Molecular Identification and Evolutionary Divergence of the Sri Lankan Sambar Deer, Rusa unicolor (Kerr 1792)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
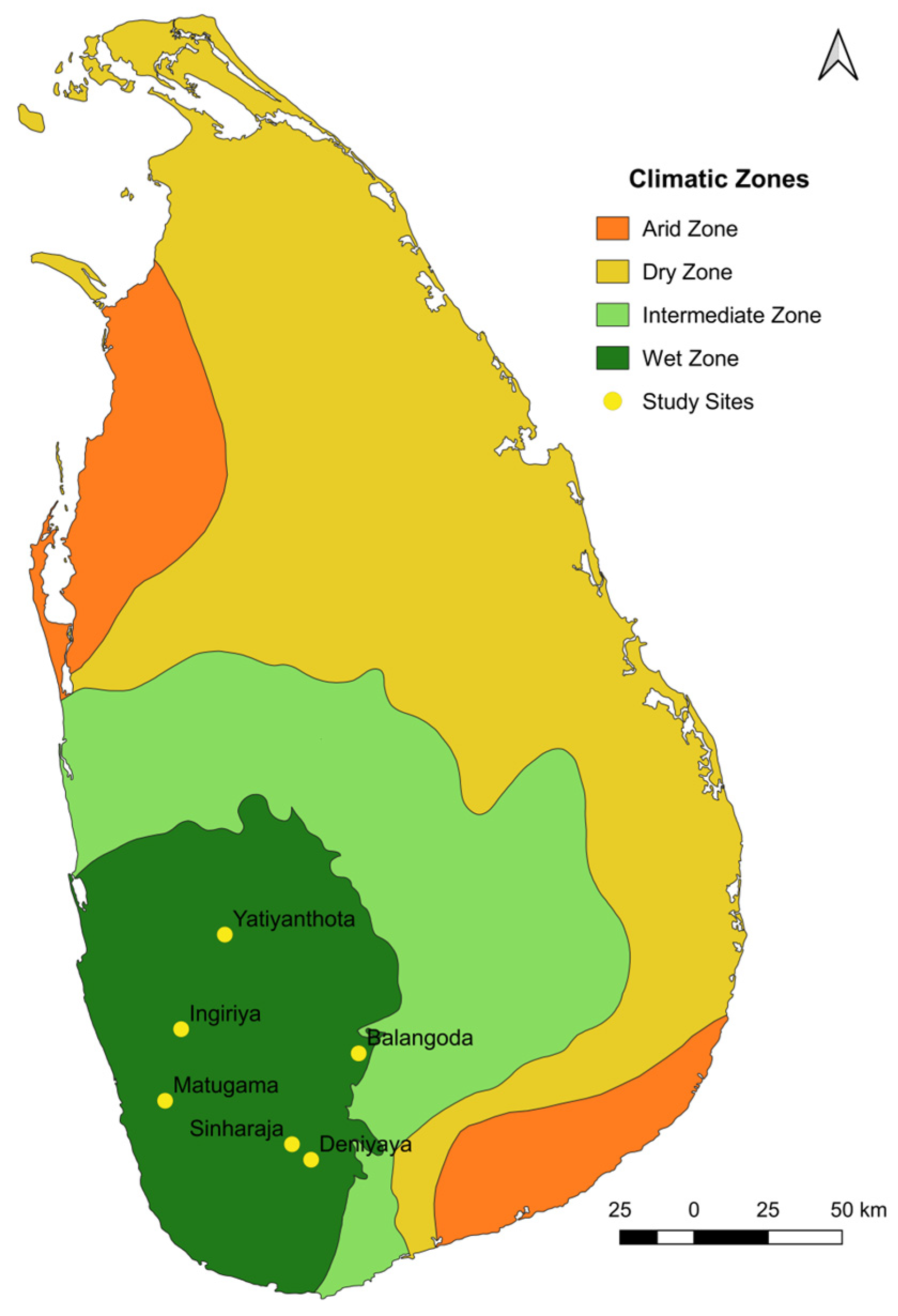
2.1. Sample Collection
2.2. DNA Extraction, Polymerase Chain Reaction (PCR), and Sequencing
2.3. Phylogenetic Analyses and Divergence Times
3. Results
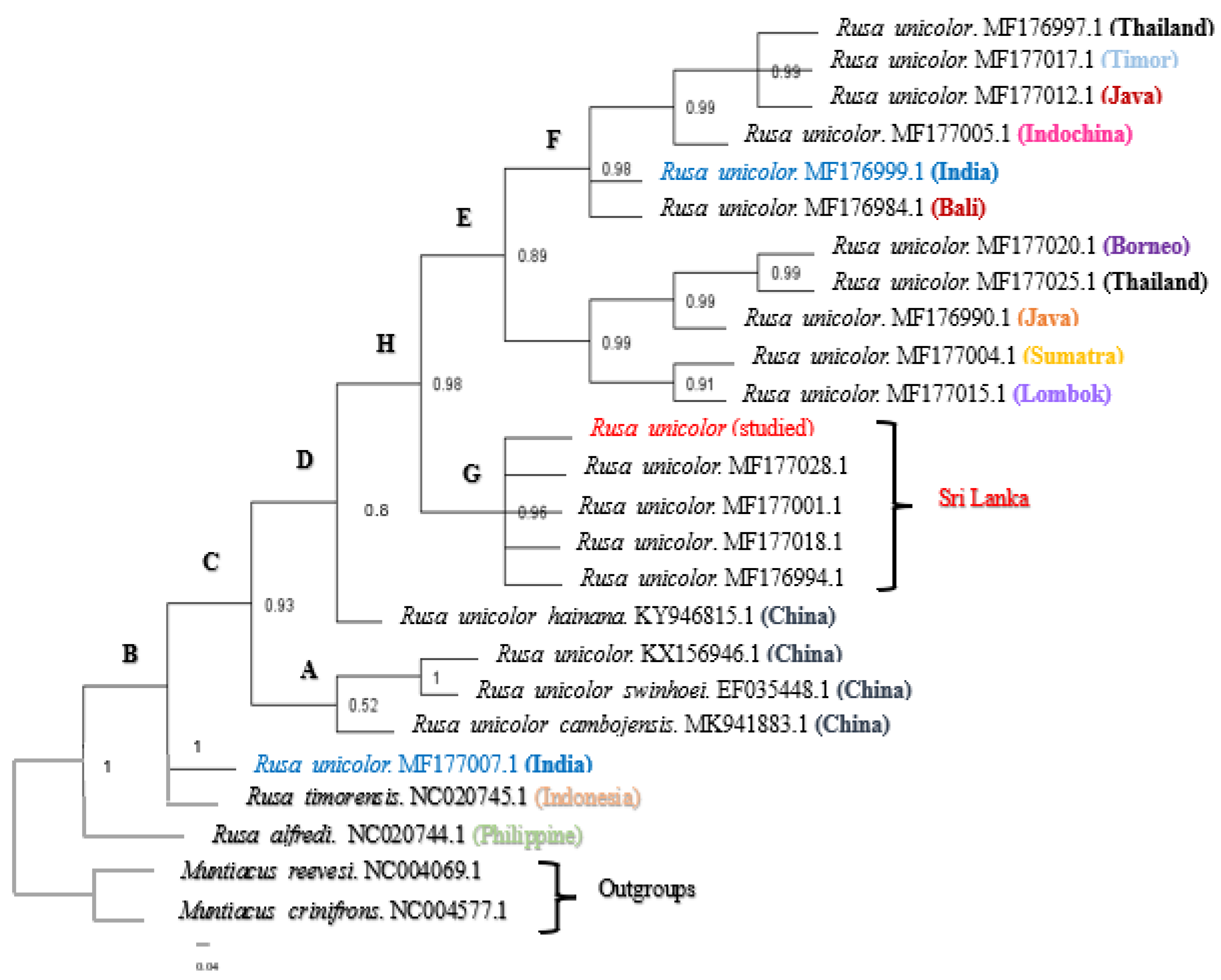
3.1. Phylogenetic Analysis
3.2. Genetic Distances
3.3. Divergence Times
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leslie, D.M. Rusa unicolor (Artiodactyla: Cervidae). Mamm. Species 2011, 43, 1–30. [Google Scholar] [CrossRef]
- Corbet, G.B.; Hill, J.E. The Mammals of the Indomalayan Region: A Systematic Review; Oxford University Press: Oxford, UK, 1992. [Google Scholar] [CrossRef]
- Ohtaishi, N.; Gao, Y. A review of the distribution of all species of deer (Tragulidae, Moschidae, and Cervidae) in China. Mammal Rev. 1990, 20, 125–144. [Google Scholar] [CrossRef]
- Chesemore, D.L. Notes on the mammals of southern Nepal. J. Mammal. 1970, 51, 162–166. [Google Scholar] [CrossRef]
- Seidensticker, J. On the ecological separation between tigers and leopards. Biotropica 1976, 8, 225–234. [Google Scholar] [CrossRef]
- Seidensticker, J. Ungulate populations in Chitwan Valley, Nepa1. Biol. Conserv. 1976, 10, 183–210. [Google Scholar] [CrossRef]
- Hsu, M.J.; Agoramoorthy, G.; Desender, K.; Baert, L.; Bonilla, H.R. Wildlife conservation in Taiwan. Conserv. Biol. 1997, 11, 834–836. [Google Scholar] [CrossRef]
- Ali, N.A.N.G.; Abdullah, M.L.; Nor, S.A.M.; Pau, T.M.; Kulaimi, N.A.M.; Naim, D.M. A review of the genus Rusa in the indo-Malayan archipelago and conservation efforts. Saudi J. Biol. Sci. 2021, 28, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Kittle, A.M.; Watson, A.C. Density of leopards (Panthera pardus kotiya) in Horton Plains National Park in the Central Highlands of Sri Lanka. Mammalia 2018, 82, 183–187. [Google Scholar] [CrossRef]
- Duckworth, J.W.; Kumar, N.S.; Pokharel, C.P.; Sagar Baral, H.; Timmins, R. Rucervus duvaucelii, The IUCN Red List of Threatened Species; IUCN: New York, NY, USA, 2015; p. 8235. [Google Scholar] [CrossRef]
- Brown, J.R.; Beckenbach, A.T.; Smith, M.J. Intraspecific DNA sequence variation of the mitochondrial control region of white sturgeon (Acipenser transmontanus). Mol. Biol. Evol. 1993, 10, 326–341. [Google Scholar] [CrossRef]
- Harrison, R.G. Animal mitochondrial DNA as a genetic marker in population and evolutionary biology. Trends Ecol. Evol. 1989, 4, 6–11. [Google Scholar] [CrossRef]
- Li, G.; Guo, W.; Zhang, Y.; Cao, G.; Wang, Z. Complete mitochondrial genome of Rusa unicolor cambojensis (Artiodactyla: Cervidae). Mitochondrial DNA Part B 2021, 6, 3372–3374. [Google Scholar] [CrossRef]
- Kumar, V.P.; Shukla, M.; Rajpoot, A.; Thakur, M.; Nigam, P.; Kumar, D.; Mehta, A.K.; Goyal, S.P. DNA barcoding as a tool for robust identification of cervids of India and its utility in wildlife forensics. Mitochondrial DNA Part B 2018, 3, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, L.; Wang, Y.; Liu, Q.; Shui, Q.; Yue, B.; Zhang, Z.; Li, J. Identification of deer species (Cervidae, Cetartiodactyla) in China using mitochondrial cytochrome c oxidase subunit I (mtDNA COI). Mitochondrial DNA Part A 2016, 27, 4240–4243. [Google Scholar] [CrossRef]
- Kumar, N.P.; Srinivasan, R.; Jambulingam, P. DNA barcoding for identification of sand flies (Diptera: Psychodidae) in India. Mol. Ecol. Resour. 2012, 12, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Gao, J. Molecular authentication of sika deer (Cervus nippon) based on allele-specific PCR. Mitochondrial DNA Part B: Resour. 2019, 4, 2231–2233. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. Partitionfinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Gernhard, T.; Hartmann, K.; Steel, M. Stochastic properties of generalised Yule models, with biodiversity applications. J. Math. Biol. 2008, 57, 713–735. [Google Scholar] [CrossRef]
- Li, K.Y.; Hsiao, C.; Yen, S.C.; Hung, C.Y.; Lin, Y.Z.; Jheng, S.W.; Yu, P.-J.; Hwang, M.-H.; Weng, G.-J.; Chen, K.-L.; et al. Phylogenetic divergence associated with climate oscillations and topology illustrates the dispersal history of Formosan sambar deer (Rusa unicolor swinhoii) in Taiwan. Mammal Res. 2023, 68, 283–294. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Pitra, C.; Fickel, J.; Meijaard, E.; Groves, C. Evolution and phylogeny of old world deer. Mol. Phylogenetics Evol. 2004, 33, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Mrázek, J.; Karlin, S. Genome signature comparisons among prokaryote, plasmid, and mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1999, 96, 9184–9189. [Google Scholar] [CrossRef]
- Wallace, D.C.; Chalkia, D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a021220. [Google Scholar] [CrossRef] [PubMed]
- Angom, S.; Gupta, S.K.; Kumar, A.; Hussain, S.A. Identification of globally threatened cervids from problematic samples using cytochrome b and control region genes. Conserv. Genet. Resour. 2015, 7, 647–650. [Google Scholar] [CrossRef]
- Roesma, D.I.; Tjong, D.H.; Janra, M.N.; Aidil, D.R. DNA barcoding of freshwater fish in Siberut Island, Mentawai Archipelago, Indonesia. Biodiversitas 2022, 23, 1795–1806. [Google Scholar] [CrossRef]
- Tello, J.G.; Moyle, R.G.; Marchese, D.J.; Cracraft, J. Phylogeny and phylogenetic classification of the tyrant flycatchers, cotingas, manakins, and their allies (Aves: Tyrannides). Cladistics 2005, 25, 429–467. [Google Scholar] [CrossRef]
- Weber, M.G.; Strauss, S.Y. Coexistence in Close Relatives: Beyond Competition and Reproductive Isolation in Sister Taxa. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 359–381. [Google Scholar] [CrossRef]
- Wedage, O.; Roberts, P.; Faulkner, P.; Crowther, A.; Douka, K.; Picin, A.; Blinkhorn, J.; Deraniyagala, S.; Boivin, N.; Petraglia, M.; et al. Late Pleistocene to early-Holocene rainforest foraging in Sri Lanka: Multidisciplinary analysis at Kitulgala Beli-lena. Quat. Sci. Rev. 2020, 2, 31. [Google Scholar] [CrossRef]
- Pybus, O.G.; Rambaut, A.; Harvey, P.H. An integrated framework for the inference of viral population history from reconstructed genealogies. Genetics 2000, 155, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Budd, G.E.; Mann, R.P. Survival and selection biases in early animal evolution and a source of systematic overestimation in molecular clocks. Interface Focus 2020, 10, 20190110. [Google Scholar] [CrossRef] [PubMed]


| Origin | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Studied sambar deer | Sri Lanka | ||||||||||||||||||||||||
| 2 | MK941883.1 | China | 0.2548 | |||||||||||||||||||||||
| 3 | KY946815.1 | China | 0.2538 | 0.0072 | ||||||||||||||||||||||
| 4 | EF035448.1 | China | 0.2532 | 0.0072 | 0.0062 | |||||||||||||||||||||
| 5 | MF176999.1 | India | 0.2531 | 0.0109 | 0.0088 | 0.0098 | ||||||||||||||||||||
| 6 | MF176994.1 | Sri Lanka | 0.2495 | 0.0145 | 0.0124 | 0.0135 | 0.0109 | |||||||||||||||||||
| 7 | MF177018.1 | Sri Lanka | 0.2495 | 0.0145 | 0.0124 | 0.0135 | 0.0109 | 0.0000 | ||||||||||||||||||
| 8 | MF177001.1 | Sri Lanka | 0.2492 | 0.0140 | 0.0119 | 0.0130 | 0.0103 | 0.0005 | 0.0005 | |||||||||||||||||
| 9 | MF177028.1 | Sri Lanka | 0.2491 | 0.0140 | 0.0119 | 0.0130 | 0.0103 | 0.0005 | 0.0005 | 0.0000 | ||||||||||||||||
| 10 | MF177015.1 | Lombok | 0.2531 | 0.0120 | 0.0093 | 0.0114 | 0.0057 | 0.0125 | 0.0125 | 0.0120 | 0.0120 | |||||||||||||||
| 11 | MF177012.1 | Java | 0.2485 | 0.0121 | 0.0089 | 0.0110 | 0.0036 | 0.0115 | 0.0115 | 0.0110 | 0.0110 | 0.0052 | ||||||||||||||
| 12 | MF177007.1 | India | 0.2541 | 0.0098 | 0.0088 | 0.0088 | 0.0103 | 0.0140 | 0.0140 | 0.0135 | 0.0135 | 0.0130 | 0.0115 | |||||||||||||
| 13 | MF177004.1 | Sumatra | 0.2538 | 0.0130 | 0.0103 | 0.0124 | 0.0046 | 0.0135 | 0.0135 | 0.0130 | 0.0130 | 0.0041 | 0.0042 | 0.0130 | ||||||||||||
| 14 | MF177025.1 | Thailand | 0.2531 | 0.0103 | 0.0072 | 0.0093 | 0.0036 | 0.0114 | 0.0114 | 0.0109 | 0.0109 | 0.0021 | 0.0031 | 0.0109 | 0.0041 | |||||||||||
| 15 | MF177017.1 | Timor | 0.2534 | 0.0124 | 0.0098 | 0.0109 | 0.0051 | 0.0140 | 0.0140 | 0.0135 | 0.0135 | 0.0026 | 0.0047 | 0.0135 | 0.0036 | 0.0026 | ||||||||||
| 16 | MF177020.1 | Borneo | 0.2541 | 0.0130 | 0.0093 | 0.0124 | 0.0046 | 0.0135 | 0.0135 | 0.0130 | 0.0130 | 0.0052 | 0.0021 | 0.0130 | 0.0041 | 0.0041 | 0.0046 | |||||||||
| 17 | MF176997.1 | Thailand | 0.2528 | 0.0124 | 0.0103 | 0.0114 | 0.0015 | 0.0124 | 0.0124 | 0.0119 | 0.0119 | 0.0073 | 0.0052 | 0.0119 | 0.0062 | 0.0051 | 0.0067 | 0.0062 | ||||||||
| 18 | MF177005.1 | Indochina | 0.2518 | 0.0111 | 0.0090 | 0.0100 | 0.0005 | 0.0111 | 0.0111 | 0.0106 | 0.0106 | 0.0063 | 0.0042 | 0.0106 | 0.0053 | 0.0042 | 0.0058 | 0.0053 | 0.0010 | |||||||
| 19 | NC004069.1 | China | 2.7543 | 0.1184 | 0.1172 | 0.1159 | 0.1153 | 0.1165 | 0.1165 | 0.1171 | 0.1171 | 0.1191 | 0.1162 | 0.1191 | 0.1146 | 0.1172 | 0.1165 | 0.1146 | 0.1146 | 0.1168 | ||||||
| 20 | NC004577.1 | China | 2.9050 | 0.1180 | 0.1168 | 0.1155 | 0.1136 | 0.1187 | 0.1187 | 0.1193 | 0.1193 | 0.1193 | 0.1171 | 0.1149 | 0.1168 | 0.1168 | 0.1174 | 0.1168 | 0.1130 | 0.1145 | 0.0646 | |||||
| 21 | KX156946.1 | China | 0.2541 | 0.0072 | 0.0051 | 0.0021 | 0.0098 | 0.0135 | 0.0135 | 0.0130 | 0.0130 | 0.0114 | 0.0099 | 0.0088 | 0.0124 | 0.0093 | 0.0119 | 0.0114 | 0.0114 | 0.0100 | 0.1159 | 0.1168 | ||||
| 22 | MF176990.1 | Java | 0.2533 | 0.0124 | 0.0088 | 0.0119 | 0.0062 | 0.0119 | 0.0119 | 0.0114 | 0.0114 | 0.0067 | 0.0036 | 0.0124 | 0.0057 | 0.0057 | 0.0062 | 0.0026 | 0.0077 | 0.0063 | 0.1141 | 0.1162 | 0.0109 | |||
| 23 | MF176984.1 | Bali | 0.2541 | 0.0135 | 0.0098 | 0.0130 | 0.0051 | 0.0140 | 0.0140 | 0.0135 | 0.0135 | 0.0057 | 0.0026 | 0.0135 | 0.0046 | 0.0046 | 0.0051 | 0.0005 | 0.0067 | 0.0058 | 0.1140 | 0.1161 | 0.0119 | 0.0021 | ||
| 24 | NC020745.1 | Indonesia | 0.2539 | 0.0070 | 0.0068 | 0.0072 | 0.0105 | 0.0088 | 0.0088 | 0.0085 | 0.0085 | 0.0110 | 0.0108 | 0.0115 | 0.0140 | 0.0113 | 0.0134 | 0.0140 | 0.0127 | 0.1194 | 0.119 | 0.0070 | 0.0128 | 0.0139 | 0.0067 | |
| 25 | NC020744.1 | Philippine | 0.255 | 0.0083 | 0.0072 | 0.0092 | 0.0110 | 0.0092 | 0.0092 | 0.0090 | 0.0090 | 0.0120 | 0.0115 | 0.0125 | 0.0155 | 0.0115 | 0.0140 | 0.0147 | 0.0130 | 0.1198 | 0.1205 | 0.0075 | 0.0133 | 0.0143 | 0.0072 | 0.0054 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayasundara, S.L.; Algewatta, H.R.; Jayawardana, S.; Perera, M.; Peiris, L.D.C. Molecular Identification and Evolutionary Divergence of the Sri Lankan Sambar Deer, Rusa unicolor (Kerr 1792). Animals 2023, 13, 2877. https://doi.org/10.3390/ani13182877
Jayasundara SL, Algewatta HR, Jayawardana S, Perera M, Peiris LDC. Molecular Identification and Evolutionary Divergence of the Sri Lankan Sambar Deer, Rusa unicolor (Kerr 1792). Animals. 2023; 13(18):2877. https://doi.org/10.3390/ani13182877
Chicago/Turabian StyleJayasundara, Subodha Lakruwani, Hirusha Randimal Algewatta, Suhada Jayawardana, Minoli Perera, and L. Dinithi C. Peiris. 2023. "Molecular Identification and Evolutionary Divergence of the Sri Lankan Sambar Deer, Rusa unicolor (Kerr 1792)" Animals 13, no. 18: 2877. https://doi.org/10.3390/ani13182877
APA StyleJayasundara, S. L., Algewatta, H. R., Jayawardana, S., Perera, M., & Peiris, L. D. C. (2023). Molecular Identification and Evolutionary Divergence of the Sri Lankan Sambar Deer, Rusa unicolor (Kerr 1792). Animals, 13(18), 2877. https://doi.org/10.3390/ani13182877






