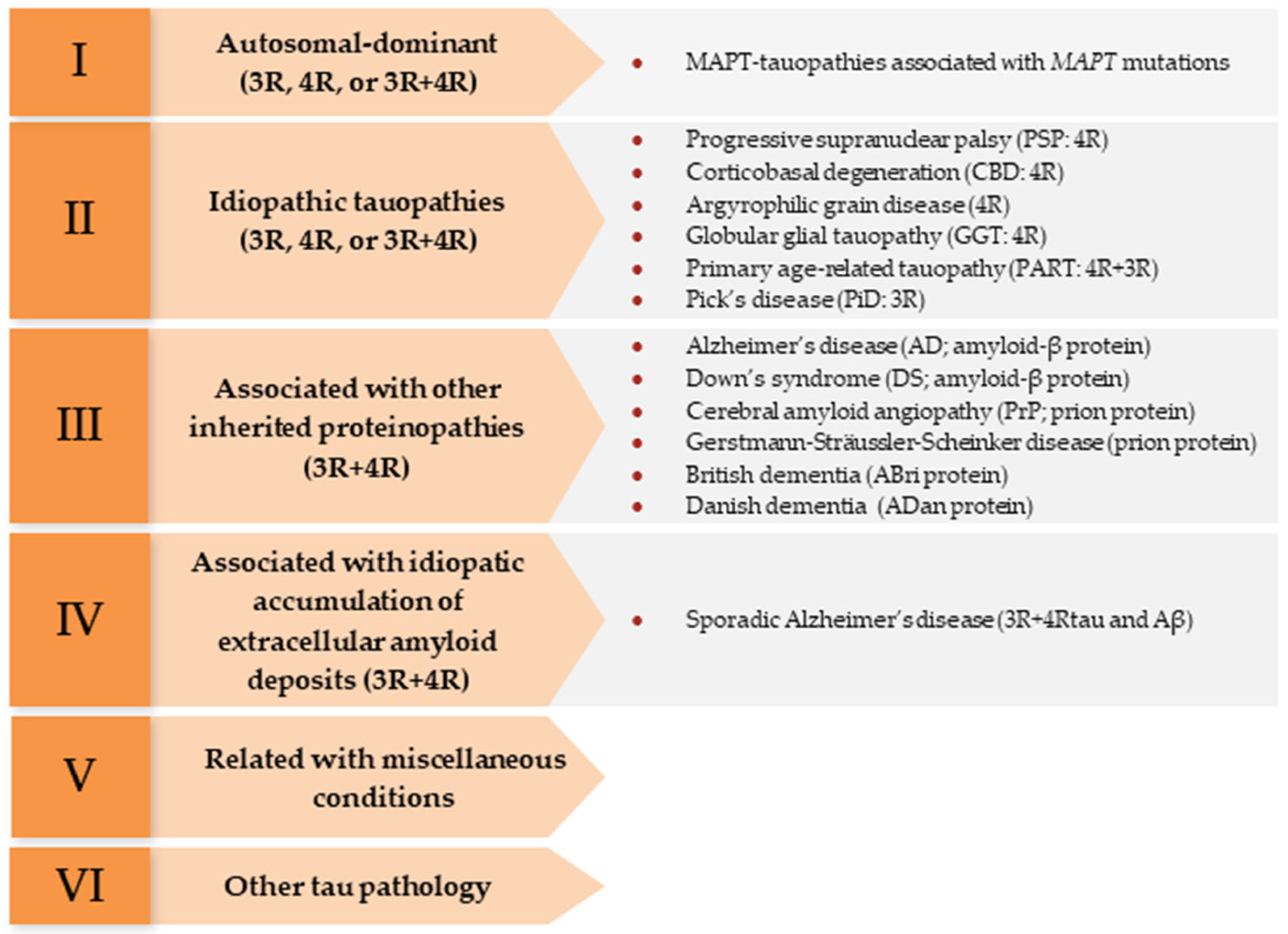
- Kovacs, G.G.; Ghetti, B.; Goedert, M. Classification of Diseases with Accumulation of Tau Protein. Neuropathol. Appl. Neurobiol. 2022, 48, e12792. [Google Scholar] [CrossRef] [PubMed]
- Ling, H. Untangling the Tauopathies: Current Concepts of Tau Pathology and Neurodegeneration. Park. Relat. Disord. 2018, 46, S34–S38. [Google Scholar] [CrossRef] [PubMed]
- Smolek, T.; Madari, A.; Farbakova, J.; Kandrac, O.; Jadhav, S.; Cente, M.; Brezovakova, V.; Novak, M.; Zilka, N. Tau Hyperphosphorylation in Synaptosomes and Neuroinflammation Are Associated with Canine Cognitive Impairment. J. Comp. Neurol. 2016, 524, 874–895. [Google Scholar] [CrossRef] [PubMed]
- Youssef, S.A.; Capucchio, M.T.; Rofina, J.E.; Chambers, J.K.; Uchida, K.; Nakayama, H.; Head, E. Pathology of the Aging Brain in Domestic and Laboratory Animals, and Animal Models of Human Neurodegenerative Diseases. Vet. Pathol. 2016, 53, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Gołaszewska, A.; Bik, W.; Motyl, T.; Orzechowski, A. Bridging the Gap between Alzheimer’s Disease and Alzheimer’s-like Diseases in Animals. Int. J. Mol. Sci. 2019, 20, 1664. [Google Scholar] [CrossRef]
- Malbon, A.J.; Sordo, L.; Wilson, L.A.; Gunn-Moore, D.; Paraschou, G.; Macintyre, N.; Schwarz, T.; McGorum, B.; Hahn, C. Alzheimer-like Pathology in the Parietal Cortex and Hippocampus of Aged Donkeys. Neurobiol. Aging 2022, 113, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Davies, E.S.; Morphew, R.M.; Cutress, D.; Morton, A.J.; McBride, S. Characterization of Microtubule-Associated Protein Tau Isoforms and Alzheimer’s Disease-like Pathology in Normal Sheep (Ovis Aries): Relevance to Their Potential as a Model of Alzheimer’s Disease. Cell. Mol. Life Sci. 2022, 79, 560. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, M.; Piccardo, P.; Ritchie, D.L.; Ironside, J.W.; Green, A.J.E.; McGovern, G. A Naturally Occurring Bovine Tauopathy Is Geographically Widespread in the UK. PLoS ONE 2015, 10, e0129499. [Google Scholar] [CrossRef][Green Version]
- Head, E.; Moffat, K.; Das, P.; Sarsoza, F.; Poon, W.W.; Landsberg, G.; Cotman, C.W.; Murphy, M.P. β-Amyloid Deposition and Tau Phosphorylation in Clinically Characterized Aged Cats. Neurobiol. Aging 2005, 26, 749–763. [Google Scholar] [CrossRef]
- Gunn-Moore, D.A.; McVee, J.; Bradshaw, J.M.; Pearson, G.R.; Head, E.; Gunn-Moore, F.J. Ageing Changes in Cat Brains Demonstrated by β-Amyloid and AT8-Immunoreactive Phosphorylated Tau Deposits. J. Feline Med. Surg. 2006, 8, 234–242. [Google Scholar] [CrossRef]
- Chambers, J.K.; Tokuda, T.; Uchida, K.; Ishii, R.; Tatebe, H.; Takahashi, E.; Tomiyama, T.; Une, Y.; Nakayama, H. The Domestic Cat as a Natural Animal Model of Alzheimer’s Disease. Acta Neuropathol. Commun. 2015, 3, 78. [Google Scholar] [CrossRef] [PubMed]
- Fiock, K.L.; Smith, J.D.; Crary, J.F.; Hefti, M.M. Β-Amyloid and Tau Pathology in the Aging Feline Brain. J. Comp. Neurol. 2019, 528, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, L.; Ando, K.; Vergara, C.; Mansour, S.; Suain, V.; Yilmaz, Z.; Reygel, A.; Gilissen, E.; Brion, J.P.; Leroy, K. A 4R Tauopathy Develops without Amyloid Deposits in Aged Cat Brains. Neurobiol. Aging 2019, 81, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Sordo, L.; Martini, A.C.; Houston, E.F.; Head, E.; Gunn-Moore, D. Neuropathology of Aging in Cats and Its Similarities to Human Alzheimer’s Disease. Front. Aging 2021, 2, 43–54. [Google Scholar] [CrossRef]
- Pakozdy, A.; Angerer, C.; Klang, A.; König, E.H.; Probst, A. Gyration of the Feline Brain: Localization, Terminology and Variability. J. Vet. Med. Ser. C Anat. Histol. Embryol. 2015, 44, 422–427. [Google Scholar] [CrossRef]
- Gallo, D.; Ruiz, A.; Sánchez-Juan, P. Genetic Architecture of Primary Tauopathies. Neuroscience 2022, 518, 27–37. [Google Scholar] [CrossRef]
- Riederer, B.M.; Mourton-Gilles, C.; Frey, P.; Delacourte, A.; Probst, A. Differential Phosphorylation of Tau Proteins during Kitten Brain Development and Alzheimer’s Disease. J. Neurocytol. 2001, 30, 145–158. [Google Scholar] [CrossRef]
- Landsberg, G.M.; Nichol, J.; Araujo, J.A. Cognitive Dysfunction Syndrome. A Disease of Canine and Feline Brain Aging. Vet. Clin. North Am. Small Anim. Pract. 2012, 42, 749–768. [Google Scholar] [CrossRef]
- Sordo, L.; Gunn-Moore, D.A. Cognitive Dysfunction in Cats: Update on Neuropathological and Behavioural Changes Plus Clinical Management. Vet. Rec. 2021, 188, 30–41. [Google Scholar] [CrossRef]
- Landsberg, G.M.; Mad’ari, A.; Žilka, N. Canine and Feline Dementia: Molecular Basis, Diagnostics and Therapy; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; ISBN 9783319532196. [Google Scholar]
- Hwang, K.; Vaknalli, R.N.; Addo-Osafo, K.; Vicente, M.; Vossel, K. Tauopathy and Epilepsy Comorbidities and Underlying Mechanisms. Front. Aging Neurosci. 2022, 14, 903973. [Google Scholar] [CrossRef]
- Ferrer, I.; Blanco, R.; Carmona, M.; Ribera, R.; Goutan, E.; Puig, B.; Rey, M.J.; Cardozo, A.; Viñals, F.; Ribalta, T. Phosphorylated Map Kinase (ERK1, ERK2) Expression Is Associated with Early Tau Deposition in Neurones and Glial Cells, but Not with Increased Nuclear DNA Vulnerability and Cell Death, in Alzheimer Disease, Pick’s Disease, Progressive Supranuclear Palsy An. Brain Pathol. 2001, 11, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Rovira, M.B.; Guerra, M.L.S.; Rey, M.J.; Costa-Jussá, F. Neuropathology and Pathogenesis of Encephalitis Following Amyloid β Immunization in Alzheimer’s Disease. Brain Pathol. 2004, 14, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kannanayakal, T.J.; Tao, H.; Vandre, D.D.; Kuret, J. Casein Kinase-1 Isoforms Differentially Associate with Neurofibrillary and Granulovacuolar Degeneration Lesions. Acta Neuropathol. 2006, 111, 413–421. [Google Scholar] [CrossRef]
- Andrés-Benito, P.; Carmona, M.; Pirla, M.J.; Torrejón-Escribano, B.; del Rio, J.A.; Ferrer, I. Dysregulated Protein Phosphorylation as Main Contributor of Granulovacuolar Degeneration at the First Stages of Neurofibrillary Tangles Pathology. Neuroscience 2021, 518, 119–140. [Google Scholar] [CrossRef]
- Köhler, C. Granulovacuolar Degeneration: A Neurodegenerative Change That Accompanies Tau Pathology. Acta Neuropathol. 2016, 132, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Taguchi, K.; Tanaka, M. Ubiquitin, Autophagy and Neurodegenerative Diseases. Cells 2020, 9, 2022. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.F.; Gan, Z.Y.; Komander, D.; Dewson, G. Ubiquitin Signalling in Neurodegeneration: Mechanisms and Therapeutic Opportunities. Cell Death Differ. 2021, 28, 570–590. [Google Scholar] [CrossRef]
- Sala-Jarque, J.; Zimkowska, K.; Ávila, J.; Ferrer, I.; del Río, J.A. Towards a Mechanistic Model of Tau-Mediated Pathology in Tauopathies: What Can We Learn from Cell-Based In Vitro Assays? Int. J. Mol. Sci. 2022, 23, 527. [Google Scholar] [CrossRef]

| Subjects | Antibody | Supplier | Reference | Host | Dilution |
|---|---|---|---|---|---|
| General markers | |||||
| All subjects | NeuN | Millipore | MAB377 | Ms | 1/100 |
| GFAP (glial fibrillary acidic protein) | Diagnostic BioSystems | RP014 | Rb | 1/400 | |
| Olig2 | Merck, Darmstadt | AB9610 | Rb | 1/500 | |
| Iba1 | Wako | 019-19741 | Rb | 1/1000 | |
| NFL-P Ser473 | Millipore | MABN2431 | Ms | 1/100 | |
| Calbindin D28K | Sigma | C8666 | Ms | 1/800 | |
| MBP (myelin basic protein) | Merck, Darmstadt | AB980 | Rb | 1/400 | |
| Protein deposits | |||||
| All subjects | 3Rtau | Upstate | 05-803 | Ms | 1/800 |
| 4Rtau | Millipore | 05-804 | Ms | 1/50 | |
| tau-P Thr181 | signalway | 11107 | Rb | 1/50 | |
| tau AT8, tau-P Ser202/Thr205 | Innogenetics | 90206 | Ms | 1/50 | |
| β-amyloid | Dako | M0872 | Ms | 1/50 | |
| Primary tauopathy cat | tau 5 | Thermo Scientific | MA5-12808 | Ms | - |
| tau-P Ser262 | Biosource | 44-7506 | Rb | 1/100 | |
| PHF1, tau-P Ser396/Ser404 | Dr. Peter Davies | Ms | 1/250 | ||
| tau-P Ser422 | Thermo Scientific | 44764 | Rb | 1/50 | |
| tau-C3 | Millipore | 36-017 | Ms | 1/100 | |
| tau-N Tyr29 | Millipore | MAB2244 | Ms | 1/200 | |
| MAP2-P Thr1620/1623 | Cell Signaling | 4544 | Rb | 1/100 | |
| Ubiquitin | Dako | Z0458 | Ms | 1/250 | |
| p62 | Transduction | 610833 | Ms | 1/100 | |
| CK1-δ | Abcam | ab85320 | Ms | 1/500 | |
| P38-P Thr180/Tyr182 | Cell Signaling | 9211 | Rb | 1/100 | |
| SAPK/JNK-P Thr183/Thr185 | Cell Signaling | 9251 | Rb | 1/25 | |
| TDP-43-P | Millipore | MABN14 | Rat | 1/100 | |
| Cat Number | Breed | Age (y) | Phosphorylated Tau |
|---|---|---|---|
| 1 | European | 1 | − |
| 2 | European | 3 | − |
| 3 | Unknown | 4 | − |
| 4 | Siamese | 5 | − |
| 5 | European | 6 | − |
| 6 | European | 9 | − |
| 7 | European | 9 | − |
| 8 | European | 10 | − |
| 9 | European | 10 | + |
| 10 | Persian | 11 | − |
| 11 | Persian | 12 | − |
| 12 | Siamese | 15 | − |
| 13 | Unknown | 17 | − |
| 14 | European | 17 | − |
| 15 | Siamese | 18 | + * |
| 16 | European | 21 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal-Palencia, L.; Font, C.; Rebollada-Merino, A.; Santpere, G.; Andrés-Benito, P.; Ferrer, I.; Pumarola, M. Primary Feline Tauopathy: Clinical, Morphological, Immunohistochemical, and Genetic Studies. Animals 2023, 13, 2985. https://doi.org/10.3390/ani13182985
Vidal-Palencia L, Font C, Rebollada-Merino A, Santpere G, Andrés-Benito P, Ferrer I, Pumarola M. Primary Feline Tauopathy: Clinical, Morphological, Immunohistochemical, and Genetic Studies. Animals. 2023; 13(18):2985. https://doi.org/10.3390/ani13182985
Chicago/Turabian StyleVidal-Palencia, Laura, Cristina Font, Agustín Rebollada-Merino, Gabriel Santpere, Pol Andrés-Benito, Isidro Ferrer, and Martí Pumarola. 2023. "Primary Feline Tauopathy: Clinical, Morphological, Immunohistochemical, and Genetic Studies" Animals 13, no. 18: 2985. https://doi.org/10.3390/ani13182985
APA StyleVidal-Palencia, L., Font, C., Rebollada-Merino, A., Santpere, G., Andrés-Benito, P., Ferrer, I., & Pumarola, M. (2023). Primary Feline Tauopathy: Clinical, Morphological, Immunohistochemical, and Genetic Studies. Animals, 13(18), 2985. https://doi.org/10.3390/ani13182985








