Phenylalanine Plays Important Roles in Regulating the Capacity of Intestinal Immunity, Antioxidants and Apoptosis in Largemouth Bass (Micropterus salmoides)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Experimental Procedures
2.3. Sample Collection and Experimental Treatment
2.4. Laboratory Determination
2.5. Statistical Analysis
3. Results
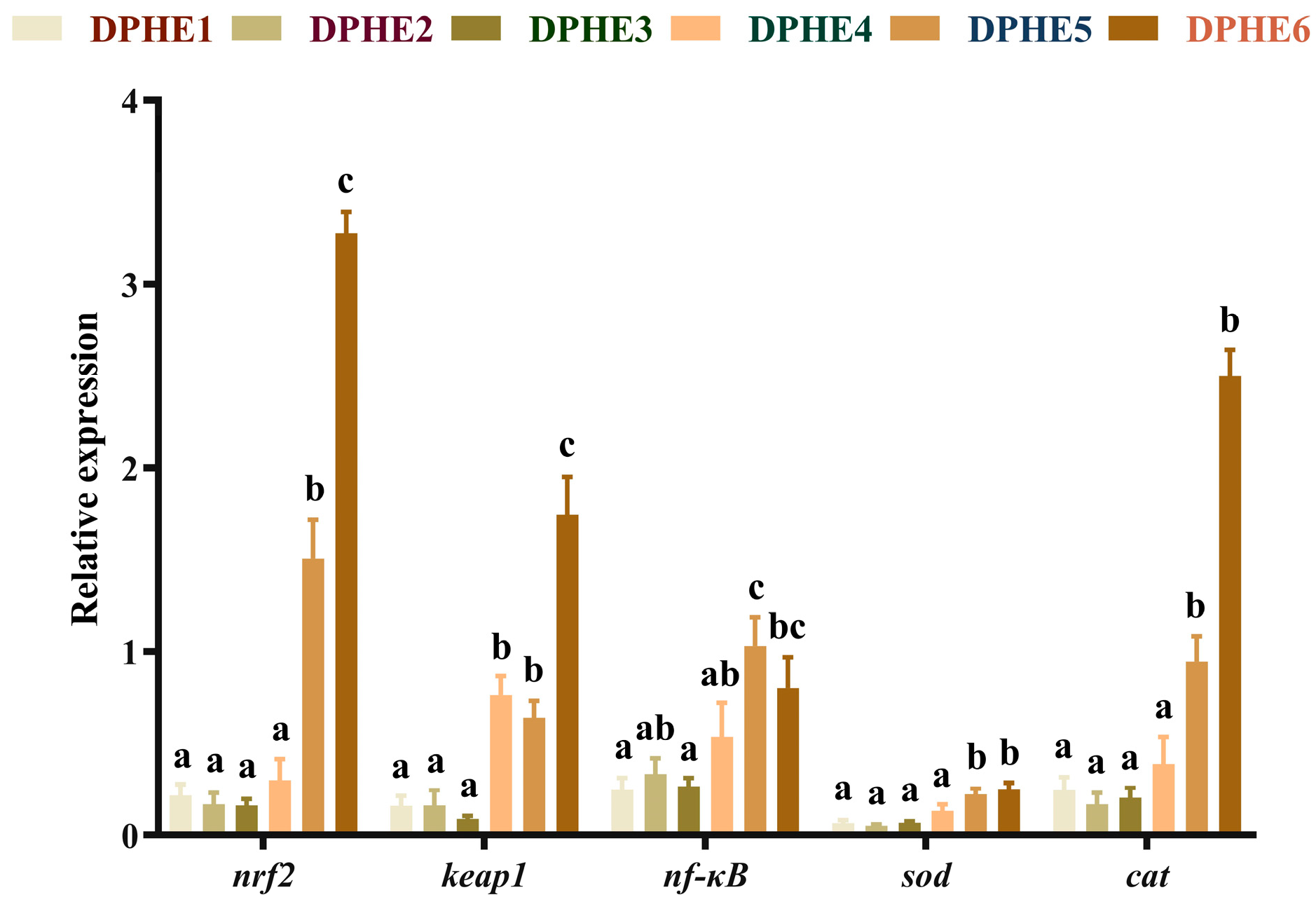
3.1. Intestinal Antioxidant Indices and Plasma Biochemical Indices
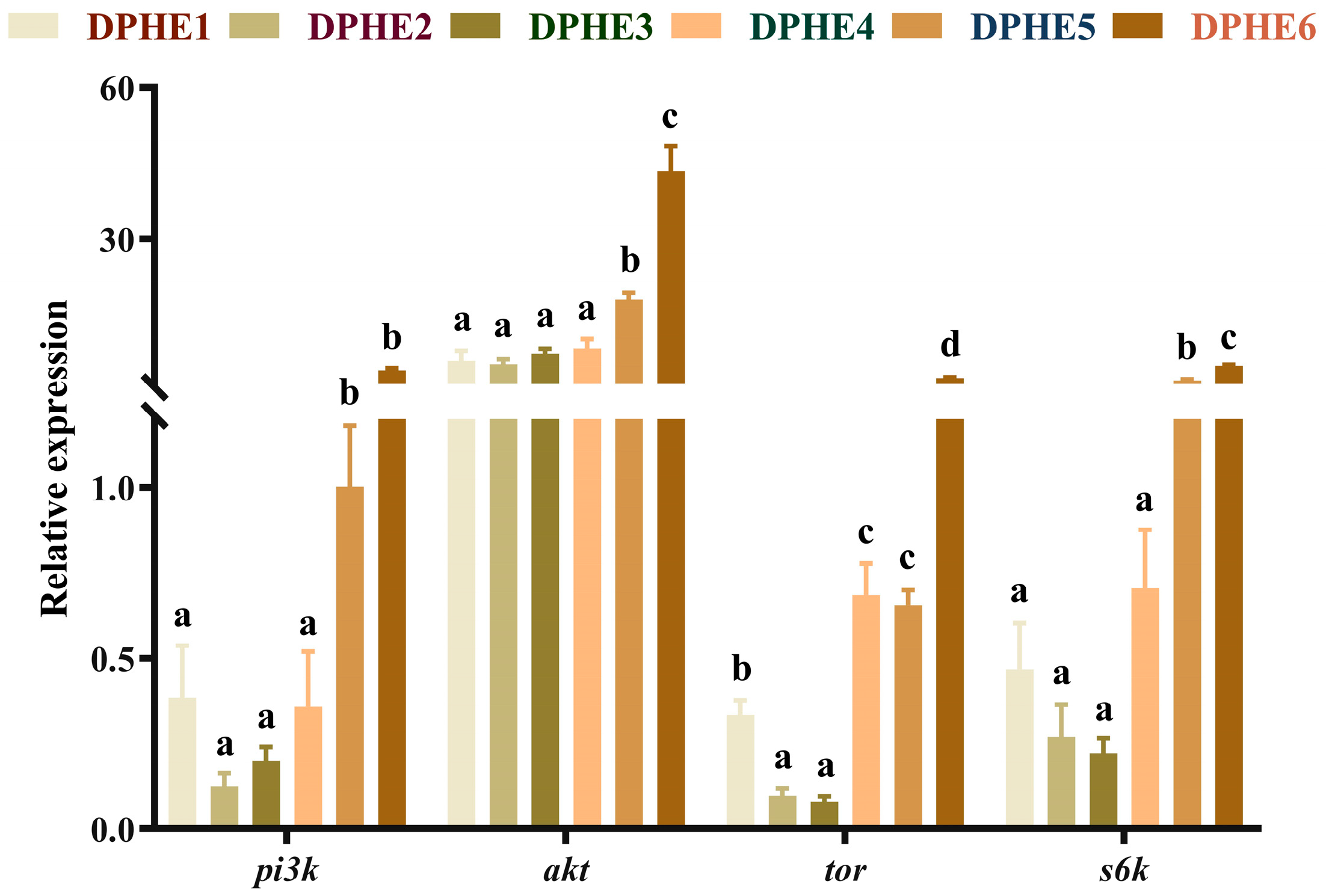
3.2. Intestinal Gene Expression of the TOR Pathway
3.3. The Expression Results of Immune-Related Genes
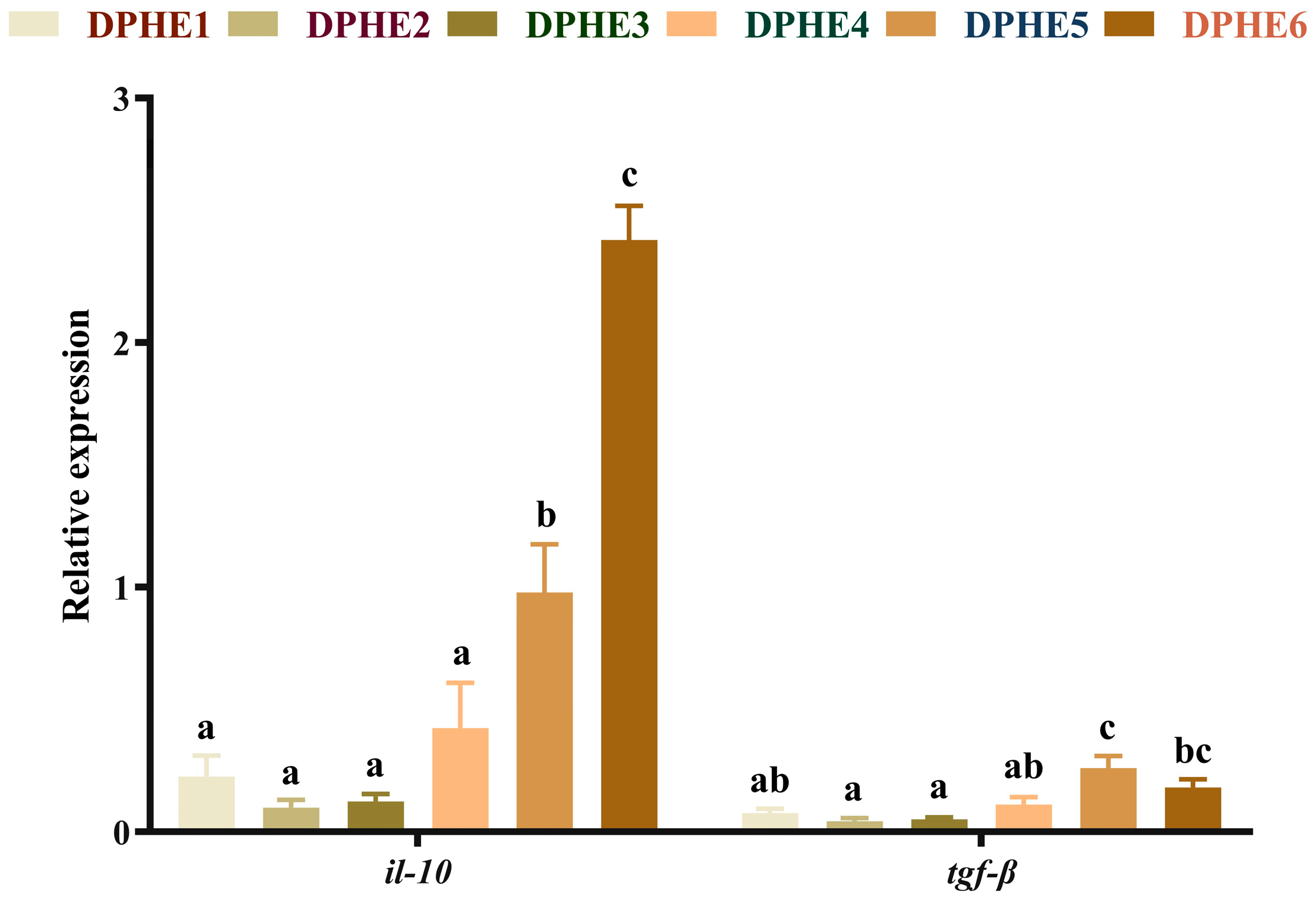
3.4. The Expression Results of Inflammatory Factors
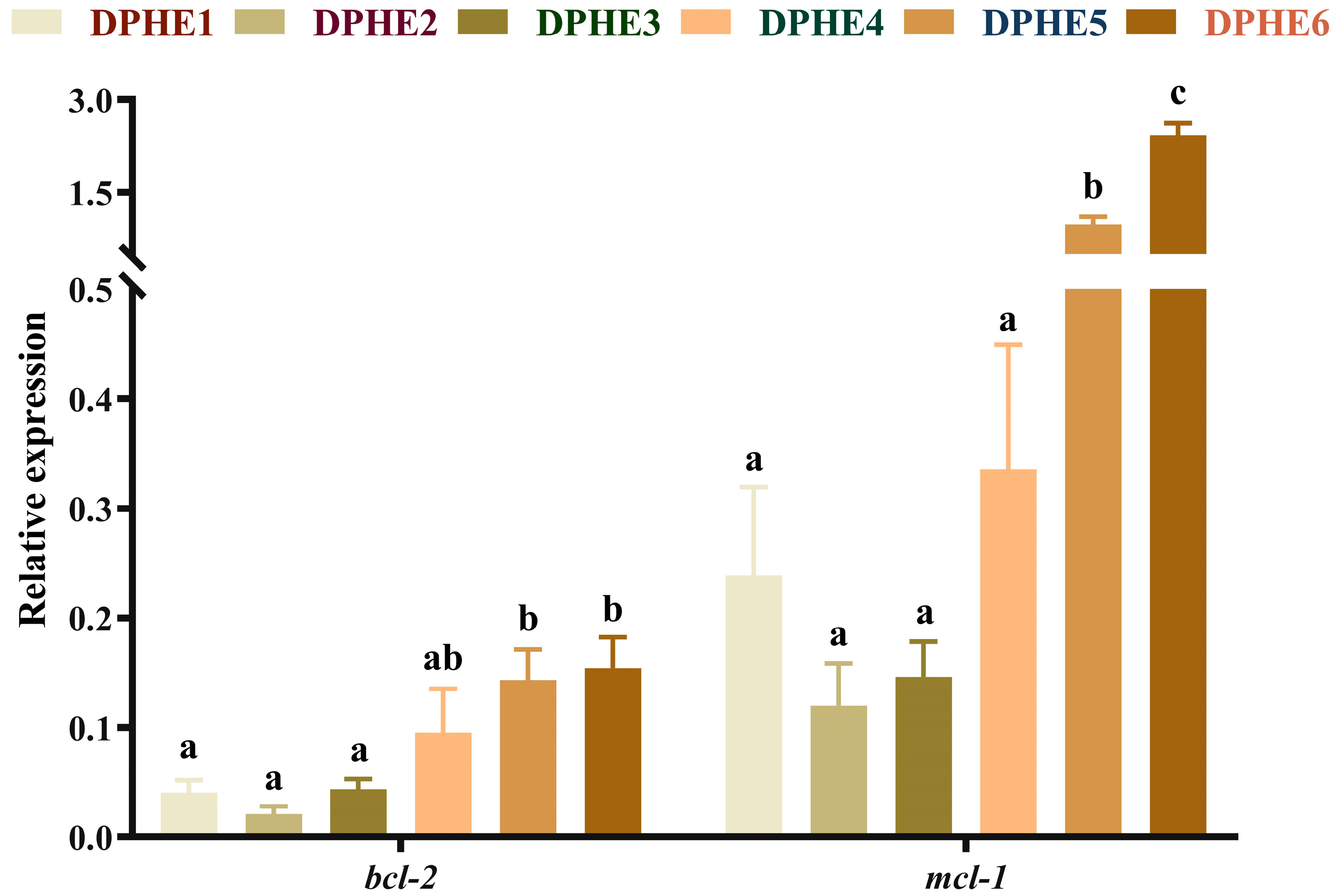
3.5. The Expression Results of Apoptosis-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bogard, J.R.; Farmery, A.K.; Little, D.C.; Fulton, E.A.; Cook, M. Will Fish Be Part of Future Healthy and Sustainable Diets? Lancet Planet. Health 2019, 3, e159–e160. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture of the People’s Republic of China. Chinese Fishery Statistical Yearbook 2022; Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2022. [Google Scholar]
- Ellison, A.R.; Uren Webster, T.M.; Rodriguez-Barreto, D.; de Leaniz, C.G.; Consuegra, S.; Orozco-terWengel, P.; Cable, J. Comparative Transcriptomics Reveal Conserved Impacts of Rearing Density on Immune Response of Two Important Aquaculture Species. Fish Shellfish. Immunol. 2020, 104, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Rohani, M.F.; Islam, S.M.; Hossain, M.K.; Ferdous, Z.; Siddik, M.A.B.; Nuruzzaman, M.; Padeniya, U.; Brown, C.; Shahjahan, M. Probiotics, Prebiotics and Synbiotics Improved the Functionality of Aquafeed: Upgrading Growth, Reproduction, Immunity and Disease Resistance in Fish. Fish Shellfish. Immunol. 2022, 120, 569–589. [Google Scholar] [CrossRef]
- He, Z.; Cheng, X.; Kyzas, G.Z.; Fu, J. Pharmaceuticals Pollution of Aquaculture and Its Management in China. J. Mol. Liq. 2016, 223, 781–789. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Yuan, Y.; Xie, Y. A Systematic Review on Antibiotics Misuse in Livestock and Aquaculture and Regulation Implications in China. Sci. Total Environ. 2021, 798, 149205. [Google Scholar] [CrossRef] [PubMed]
- Pohlenz, C.; Gatlin, D.M. Interrelationships between Fish Nutrition and Health. Aquaculture 2014, 431, 111–117. [Google Scholar] [CrossRef]
- Kelly, B.; Pearce, E.L. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011; p. 13039. ISBN 978-0-309-16338-5. [Google Scholar]
- Udenfriend, S.; Cooper, J.R. The Enzymatic Conversion of Phenylalanine to Tyrosine. J. Biol. Chem. 1952, 194, 503–511. [Google Scholar] [CrossRef]
- Ryan, W.L. Inhibition of the Immune Response by Phenylalanine: Application to Skin Transplantation. JAMA 1965, 191, 295. [Google Scholar] [CrossRef]
- Haubeck, H.D.; Lorkowski, G.; Kölsch, E.; Röschenthaler, R. Immunosuppression by Ochratoxin A and Its Prevention by Phenylalanine. Appl. Environ. Microbiol. 1981, 41, 1040–1042. [Google Scholar] [CrossRef]
- Feng, L.; Li, W.; Liu, Y.; Jiang, W.-D.; Kuang, S.-Y.; Jiang, J.; Tang, L.; Wu, P.; Tang, W.-N.; Zhang, Y.-A.; et al. Dietary Phenylalanine-Improved Intestinal Barrier Health in Young Grass Carp (Ctenopharyngodon Idella) Is Associated with Increased Immune Status and Regulated Gene Expression of Cytokines, Tight Junction Proteins, Antioxidant Enzymes and Related Signalling Molecules. Fish Shellfish. Immunol. 2015, 45, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, W.; Liu, Y.; Jiang, W.-D.; Kuang, S.-Y.; Wu, P.; Jiang, J.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; et al. Protective Role of Phenylalanine on the ROS-Induced Oxidative Damage, Apoptosis and Tight Junction Damage via Nrf2, TOR and NF-ΚB Signalling Molecules in the Gill of Fish. Fish Shellfish. Immunol. 2017, 60, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Gong, Q.; Lai, S.; Cheng, Z.; Chen, Z.; Zheng, J.; Peng, B. Phenylalanine Enhances Innate Immune Response to Clear Ceftazidime-Resistant Vibrio alginolyticus in Danio Rerio. Fish Shellfish. Immunol. 2019, 84, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Wretlind, K.A.J. The Availability for Growth and the Toxicity of L- and D-Phenylalanine. Acta Physiol. Scand. 1952, 25, 276–285. [Google Scholar] [CrossRef]
- Antuna-Puente, B.; Fellahi, S.; McAvoy, C.; Fève, B.; Bastard, J.-P. Interleukins in Adipose Tissue: Keeping the Balance. Mol. Cell. Endocrinol. 2022, 542, 111531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Liu, S.; Zhang, S.; Lu, S.; Liu, H.; Han, S.; Jiang, H.; Zhang, Y. Effects of Dietary Arginine on Growth Performance, Digestion, Absorption Ability, Antioxidant Capability, Gene Expression of Intestinal Protein Synthesis, and Inflammation-Related Genes of Triploid Juvenile Oncorhynchus Mykiss Fed a Low-Fishmeal Diet. Aquac. Nutr. 2022, 2022, 3793727. [Google Scholar] [CrossRef]
- Liang, H.; Mokrani, A.; Ji, K.; Ge, X.; Ren, M.; Pan, L.; Sun, A. Effects of Dietary Arginine on Intestinal Antioxidant Status and Immunity Involved in Nrf2 and NF-ΚB Signaling Pathway in Juvenile Blunt Snout Bream, Megalobrama amblycephala. Fish Shellfish. Immunol. 2018, 82, 243–249. [Google Scholar] [CrossRef]
- Xu, C.; Huang, X.-P.; Guan, J.-F.; Chen, Z.-M.; Ma, Y.-C.; Xie, D.-Z.; Ning, L.-J.; Li, Y.-Y. Effects of Dietary Leucine and Valine Levels on Growth Performance, Glycolipid Metabolism and Immune Response in Tilapia GIFT Oreochromis niloticus. Fish Shellfish. Immunol. 2022, 121, 395–403. [Google Scholar] [CrossRef]
- Yang, P.; Wang, W.; Chi, S.; Mai, K.; Song, F.; Wang, L. Effects of Dietary Lysine on Regulating GH-IGF System, Intermediate Metabolism and Immune Response in Largemouth Bass (Micropterus salmoides). Aquac. Rep. 2020, 17, 100323. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Zhang, J.; Liu, R.; Zhao, H.; Shan, S.; Yang, G. Identification of Three Inflammatory Caspases in Common Carp (Cyprinus carpio L.) and Its Role in Immune Response against Bacterial Infection. Fish Shellfish. Immunol. 2022, 131, 590–601. [Google Scholar] [CrossRef]
- Yamada, H.; Arai, T.; Endo, N.; Yamashita, K.; Fukuda, K.; Sasada, M.; Uchiyama, T. LPS-Induced ROS Generation and Changes in Glutathione Level and Their Relation to the Maturation of Human Monocyte-Derived Dendritic Cells. Life Sci. 2006, 78, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Guo, Q.; Duan, X.; Xu, Z.; Wang, Q. L-Arginine Inhibited Apoptosis of Fish Leukocytes via Regulation of NF-ΚB-Mediated Inflammation, NO Synthesis, and Anti-Oxidant Capacity. Biochimie 2019, 158, 62–72. [Google Scholar] [CrossRef]
- Pan, F.-Y.; Feng, L.; Jiang, W.-D.; Jiang, J.; Wu, P.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; Zhou, X.-Q.; et al. Methionine Hydroxy Analogue Enhanced Fish Immunity via Modulation of NF-ΚB, TOR, MLCK, MAPKs and Nrf2 Signaling in Young Grass Carp (Ctenopharyngodon idella). Fish Shellfish. Immunol. 2016, 56, 208–228. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhu, W.; Chen, T.; Cui, W.; Li, X.; Xu, S. Paraquat Induces Apoptosis, Programmed Necrosis, and Immune Dysfunction in CIK Cells via the PTEN/PI3K/AKT Axis. Fish Shellfish. Immunol. 2022, 130, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, X.; Wang, L.; Du, C.; Hou, C.; Liu, F.; Xie, Q.; Lou, B.; Jin, S.; Zhu, J. Establishment of the First Cell Line from the Small Yellow Croaker (Larimichthys polyactis) and Its Application in Unraveling the Mechanism of ROS-Induced Apoptosis under Hypoxia. Aquaculture 2023, 563, 738900. [Google Scholar] [CrossRef]
- Yang, S.; Luo, J.; Huang, Y.; Yuan, Y.; Cai, S. Effect of Sub-Lethal Ammonia and Nitrite Stress on Autophagy and Apoptosis in Hepatopancreas of Pacific Whiteleg Shrimp Litopenaeus vannamei. Fish Shellfish. Immunol. 2022, 130, 72–78. [Google Scholar] [CrossRef]
- Bai, J.; Lutz-Carrillo, D.J.; Quan, Y.; Liang, S. Taxonomic Status and Genetic Diversity of Cultured Largemouth Bass Micropterus salmoides in China. Aquaculture 2008, 278, 27–30. [Google Scholar] [CrossRef]
- Rahman, M.M.; Li, X.; Sharifuzzaman, S.M.; He, M.; Poolsawat, L.; Yang, H.; Leng, X. Dietary Threonine Requirement of Juvenile Largemouth Bass, Micropterus salmoides. Aquaculture 2021, 543, 736884. [Google Scholar] [CrossRef]
- Montelongo-Alfaro, I.O.; Amador-Cano, G.; Rábago-Castro, J.L.; Sánchez-Martínez, J.G.; Benavides-González, F.; Gojon-Báez, H.H. A Preliminary Study of Largemouth Bass Virus in Mexico. J. Wildl. Dis. 2019, 55, 516. [Google Scholar] [CrossRef]
- Woodland, J.E.; Brunner, C.J.; Noyes, A.D.; Grizzle, J.M. Experimental Oral Transmission of Largemouth Bass Virus. J. Fish Dis. 2002, 25, 669–672. [Google Scholar] [CrossRef]
- Yi, C.; Liang, H.; Xu, G.; Zhu, J.; Wang, Y.; Li, S.; Ren, M.; Chen, X. Appropriate Dietary Phenylalanine Improved Growth, Protein Metabolism and Lipid Metabolism, and Glycolysis in Largemouth Bass (Micropterus salmoides). Fish Physiol. Biochem. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Liang, H.; Ge, X.; Xia, D.; Pan, L.; Mi, H.; Ren, M. A Study of the Potential Effect of Yellow Mealworm (Tenebrio Molitor) Substitution for Fish Meal on Growth, Immune and Antioxidant Capacity in Juvenile Largemouth Bass (Micropterus Salmoides). Fish Shellfish. Immunol. 2022, 120, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Martyniuk, C.J.; Spade, D.J.; Blum, J.L.; Kroll, K.J.; Denslow, N.D. Methoxychlor Affects Multiple Hormone Signaling Pathways in the Largemouth Bass (Micropterus Salmoides) Liver. Aquat. Toxicol. 2011, 101, 483–492. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, J.; Chen, F.; Tang, X.; Liao, L.; Liu, Q.; Luo, J.; Du, Z.; Li, Z.; Luo, W.; et al. High Carbohydrate Diet Induced Endoplasmic Reticulum Stress and Oxidative Stress, Promoted Inflammation and Apoptosis, Impaired Intestinal Barrier of Juvenile Largemouth Bass (Micropterus Salmoides). Fish Shellfish. Immunol. 2021, 119, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Kiron, V. Fish Immune System and Its Nutritional Modulation for Preventive Health Care. Anim. Feed. Sci. Technol. 2012, 173, 111–133. [Google Scholar] [CrossRef]
- Mauel, M.J.; Miller, D.L.; Merrill, A.L. Hematologic and Plasma Biochemical Values of Healthy Hybrid Tilapia (Oreochromis aureus × Oreochromis nilotica) Maintained in a Recirculating System. J. Zoo Wildl. Med. 2007, 38, 420–424. [Google Scholar] [CrossRef]
- Peng, Z.; Yan, L.; Wei, L.; Gao, X.; Shi, L.; Ren, T.; Wang, W.; Han, Y. Effect of Dietary Chicken Gut Meal Levels on Growth Performance, Plasma Biochemical Parameters, Digestive Ability and Fillet Quality of Cyprinus Carpio. Aquac. Rep. 2022, 24, 101183. [Google Scholar] [CrossRef]
- Li, W.; Feng, L.; Liu, Y.; Jiang, W.-D.; Kuang, S.-Y.; Jiang, J.; Li, S.-H.; Tang, L.; Zhou, X.-Q. Effects of Dietary Phenylalanine on Growth, Digestive and Brush Border Enzyme Activities and Antioxidant Capacity in the Hepatopancreas and Intestine of Young Grass Carp (Ctenopharyngodon idella). Aquacult. Nutr. 2015, 21, 913–925. [Google Scholar] [CrossRef]
- Kumar, S.; Sahu, N.P.; Pal, A.K.; Sagar, V.; Sinha, A.K.; Baruah, K. Modulation of Key Metabolic Enzyme of Labeo Rohita (Hamilton) Juvenile: Effect of Dietary Starch Type, Protein Level and Exogenous α-Amylase in the Diet. Fish Physiol. Biochem. 2009, 35, 301–315. [Google Scholar] [CrossRef]
- Michelato, M.; Furuya, W.M.; Graciano, T.S.; Vidal, L.V.O.; Xavier, T.O.; de Moura, L.B.; Furuya, V.R.B. Digestible Methionine + Cystine Requirement for Nile Tilapia from 550 to 700 g. R. Bras. Zootec. 2013, 42, 7–12. [Google Scholar] [CrossRef]
- Busch, C.J.; Binder, C.J. Malondialdehyde Epitopes as Mediators of Sterile Inflammation. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 398–406. [Google Scholar] [CrossRef]
- Liang, H.; Xu, P.; Xu, G.; Zhang, L.; Huang, D.; Ren, M.; Zhang, L. Histidine Deficiency Inhibits Intestinal Antioxidant Capacity and Induces Intestinal Endoplasmic-Reticulum Stress, Inflammatory Response, Apoptosis, and Necroptosis in Largemouth Bass (Micropterus salmoides). Antioxidants 2022, 11, 2399. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Wang, C.; Zhao, Z.; Luo, L.; Du, X.; Xu, Q. Effect of N-Carbamoylglutamate Supplementation on the Growth Performance, Antioxidant Status and Immune Response of Mirror Carp (Cyprinus carpio) Fed an Arginine-Deficient Diet. Fish Shellfish. Immunol. 2019, 84, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Zou, Z.; Li, D.; Zhu, J.; Yue, Y.; Yang, H. Effect of Dietary Phenylalanine Level on Growth Performance, Body Composition, and Biochemical Parameters in Plasma of Juvenile Hybrid Tilapia, Oreochromis niloticus × Oreochromis aureus. J. World Aquacult. Soc. 2020, 51, 437–451. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Z.; Misra, B.R.; Zhang, L.; Cao, Z.; Yamamoto, M.; Trush, M.A.; Misra, H.P.; Li, Y. Nuclear Factor E2-Related Factor 2-Dependent Myocardiac Cytoprotection Against Oxidative and Electrophilic Stress. Cardiovasc. Toxicol. 2008, 8, 71–85. [Google Scholar] [CrossRef]
- Kobayashi, M.; Itoh, K.; Suzuki, T.; Osanai, H.; Nishikawa, K.; Katoh, Y.; Takagi, Y.; Yamamoto, M. Identification of the Interactive Interface and Phylogenic Conservation of the Nrf2-Keap1 System: Conserved Regulation of Cytoprotective Genes. Genes Cells 2002, 7, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xiao, J.-H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxidative Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, H.; Longshaw, M.; Wang, J.; Ge, X.; Zhu, J.; Li, S.; Ren, M. Effects of Replacing Fishmeal with Methanotroph (Methylococcus capsulatus, Bath) Bacteria Meal (FeedKind®) on Growth and Intestinal Health Status of Juvenile Largemouth Bass (Micropterus salmoides). Fish Shellfish. Immunol. 2022, 122, 298–305. [Google Scholar] [CrossRef]
- Weichhart, T.; Costantino, G.; Poglitsch, M.; Rosner, M.; Zeyda, M.; Stuhlmeier, K.M.; Kolbe, T.; Stulnig, T.M.; Hörl, W.H.; Hengstschläger, M.; et al. The TSC-MTOR Signaling Pathway Regulates the Innate Inflammatory Response. Immunity 2008, 29, 565–577. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation Meets Ubiquitination: The Control of NF-ΚB Activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New Developments in Fish Amino Acid Nutrition: Towards Functional and Environmentally Oriented Aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Chrousos, G.P. Stress Hormones, Proinflammatory and Antiinflammatory Cytokines, and Autoimmunity. Ann. N. Y. Acad. Sci. 2002, 966, 290–303. [Google Scholar] [CrossRef]
- Leonel, A.J.; Teixeira, L.G.; Oliveira, R.P.; Santiago, A.F.; Batista, N.V.; Ferreira, T.R.; Santos, R.C.; Cardoso, V.N.; Cara, D.C.; Faria, A.M.C.; et al. Antioxidative and Immunomodulatory Effects of Tributyrin Supplementation on Experimental Colitis. Br. J. Nutr. 2013, 109, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.-J.; Han, L.-H.; Cong, R.-S.; Liang, J. Caspase Family Proteases and Apoptosis. ABBS 2005, 37, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Shore, G.C.; Nguyen, M. Bcl-2 Proteins and Apoptosis: Choose Your Partner. Cell 2008, 135, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, X.; Yuan, X. Phenylalanine Activates the Mitochondria-Mediated Apoptosis through the RhoA/Rho-Associated Kinase Pathway in Cortical Neurons: RhoA Involved Apoptosis by Phenylalanine in Neurons. Eur. J. Neurosci. 2007, 25, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. Therapeutic Potential of Inhibition of the NF-ΚB Pathway in the Treatment of Inflammation and Cancer. J. Clin. Investig. 2001, 107, 135–142. [Google Scholar] [CrossRef]


| Ingredients (%) | |
|---|---|
| Fish meal a | 25 |
| Rapeseed meal a | 8 |
| Soybean meal a | 6 |
| Corn gluten meal a | 8 |
| Wheat flour a | 16 |
| Rice bran | 8 |
| Fish oil | 5 |
| Sleeve-Fish Ointment | 2 |
| Amino acid premix b | 13.02 |
| Choline chloride | 0.1 |
| Vitamin premix c | 1 |
| Mineral premix d | 1 |
| Calcium dihydrogen phosphate | 1 |
| Microcrystalline cellulose | 4.57 |
| Ethoxy quinoline | 0.01 |
| Glycine | * |
| Phenylalanine | ** |
| Vitamin C | 0.05 |
| Total | 100 |
| Actual phenylalanine level | *** |
| Actual tyrosine level | 1.32–1.34 |
| Gene | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) | Accession Number/Reference |
|---|---|---|---|
| pi3k | CTCACCATGGAGGATGGACC | ACGGTGGGAGTGGAGGTTTA | Cluster-21914.23096 |
| akt | AGCGCACCTTCCATGTAGAC | GGCTATTTGCCACTTGCTGG | AXE72881.1 |
| tor | CCATCCTCAACCTACTTCC | CTCTCCTTCTCCTTCTTCAG | Cluster-21914.16479 |
| s6k | GTAATGCAAAGGACACGGCG | GTTCCCCACCGCTCAGATAC | XP_010747297.3 |
| keap1 | CGTACGTCCAGGCCTTACTC | TGACGGAAATAACCCCCTGC | Cluster-21914.26115 |
| nrf2 | AGAGACATTCGCCGTAGA | TCGCAGTAGAGCAATCCT | NM_212855.2 |
| nf-κB | CCACTCAGGTGTTGGAGCTT | TCCAGAGCACGACACACTTC | Cluster-21914.7253 |
| il-10 | CGGCACAGAAATCCCAGAGC | CAGCAGGCTCACAAAATAAACATCT | Yang et al., 2020 [21] |
| tgf-β | GCTCAAAGAGAGCGAGGATG | TCCTCTACCATTCGCAATCC | [34] |
| il-1β | CGTGACTGACAGCAAAAAGAGG | GATGCCCAGAGCCACAGTTC | [34] |
| tnf-α | CTTCGTCTACAGCCAGGCATCG | TTTGGCACACCGACCTCACC | [34] |
| il-8 | TCGGTCCTCCTGGGTGAAAA | GTGCTCCTTCCTGCTGATGTA | Cluster-21914.20189 |
| sod | TGGCAAGAACAAGAACCACA | CCTCTGATTTCTCCTGTCACC | [35] |
| cat | CTATGGCTCTCACACCTTC | TCCTCTACTGGCAGATTCT | MK614708.1 |
| caspase-3s | GAGGCGATGGACAAGAGTCA | CACAGACGAATGAAGCGTGG | XM_038713063.1 |
| caspase-8 | GAGACAGACAGCAGACAACCA | TTCCATTTCAGCAAACACATC | [36] |
| caspase-9 | CTGGAATGCCTTCAGGAGACGGG | GGGAGGGGCAAGACAACAGGGTG | [36] |
| bax | ACTTTGGATTACCTGCGGGA | TGCCAGAAATCAGGAGCAGA | [36] |
| bcl-2 | CCAACGTCATGGTTGTCATGG | GTGGAGCCAACCAGGAATCT | Cluster-21914.31403 |
| mcl-1 | GTGGCCAACAATGAGAAGGC | AGGAGTCTCTGTTCGTCCGT | Cluster-21914.26326 |
| β-actin | ATGCAGAAGGAGATCACAGCCT | AGTATTTACGCTCAGGTGGGG | AF253319.1 |
| Parameters | Dietary Phenylalanine Group | |||||
|---|---|---|---|---|---|---|
| DPHE1 | DPHE2 | DPHE3 | DPHE4 | DPHE5 | DPHE6 | |
| T-SOD 2 (U/mgprot) | 4.75 ± 0.18 a | 5.79 ± 0.12 ab | 6.77 ± 0.10 bc | 7.29 ± 0.38 c | 6.46 ± 0.94 bc | 5.38 ± 0.66 ab |
| MDA 2 (nmol/mgprot) | 6.40 ± 0.94 b | 4.17 ± 0.16 a | 5.10 ± 0.60 ab | 6.68 ± 0.47 b | 6.99 ± 0.32 b | 7.18 ± 0.28 b |
| GSH 2 (umol/gprot) | 41.19 ± 2.59 a | 35.00 ± 2.21 a | 58.09 ± 4.22 bc | 80.84 ± 2.23 d | 67.21 ± 5.34 c | 46.92 ± 4.77 ab |
| T-AOC 2 (mmol/g) | 0.66 ± 0.05 ab | 0.75 ± 0.08 ab | 0.72 ± 0.03 ab | 0.80 ± 0.04 b | 0.68 ± 0.03 ab | 0.59 ± 0.03 a |
| CAT 2 (U/mgprot) | 74.78 ± 11.12 a | 81.27 ± 9.35 ab | 96.82 ± 9.12 ab | 98.21 ± 17.01 ab | 98.49 ± 13.68 ab | 130.41 ± 13.91 b |
| Parameters | Dietary Phenylalanine Group | |||||
|---|---|---|---|---|---|---|
| DPHE1 | DPHE2 | DPHE3 | DPHE4 | DPHE5 | DPHE6 | |
| ALP 2 (U/L) | 23.36 ± 1.49 a | 41.06 ± 3.68 b | 37.26 ± 2.51 b | 34.81 ± 4.00 ab | 37.50 ± 4.52 b | 27.32 ± 1.93 ab |
| ALB 2 (g/L) | 9.47 ± 0.40 a | 12.88 ± 0.44 b | 13.94 ± 1.14 b | 12.71 ± 0.58 b | 12.06 ± 0.76 b | 13.46 ± 0.60 b |
| ALT 2 (U/L) | 0.83 ± 0.16 a | 1.03 ± 0.20 ab | 1.14 ± 0.20 ab | 1.81 ± 0.27 b | 1.37 ± 0.28 ab | 1.08 ± 0.17 ab |
| AST 2 (U/L) | 18.38 ± 2.05 a | 21.18 ± 1.73 a | 22.48 ± 1.13 a | 30.02 ± 0.99 b | 23.02 ± 1.58 a | 19.93 ± 1.58 a |
| LDL-C 2 (mmol/L) | 0.94 ± 0.13 a | 0.93 ± 0.05 a | 1.00 ± 0.10 a | 0.92 ± 0.07 a | 0.91 ± 0.06 a | 1.15 ± 0.06 a |
| HDL-C 2 (mmol/L) | 2.49 ± 0.11 a | 3.62 ± 0.13 c | 3.66 ± 0.13 c | 3.36 ± 0.15 bc | 2.92 ± 0.13 ab | 3.59 ± 0.16 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, C.; Liang, H.; Huang, D.; Yu, H.; Xue, C.; Gu, J.; Chen, X.; Wang, Y.; Ren, M.; Zhang, L. Phenylalanine Plays Important Roles in Regulating the Capacity of Intestinal Immunity, Antioxidants and Apoptosis in Largemouth Bass (Micropterus salmoides). Animals 2023, 13, 2980. https://doi.org/10.3390/ani13182980
Yi C, Liang H, Huang D, Yu H, Xue C, Gu J, Chen X, Wang Y, Ren M, Zhang L. Phenylalanine Plays Important Roles in Regulating the Capacity of Intestinal Immunity, Antioxidants and Apoptosis in Largemouth Bass (Micropterus salmoides). Animals. 2023; 13(18):2980. https://doi.org/10.3390/ani13182980
Chicago/Turabian StyleYi, Changguo, Hualiang Liang, Dongyu Huang, Heng Yu, Chunyu Xue, Jiaze Gu, Xiaoru Chen, Yongli Wang, Mingchun Ren, and Lu Zhang. 2023. "Phenylalanine Plays Important Roles in Regulating the Capacity of Intestinal Immunity, Antioxidants and Apoptosis in Largemouth Bass (Micropterus salmoides)" Animals 13, no. 18: 2980. https://doi.org/10.3390/ani13182980
APA StyleYi, C., Liang, H., Huang, D., Yu, H., Xue, C., Gu, J., Chen, X., Wang, Y., Ren, M., & Zhang, L. (2023). Phenylalanine Plays Important Roles in Regulating the Capacity of Intestinal Immunity, Antioxidants and Apoptosis in Largemouth Bass (Micropterus salmoides). Animals, 13(18), 2980. https://doi.org/10.3390/ani13182980







