Consider the Source: The Impact of Social Mixing on Drylot Housed Steer Behavior and Productivity
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
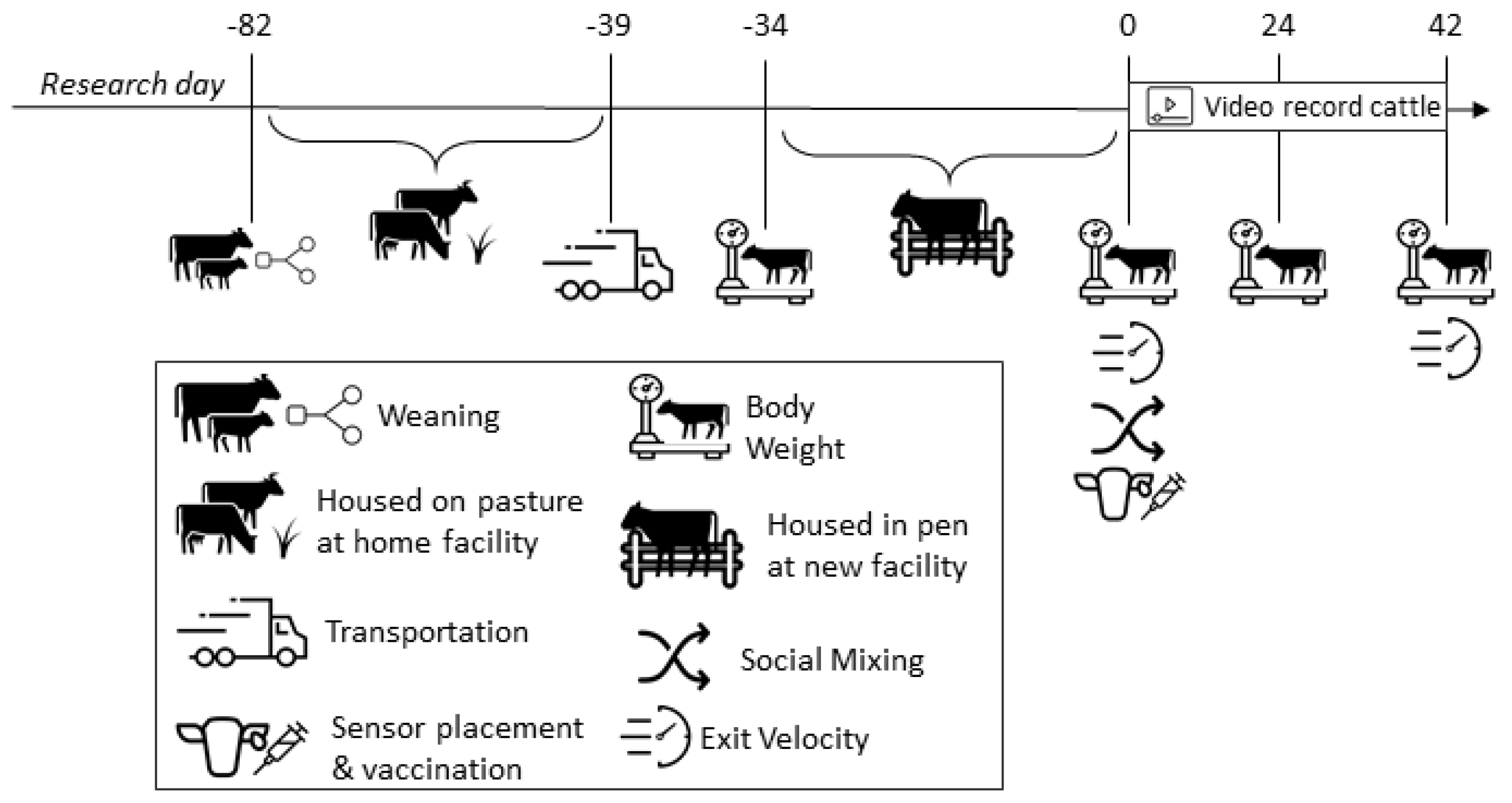
2.1. Animals and Housing
2.2. Data Collection
2.3. Statistical Analyses
3. Results
3.1. Productivity
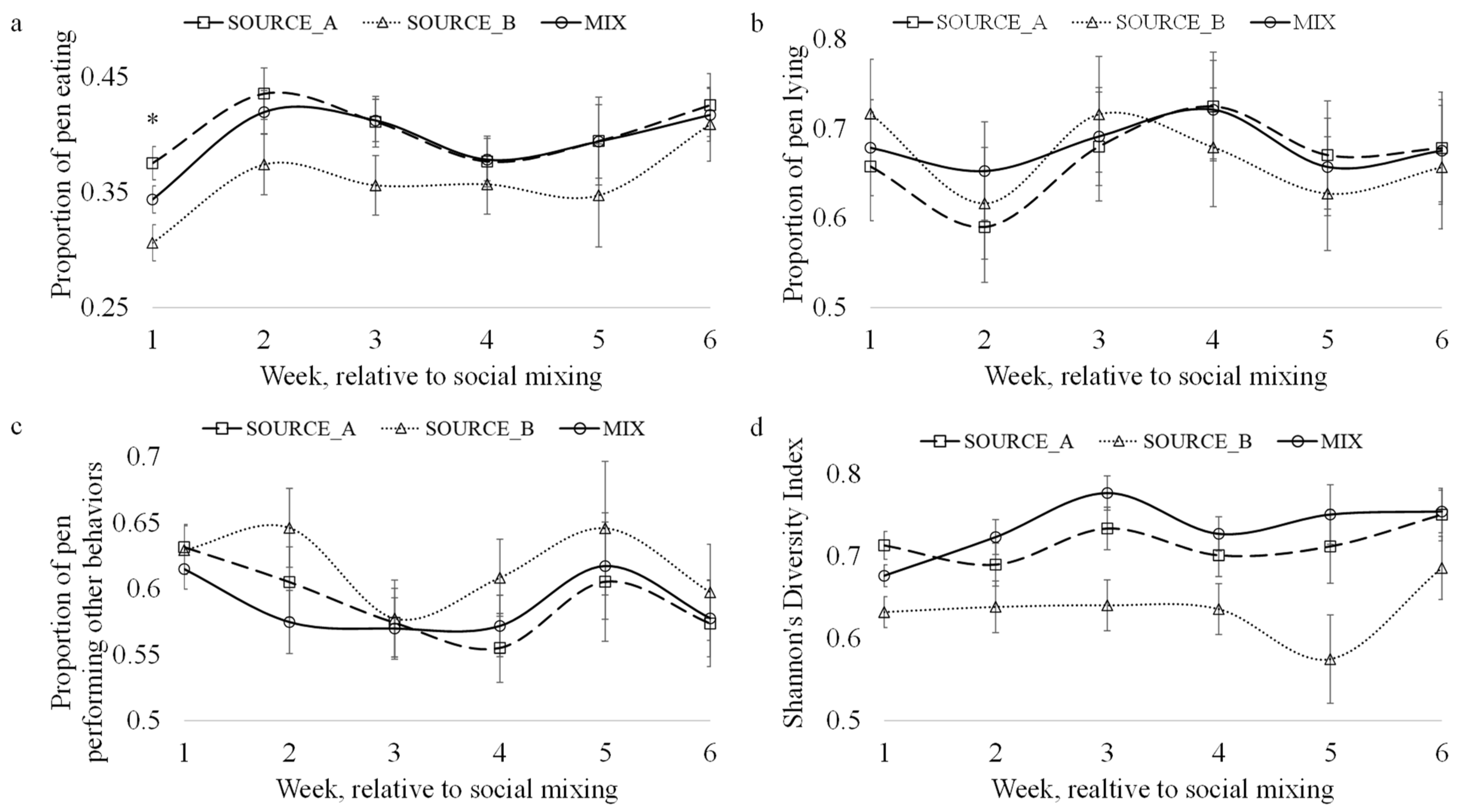
3.2. Core Behaviors and Herd Synchrony
3.3. Rumination and Activity
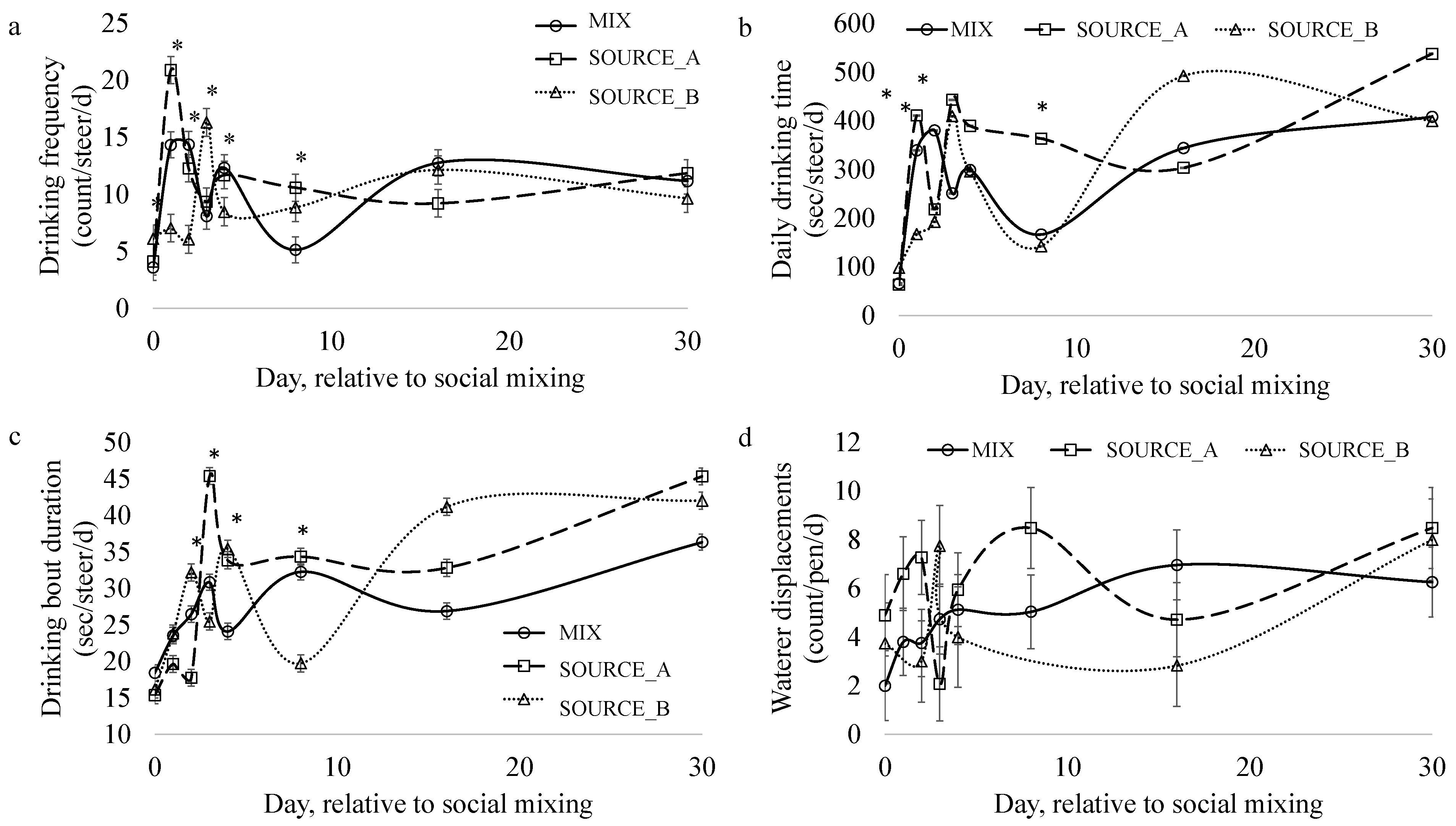
3.4. Drinking Behavior
3.4.1. Drinking Bout Frequency
3.4.2. Daily Drinking Duration
3.4.3. Drinking Bout Duration
3.4.4. Waterer Displacements
3.5. Social Behavior
4. Discussion
4.1. Impact of Social Mixing on Productivity
4.2. Impact of Social Mixing on Behavior
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reinhardt, V.; Reinhardt, A. Cohesive relationships in a cattle herd (Bos indicus). Behaviour 1981, 77, 121–150. [Google Scholar] [CrossRef]
- Ribble, C.S.; Meek, A.H.; Janzen, E.D.; Guichon, P.T.; Jim, G.K. Effect of time of year, weather, and the pattern of auction market sales on fatal fibrinous pneumonia (shipping fever) in calves in a large feedlot in Alberta (1985-1988). Can. J. Vet. Res. 1995, 59, 167. [Google Scholar] [PubMed]
- Step, D.; Krehbiel, C.; DePra, H.; Cranston, J.; Fulton, R.; Kirkpatrick, J.; Gill, D.; Payton, M.; Montelongo, M.; Confer, A. Effects of commingling beef calves from different sources and weaning protocols during a forty-two-day receiving period on performance and bovine respiratory disease. J. Anim. Sci. 2008, 86, 3146–3158. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.B.; Nordlund, K.V. Behavioral needs of the transition cow and considerations for special needs facility design. Vet. Clin. Food Anim. Pract. 2004, 20, 495–520. [Google Scholar] [CrossRef]
- Peden, R.S.; Turner, S.P.; Boyle, L.A.; Camerlink, I. The translation of animal welfare research into practice: The case of mixing aggression between pigs. Appl. Anim. Behav. Sci. 2018, 204, 1–9. [Google Scholar] [CrossRef]
- Proudfoot, K.; Habing, G. Social stress as a cause of diseases in farm animals: Current knowledge and future directions. Vet. J. 2015, 206, 15–21. [Google Scholar] [CrossRef]
- Estevez, I.; Andersen, I.-L.; Nævdal, E. Group size, density and social dynamics in farm animals. Appl. Anim. Behav. Sci. 2007, 103, 185–204. [Google Scholar] [CrossRef]
- Konggaard, S.; Krohn, C. Undersogelser over foderoptagelse og social adfaerd hos gruppefodrede koer i losdrift. iII. forste kalvs koer i gruppe for sig eller i gruppe med aeldre koer. Beret. Fra Statens Husdyrbrugsfors. 1978. [Google Scholar]
- Grant, R.; Albright, J. Effect of animal grouping on feeding behavior and intake of dairy cattle. J. Dairy Sci. 2001, 84, E156–E163. [Google Scholar] [CrossRef]
- Schein, M.W.; Fohrman, M.H. Social dominance relationships in a herd of dairy cattle. Br. J. Anim. Behav. 1955, 3, 45–55. [Google Scholar] [CrossRef]
- Hubbard, A.J.; Foster, M.J.; Daigle, C.L. Impact of social mixing on beef and dairy cattle—A scoping review. Appl. Anim. Behav. Sci. 2021, 241, 105389. [Google Scholar] [CrossRef]
- Burrow, H. Effect of intensive handling of zebu crossbred weaner calves on temperament. In Proceedings of the Conference of the Australian Association of Animal Breeding and Genetics, Melbourne, Australia, 24–27 June 1991. [Google Scholar]
- Daigle, C.L.; Hubbard, A.J.; Grandin, T. The use of traditional fear tests to evaluate different emotional circuits in cattle. J. Vis. Exp. 2020, 158, e60641. [Google Scholar]
- Mitlöhner, F.; Morrow-Tesch, J.; Wilson, S.; Dailey, J.; McGlone, J. Behavioral sampling techniques for feedlot cattle. J. Anim. Sci. 2001, 79, 1189–1193. [Google Scholar] [CrossRef]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949. [Google Scholar]
- Cronin, K.; Ross, S. Technical contribution: A cautionary note on the use of behavioural diversity (H-Index) in animal welfare science. Anim. Welf. 2019, 28, 157–164. [Google Scholar] [CrossRef]
- Collier, R.; Eley, R.; Sharma, A.; Pereira, R.; Buffington, D. Shade management in subtropical environment for milk yield and composition in Holstein and Jersey cows. J. Dairy Sci. 1981, 64, 844–849. [Google Scholar] [CrossRef]
- Burrow, H.; Dillon, R. Relationships between temperament and growth in a feedlot and commercial carcass traits of Bos indicus crossbreds. Aust. J. Exp. Agric. 1997, 37, 407–411. [Google Scholar] [CrossRef]
- Broom, D.M. Cognitive ability and awareness in domestic animals and decisions about obligations to animals. Appl. Anim. Behav. Sci. 2010, 126, 1–11. [Google Scholar] [CrossRef]
- Agnew, L.L.; Colditz, I.G. Development of a method of measuring cellular stress in cattle and sheep. Vet. Immunol. Immunopathol. 2008, 123, 197–204. [Google Scholar] [CrossRef]
- Arthington, J.; Eicher, S.; Kunkle, W.; Martin, F. Effect of transportation and commingling on the acute-phase protein response, growth, and feed intake of newly weaned beef calves. J. Anim. Sci. 2003, 81, 1120–1125. [Google Scholar] [CrossRef]
- Costa, J.; Von Keyserlingk, M.; Weary, D. Invited review: Effects of group housing of dairy calves on behavior, cognition, performance, and health. J. Dairy Sci. 2016, 99, 2453–2467. [Google Scholar] [CrossRef] [PubMed]
- Kavelaars, A.; Heijnen, C.J.; Tennekes, R.; Bruggink, J.E.; Koolhaas, J.M. Individual behavioral characteristics of wild-type rats predict susceptibility to experimental autoimmune encephalomyelitis. Brain Behav. Immun. 1999, 13, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J. Individual coping strategies and vulnerability to stress pathology. Homeost. Health Dis. 1994, 35, 24–27. [Google Scholar]
- Bolhuis, J.E.; Parmentier, H.K.; Schouten, W.G.; Schrama, J.W.; Wiegant, V.M. Effects of housing and individual coping characteristics on immune responses of pigs. Physiol. Behav. 2003, 79, 289–296. [Google Scholar] [CrossRef]
- Hessing, M.J. Individual Behavioural Characteristics in Pigs and Their Consequences for Pig Husbandry; Wageningen University and Research: Wageningen, The Netherlands, 1994. [Google Scholar]
- Hessing, M.; Coenen, G.; Vaiman, M.; Renard, C. Individual differences in cell-mediated and humoral immunity in pigs. Vet. Immunol. Immunopathol. 1995, 45, 97–113. [Google Scholar] [CrossRef]
- Janczak, A.M.; Pedersen, L.J.; Rydhmer, L.; Bakken, M. Relation between early fear-and anxiety-related behaviour and maternal ability in sows. Appl. Anim. Behav. Sci. 2003, 82, 121–135. [Google Scholar] [CrossRef]
- Thodberg, K.; Jensen, K.H.; Herskin, M.S. Nursing behaviour, postpartum activity and reactivity in sows: Effects of farrowing environment, previous experience and temperament. Appl. Anim. Behav. Sci. 2002, 77, 53–76. [Google Scholar] [CrossRef]
- Koolhaas, J.; Van Reenen, C. Animal behavior and well-being symposium: Interaction between coping style/personality, stress, and welfare: Relevance for domestic farm animals. J. Anim. Sci. 2016, 94, 2284–2296. [Google Scholar] [CrossRef]
- King, D.; Pfeiffer, C.S.; Randel, R.; Welsh Jr, T.; Oliphint, R.; Baird, B.; Curley Jr, K.; Vann, R.; Hale, D.; Savell, J. Influence of animal temperament and stress responsiveness on the carcass quality and beef tenderness of feedlot cattle. Meat Sci. 2006, 74, 546–556. [Google Scholar] [CrossRef]
- Petherick, J.C.; Holroyd, R.; Doogan, V.; Venus, B. Productivity, carcass and meat quality of lot-fed Bos indicus cross steers grouped according to temperament. Aust. J. Exp. Agric. 2002, 42, 389–398. [Google Scholar] [CrossRef]
- Voisinet, B.; Grandin, T.; Tatum, J.; O’connor, S.; Struthers, J. Feedlot cattle with calm temperaments have higher average daily gains than cattle with excitable temperaments. J. Anim. Sci. 1997, 75, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Burdick, N.; Randel, R.; Carroll, J.; Welsh, T. Interactions between temperament, stress, and immune function in cattle. Int. J. Zool. 2011, 2011, 373197. [Google Scholar] [CrossRef]
- Fell, L.; Colditz, I.; Walker, K.; Watson, D. Associations between temperament, performance and immune function in cattle entering a commercial feedlot. Aust. J. Exp. Agric. 1999, 39, 795–802. [Google Scholar] [CrossRef]
- Oliphint, R.A. Evaluation of the Inter-Relationships of Temperament, Stress Responsiveness and Immune Function in Beef Calves; Texas A&M University: College Station, TX, USA, 2006. [Google Scholar]
- Cooke, R.; Arthington, J.; Araujo, D.; Lamb, G. Effects of acclimation to human interaction on performance, temperament, physiological responses, and pregnancy rates of Brahman-crossbred cows. J. Anim. Sci. 2009, 87, 4125–4132. [Google Scholar] [CrossRef]
- Curley Jr, K.; Paschal, J.C.; Welsh Jr, T.; Randel, R.D. Exit velocity as a measure of cattle temperament is repeatable and associated with serum concentration of cortisol in Brahman bulls. J. Anim. Sci. 2006, 84, 3100–3103. [Google Scholar] [CrossRef]
- Von Keyserlingk, M.; Olenick, D.; Weary, D. Acute behavioral effects of regrouping dairy cows. J. Dairy Sci. 2008, 91, 1011–1016. [Google Scholar] [CrossRef]
- Bristow, D.J.; Holmes, D.S. Cortisol levels and anxiety-related behaviors in cattle. Physiol. Behav. 2007, 90, 626–628. [Google Scholar] [CrossRef]
- Haley, D.; Rushen, J.; Passillé, A.d. Behavioural indicators of cow comfort: Activity and resting behaviour of dairy cows in two types of housing. Can. J. Anim. Sci. 2000, 80, 257–263. [Google Scholar] [CrossRef]
- Stone, A.; Jones, B.; Becker, C.; Bewley, J. Influence of breed, milk yield, and temperature-humidity index on dairy cow lying time, neck activity, reticulorumen temperature, and rumination behavior. J. Dairy Sci. 2017, 100, 2395–2403. [Google Scholar] [CrossRef]
- Guan, L.L.; Nkrumah, J.D.; Basarab, J.A.; Moore, S.S. Linkage of microbial ecology to phenotype: Correlation of rumen microbial ecology to cattle’s feed efficiency. FEMS Microbiol. Lett. 2008, 288, 85–91. [Google Scholar] [CrossRef]
- Hernandez-Sanabria, E.; Guan, L.L.; Goonewardene, L.A.; Li, M.; Mujibi, D.F.; Stothard, P.; Moore, S.S.; Leon-Quintero, M.C. Correlation of particular bacterial PCR-denaturing gradient gel electrophoresis patterns with bovine ruminal fermentation parameters and feed efficiency traits. Appl. Environ. Microbiol. 2010, 76, 6338–6350. [Google Scholar] [CrossRef] [PubMed]
- Myer, P.; Freetly, H.; Wells, J.; Smith, T.; Kuehn, L. Analysis of the gut bacterial communities in beef cattle and their association with feed intake, growth, and efficiency. J. Anim. Sci. 2017, 95, 3215–3224. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Aminov, R.; Nagamine, T.; Matsui, H.; Nakamura, M.; Benno, Y. Diet-dependent shifts in the bacterial population of the rumen revealed with real-time PCR. Appl. Environ. Microbiol. 2001, 67, 2766–2774. [Google Scholar] [CrossRef] [PubMed]
- Malmuthuge, N.; Griebel, P.J.; Guan, L.L. The gut microbiome and its potential role in the development and function of newborn calf gastrointestinal tract. Front. Vet. Sci. 2015, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Shane, D.D.; White, B.J.; Larson, R.L.; Amrine, D.E.; Kramer, J.L. Probabilities of cattle participating in eating and drinking behavior when located at feeding and watering locations by a real time location system. Comput. Electron. Agric. 2016, 127, 460–466. [Google Scholar] [CrossRef]
- Ruuska, S.; Kajava, S.; Mughal, M.; Zehner, N.; Mononen, J. Validation of a pressure sensor-based system for measuring eating, rumination and drinking behaviour of dairy cattle. Appl. Anim. Behav. Sci. 2016, 174, 19–23. [Google Scholar] [CrossRef]
- McDonald, P.V.; von Keyserlingk, M.A.; Weary, D.M. Hot weather increases competition between dairy cows at the drinker. J. Dairy Sci. 2020, 103, 3447–3458. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Hsu, J.-T.; Ding, S.-T.; Rustia, D.J.A.; Lin, T.-T. Assessment of dairy cow heat stress by monitoring drinking behaviour using an embedded imaging system. Biosyst. Eng. 2020, 199, 97–108. [Google Scholar] [CrossRef]
- Capik, S.F.; White, B.J.; Larson, R.L.; Van Engen, N.; Coetzee, J.F. Effect of meloxicam administration on movement, feeding, and drinking behaviors of transported and nontransported cattle. Am. J. Vet. Res. 2017, 78, 1437–1443. [Google Scholar] [CrossRef]
- Welp, T.; Rushen, J.; Kramer, D.; Festa-Bianchet, M.; De Passille, A. Vigilance as a measure of fear in dairy cattle. Appl. Anim. Behav. Sci. 2004, 87, 1–13. [Google Scholar] [CrossRef]
- Boland, C.R. An experimental test of predator detection rates using groups of free-living emus. Ethology 2003, 109, 209–222. [Google Scholar] [CrossRef]
- Hunter, L.; Skinner, J. Vigilance behaviour in African ungulates: The role of predation pressure. Behaviour 1998, 195–211. [Google Scholar] [CrossRef]
- Quenette, P. Functions of vigilance behaviour in mammals: A review. Acta Oecologica 1990, 11, 801–818. [Google Scholar]
- Lung, M.A.; Childress, M.J. The influence of conspecifics and predation risk on the vigilance of elk (Cervus elaphus) in Yellowstone National Park. Behav. Ecol. 2007, 18, 12–20. [Google Scholar] [CrossRef]
- Kluever, B.M.; Breck, S.W.; Howery, L.D.; Krausman, P.R.; Bergman, D.L. Vigilance in cattle: The influence of predation, social interactions, and environmental factors. Rangel. Ecol. Manag. 2008, 61, 321–328. [Google Scholar] [CrossRef]
- Clayton, D.A. Socially facilitated behavior. Q. Rev. Biol. 1978, 53, 373–392. [Google Scholar] [CrossRef]
- Krause, J.; Ruxton, G.D.; Ruxton, G.; Ruxton, I.G. Living in Groups; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Šárová, R.; Špinka, M.; Panamá, J.L.A. Synchronization and leadership in switches between resting and activity in a beef cattle herd—a case study. Appl. Anim. Behav. Sci. 2007, 108, 327–331. [Google Scholar] [CrossRef]
- Miller, K.; Wood-Gush, D. Some effects of housing on the social behaviour of dairy cows. Anim. Sci. 1991, 53, 271–278. [Google Scholar] [CrossRef]
- Rook, A.; Huckle, C. Synchronization of ingestive behaviour by grazing dairy cows. Anim. Sci. 1995, 60, 25–30. [Google Scholar] [CrossRef]
- Bouissou, M.-F. The social behaviour of cattle. In Social Behaviour in Farm Animals; CABI: New York, NY, USA, 2001. [Google Scholar]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Wasilewski, A. Friendship in Ungulates? Sociopositive Relationships between Non-Related Herd Members of the Same Species; Philipps-Universitat Marburg: Marburg, Germany, 2003. [Google Scholar]
- Daigle, C.; Jackson, B.; Gill, R.; Wickersham, T.; Sawyer, J. Impact of exercise on productivity, behavior, and immune functioning of weaned Bos indicus–cross calves housed in drylots. J. Anim. Sci. 2017, 95, 5230–5239. [Google Scholar] [CrossRef] [PubMed]
- Daigle, C.L.; Mathias, A.J.; Ridge, E.E.; Gill, R.; Wickersham, T.A.; Sawyer, J.E. Case Study: Effect of exercise programs during receiving in a commercial feedlot on behavior and productivity of Brahman crossbred calves: Results from a commercial environment and a comparison to the research environment. Prof. Anim. Sci. 2018, 34, 653–663. [Google Scholar] [CrossRef]
- Hasegawa, N.; Nishiwaki, A.; Sugawara, K.; Ito, I. The effects of social exchange between two groups of lactating primiparous heifers on milk production, dominance order, behavior and adrenocortical response. Appl. Anim. Behav. Sci. 1997, 51, 15–27. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Mutoh, Y.; Umetsu, R. Interrelationships among maintenance behaviour, agonistic behaviour and live weight change in a beef cattle herd after introducing a strange cow. J. Fac. Agric.-Kyushu Univ. 1993, 37, 209–218. [Google Scholar] [CrossRef]
- Lozada, C.; Toro, G.; Daigle, C. Heifers are from Venus, steers are from Mars: Sex differences in social behaviors in drylot housed Brahman cattle exposed to a cattle brush. In Proceedings of the ASAS-CSAS-SSAS Annual Meeting and Trade Show, Louisville, KY, USA, 14–17 July 2021. [Google Scholar]
- Reinhardt, C.; Reinhardt, A.; Reinhardt, V. Social behaviour and reproductive performance in semi-wild Scottish Highland cattle. Appl. Anim. Behav. Sci. 1986, 15, 125–136. [Google Scholar] [CrossRef]
- Jensen, M.B. The role of social behavior in cattle welfare. In Advances in Cattle Welfare; Elsevier: Amsterdam, The Netherlands, 2018; pp. 123–155. [Google Scholar]
- Price, E.O. Principles and Applications of Domestic Animal Behavior: An Introductory Text; CABI: Wallingford, UK, 2008. [Google Scholar]


| Item | % of Diet (Dry Matter Basis) |
|---|---|
| Ingredient | |
| Sorghum–Sudan grass hay | 17.00% |
| Corn stalks | 10.00% |
| Steamed flaked corn | 36.15% |
| Sweet Bran | 28.25% |
| Pellet Supplement | 3.50% |
| Urea | 0.75% |
| Corn oil | 3.80% |
| Limestone | 0.55% |
| Nutrient content | |
| NEm, Mcal/kg NEg, Mcal/kg | 0.81 0.52 |
| Total Digestible Nutrients, % | 73.7 |
| Rumen Degradeable Protien, % | 17.4 |
| Fat, % | 6.3 |
| Ca:P ratio | 1.31 |
| Behavior | Definition |
|---|---|
| Eating | Steer has its head in a feed bunk. |
| Drinking | Steer has its head in a water trough and appears to be swallowing. |
| Lying | Steer is recumbent, i.e., not supported by legs (lying laterally or sternally). |
| Standing | Steer is standing on four legs without locomotion. |
| Allogrooming | Licking movements carried out by one steer on the body of another, characterized by repetitive back-and-forth head movements performed by the actor in direct contact with the reactor. Once the actor stops grooming the reactor for more than 10s, the bout is finished. |
| Headbutting | Head of one steer connects with the body of another steer. |
| Prolonged headbutt | A prolonged headbutt was recorded when the actor initiated head-to-head or head-to-neck contact with another steer that lasted longer than 5s. It is characterized by continuous direct contact between both steers, wherein steers potentially pushed or maneuvered against each other. When steers lost contact for more than 10s, the bout was finished. |
| Mounting | Steer positions body on the top of another steer’s topline |
| Feed bunk displacement | A displacement was recorded when a butt or a push from the “actor” resulted in the complete withdrawal of the head of another individual (the “recipient”) from the feed bunk 1. |
| Waterer displacement | A displacement was recorded when a butt or a push from the “actor” resulted in the complete withdrawal of the head of another individual (the “recipient”) from the waterer 1. |
| Item | MIX | SOURCE_B | SOURCE_A | SEM | p-Value |
|---|---|---|---|---|---|
| ADG 1, kg | 1.50 | 1.57 | 1.59 | 0.05 | 0.59 |
| DMI 2, kg | 10.72 | 10.58 | 10.78 | 0.32 | 0.48 |
| G:F 3 | 0.14 | 0.15 | 0.14 | 0.04 | 0.48 |
| Item | SOURCE_B MIX | SOURCE_BNOMIX | SOURCE_AMIX | SOURCE_ANOMIX | SEM | p-Value |
|---|---|---|---|---|---|---|
| Drinking Behavior 1 | ||||||
| Bout frequency, count/steer | 8.04 a | 8.30 a | 10.52 b | 9.93 b | 1.08 | 0.04 |
| Total time, sec/steer | 223.65 | 244.06 | 276.88 | 299.26 | 1.10 | 0.80 |
| Mean bout duration, sec/bout/steer | 27.89 | 29.38 | 26.50 | 30.13 | 1.07 | 0.48 |
| ADG 2, kg | 1.41 | 1.59 | 1.60 | 1.57 | 0.039 | 0.10 |
| EV 3, m/s | 2.45 a | 2.34 a | 1.26 b | 1.49 b | 0.16 | <0.0001 |
| Rumination 4, min/steer/day | 477 | 487 | 527 | 523 | 19.44 | 0.21 |
| Activity 4, min/steer/day | 340.89 | 326.09 | 338.15 | 334.00 | 9.12 | 0.41 |
| Treatment | |||||
|---|---|---|---|---|---|
| Item | MIX | SOURCE_B | SOURCE_A | SEM | p-Value |
| EV 1, m/s | 1.86 a | 2.34 b | 1.48 a | 0.22 | 0.02 |
| Herd behavior 2, proportion of pen | |||||
| Lying | 0.68 | 0.67 | 0.67 | 0.02 | 0.614 |
| Eating | 0.39 a | 0.36 b | 0.40 a | 0.01 | 0.005 |
| Other | 0.59 | 0.62 | 0.59 | 0.01 | 0.059 |
| Shannon–Weiner Diversity Index 3 | 0.72 a | 0.64 b | 0.72 a | 0.01 | <0.0001 |
| Social Behavior 4 | |||||
| Allogrooming (bout/pen/d) | 19.97 a | 12.09 b | 21.34 a | 0.13 | 0.000 |
| Feedbunk displacement (count/pen/d) | 20.13 | 17.76 | 20.29 | 0.25 | 0.936 |
| Waterer displacement (count/pen/d) | 4.43 | 4.73 | 5.48 | 0.17 | 0.581 |
| Headbutt (count/pen/d) | 45.80 | 52.67 | 56.86 | 0.17 | 0.709 |
| Long headbutt (count/pen/d) | 63.37 | 41.94 | 63.88 | 7.50 | 0.090 |
| Mounting (count/pen/d) | 4.92 | 4.32 | 4.16 | 0.21 | 0.764 |
| Rumination duration 5, min/d | 500.24 b | 489.97 b | 518.98 a | 31.6 | 0.0004 |
| Activity duration 5, min/d | 342.03 a | 327.74 b | 336.00 a | 4.43 | <0.0001 |
| Drinking behavior 4 | |||||
| Daily bout frequency, count/steer | 9.26 | 8.84 | 10.36 | 0.11 | 0.782 |
| Total daily time drinking, count/steer | 249.44 | 243.81 | 298.75 | 0.09 | 0.302 |
| Mean daily bout duration, sec/bout | 26.84 | 28.03 | 28.39 | 0.09 | 0.639 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daigle, C.L.; Sawyer, J.E.; Cooke, R.F.; Jennings, J.S. Consider the Source: The Impact of Social Mixing on Drylot Housed Steer Behavior and Productivity. Animals 2023, 13, 2981. https://doi.org/10.3390/ani13182981
Daigle CL, Sawyer JE, Cooke RF, Jennings JS. Consider the Source: The Impact of Social Mixing on Drylot Housed Steer Behavior and Productivity. Animals. 2023; 13(18):2981. https://doi.org/10.3390/ani13182981
Chicago/Turabian StyleDaigle, Courtney L., Jason E. Sawyer, Reinaldo F. Cooke, and Jenny S. Jennings. 2023. "Consider the Source: The Impact of Social Mixing on Drylot Housed Steer Behavior and Productivity" Animals 13, no. 18: 2981. https://doi.org/10.3390/ani13182981
APA StyleDaigle, C. L., Sawyer, J. E., Cooke, R. F., & Jennings, J. S. (2023). Consider the Source: The Impact of Social Mixing on Drylot Housed Steer Behavior and Productivity. Animals, 13(18), 2981. https://doi.org/10.3390/ani13182981






