1. Introduction
The concept of ecotourism stems from the belief that environmentally responsible tourism can both be a financial boon to local populations and help conserve the environment [
1,
2]. However, many ecotourism operations have been shown to damage local wildlife through anthropogenic disturbances such as increased pollution [
3], disruption of daily wildlife routines and social behavior [
4], increased risk of disease transmission [
5,
6,
7], and indications of increased stress in wildlife [
8,
9,
10,
11]. These risks are more concerning at sites that feature primates, whose conservation status is often considered as an indicator of the overall health of their ecosystems [
12]. However, while practices in many ecotourism locations are designed to reduce potential harm from anthropogenic factors, managers and researchers at sites in operation for decades often do not consider measuring potential increases in primate stress as a need [
13]; see also [
14] for review. Often, this is because they assume that, due to decades of apparent habituation (i.e., cessation of behavioral responses to a once-novel situation), primates targeted for tourism no longer experience harmful consequences of prolonged exposure to tourists or to the researchers [
15]. Unfortunately, this assumption is risky given evidence that unavoidable, chronic exposure of primates to humans can be associated with behavioral and physiological manifestations of stress [
11,
16,
17]. Increased research into behavioral and physiological responses of individual primates to tourism, while carefully controlling for potentially confounding factors, can help identify ecotourism practices that need modification.
1.1. Stress Physiology—Acute vs. Chronic
Organisms maintain an internal equilibrium (homeostasis) through coordinated physiological responses to stimuli that are perceived to threaten their normal function (i.e., stressors) [
18]. Acute stressors activate a cascade of physiological responses [
19], including the secretion of glucocorticoids (cortisol or corticosterone) [
20]. These physiological responses can be adaptive by meeting short-term increases in metabolic demand needed for an effective “fight or flight” response [
21]. When an animal is not experiencing a stressor, it usually displays relatively low glucocorticoid levels. Normal responses to acute stressors (i.e., acute stress) generally involve short-term increases in glucocorticoids within a few hours for salivary levels (e.g., within 2 h:
Ovis aries, [
22]; ~2 h:
Pan Paniscus, [
23]); within 24 h for urinary levels:
Macaca fascicularis,
Pan troglodytes [
24]; and within a few days for fecal levels (e.g., ~36 h:
Macaca nigra [
25] or ~48–72 h:
Pongo pygmaeus morio, [
26]). Fecal glucocorticoid levels then return to undisturbed levels quickly after the stressor is no longer perceived (
P.p. morio: [
26];
M. fasicularis,
P. troglodytes: [
24]; and
M. nigra: [
25]).
However, when an organism experiences stressors for extended durations (i.e., chronic stress), the prolonged release of cortisol is energetically expensive and disruptive to other physiological processes, including immune function, reproduction, and growth [
21]. Chronic stress responses in primates are marked by high baseline glucocorticoid levels with no or minimal increases when exposed to an acute stressor and/or a delay in the return to undisturbed output levels following the end of a stressor [
27,
28].
While the harmful effects of chronic stress on health and fitness are well documented, researchers are still uncovering the effects of frequent acute glucocorticoid responses on health and fitness. For example, frequent acute physiological stress can potentially disrupt reproductive endocrine processes and affect fertility [
29,
30,
31,
32,
33].
1.2. Measuring Physiological Stress in Wildlife
In this study, we asked whether glucocorticoid levels were affected by tourism in wild, apparently habituated Sulawesi black crested macaques (
M. nigra) in the Tangkoko Nature Reserve by measuring fecal glucocorticoid metabolites (FGCMs) as a proxy for the physiological stress response. Currently, the least invasive way to measure glucocorticoid levels in wildlife is through feces [
34]; see also [
35] for review. Cortisol itself is generally not present in feces; depending on the species, it breaks down into several metabolites. FGCM can indicate average cortisol levels over a day. In many mammals, including several primates, glucocorticoid metabolites in feces peak about 24–48 h after a stressful event is no longer perceived [
25,
36,
37] and return to undisturbed levels about 48–72 h after the peak [
24,
26,
37].
Properly timing the collection of samples can help uncover whether responses are consistent with acute or chronic stress responses to anthropogenic stress. It is also important to control for other influences on glucocorticoid levels. Notably, higher-than-normal glucocorticoid levels indicate that demands for energy expenditure and mobilization are high, and that may be due to any number of factors [
38]. In addition to real or perceived risks of tourism, FGCM levels in our macaque study groups could increase with environmental pressures, e.g., food resources [
39], extreme weather [
40,
41], reproductive hormones (estradiol: [
33,
42,
43]; testosterone: [
44,
45]), vigorous physical activity [
46] and others. Thus, researchers can use measurements of FGCM levels to shed light on sources of anthropogenic stress in ecotourist sites provided they control for other factors that potentially increase metabolic demand.
1.3. Evidence of Physiological Stress Related to Primate Ecotourism
A recent meta-analysis of anthropogenic impacts on physiological stress in wild primates by Kaisin et al. [
16] found that primates living in sites experiencing various anthropogenic disturbances exhibited higher glucocorticoid levels than those who were not. However, they saw this only under the conditions of habitat loss and hunting. While they did not find an overall significant effect when looking at tourism, there are several examples from the literature that indicate increases in physiological stress related to tourism. For example, wild
P.p. morio showed an increase in FGCM following tourist visits [
26]. Additionally, Shutt et al. [
11] found that two Western lowland gorilla (
Gorilla gorilla gorilla) groups that were apparently habituated to tourism (one recently and one for a longer period of time) had higher FGCM levels than a non-human-contacted unhabituated group. Also, in these same tourism-designated groups, when tourists violated the “no closer than 7 m to the group” rule,
G.g. gorilla FGCM levels increased. Barbary macaques (
Macaca sylvanus) also had higher fecal glucocorticoids after aggressive interactions with tourists [
47]. At this time, it is not clear whether the inconsistencies in findings among studies (e.g., [
16] vs. [
11,
26,
47]) are due to species differences, the lack of control for other factors that may affect metabolic demand as described above, or other issues. As such, they highlight the need to explore physiological stress responses to tourism using a variety of primate species and circumstances while controlling for possibly confounding factors.
1.4. Macaca Nigra and Tourism in Tangkoko Nature Reserve
One species in which such questions can be ideally examined is the Sulawesi black crested macaque,
M. nigra, living in Northeast Sulawesi, Indonesia. Black crested macaque social organization is female-philopatric and female-bonded [
48] with nonseasonal breeding, but with a tendency toward birth peaks between January and May [
49]. The macaques use a variety of habitats, subsist primarily on fruit supplemented with plant parts as well as invertebrate and vertebrate prey [
50], and live in large multimale, multifemale groups. They are diurnal and semiterrestrial, spending 59% of their day traveling, foraging, and feeding, with the remaining time spent resting and socializing [
51]. Male crested macaques disperse at sexual maturity and secondarily at intervals throughout adulthood [
52]. For additional details on
M. nigra and current status, see [
53,
54].
The Indonesian government and local villagers of Batu Putih jointly manage ecotourism in Tangkoko Nature Reserve (TNR). Local villagers earn income directly from the government by serving as park employees, rangers, and firefighters. Tourists also provide income by paying for lodging, food, and guides. In the last 25 years, the number of inns in the village has grown from one to eight, with more planned and several restaurants in the works. Prior to the COVID pandemic, many Batu Putih citizens used tourism as their primary source of income, and fewer took jobs in the nearby gold mines (personal communication with local people, 2017). With Indonesia opening back up to visitors, tourists are returning to TNR (D.B., personal communication with local people, 2023).
For over three decades, unfamiliar humans (tourists) have visited several crested macaque social groups almost daily. Park rules constantly change, but in general, guides must remain with tourists while they are inside TNR, unless tourists wish to go to the beach. Tourist groups range in size from 2 to 25 individuals (with cruise ship tour groups and school groups increasing this to 100). Although trails exist to help traverse the forest, tourists do not need to remain on them. They explore the forest from dawn until dusk and can walk into a group of macaques unhindered. Although a “research-only” zone exists to protect some macaque groups from frequent tourist visits, guides sometimes lead tourist groups to a giant strangler fig in the center of this zone. Along the way, tourist groups may occasionally encounter a macaque group that is designated for research only, in which case researchers within the Macaca Nigra Project (MNP—our research site manager and research sponsor) can request that they leave the area as quickly and quietly as possible. Macaque groups less habituated to tourism also tend to move farther away from or actively avoid tourists (D.B., personal observation).
Two distinct M. nigra groups most often occupy the tourist zone. During the low tourist (rainy) season, these macaque groups are exposed to an average of two tourist groups per day. During the high tourist (dry) season, they can be exposed to as many as seven tourist groups per day, often more than one at a time. The closer a macaque group is to the main entrance, the more likely it is to encounter tourists. Park rules prohibit feeding and interacting with macaques and discourage flash photography; however, guides rarely enforce these rules and sometimes actively encourage feeding and touching of the macaques.
1.5. Other Anthropological Influences
Batu Putih villagers keep gardens directly abutting the reserve that have historically been a sought-after food source for M. nigra. When MNP began research inside TNR, the program directors recognized the importance of employing someone whose sole job was to defend the village against crop foraging using nonlethal methods. Any macaque group that ranges close to the village border experiences nonlethal crop defense to encourage them to return deeper into the TNR.
Prior research examined the effects of tourism on crested macaque behavior and physiology inside TNR, but none have investigated physiological responses on an individual level. Paulsen [
55] found that crested macaques displayed aggressive behaviors more frequently and escalated aggression more quickly when in the presence of tourists, and also that fecal cortisol metabolite levels (pooled by group) were lower in a group experiencing intermediate numbers of tourists than either a group experiencing more tourists or a group experiencing almost no tourism. More recently, Bertrand et al. [
56] found that
M. nigra groups displayed behavioral changes when exposed to tourists. Specifically, macaques showed signs of behavioral inhibition (a general decrease in several behaviors indicative of vigilance) when more tourists were present in the forest. They also showed signs of both inhibition and increases in stress related behavior (for example aggression) when tourists were directly present in groups, similar to how primates perceive predators posing varying degrees of risk.
Here, we test the general hypothesis that levels of FGCM are associated with aspects of tourism in three apparently habituated groups of wild
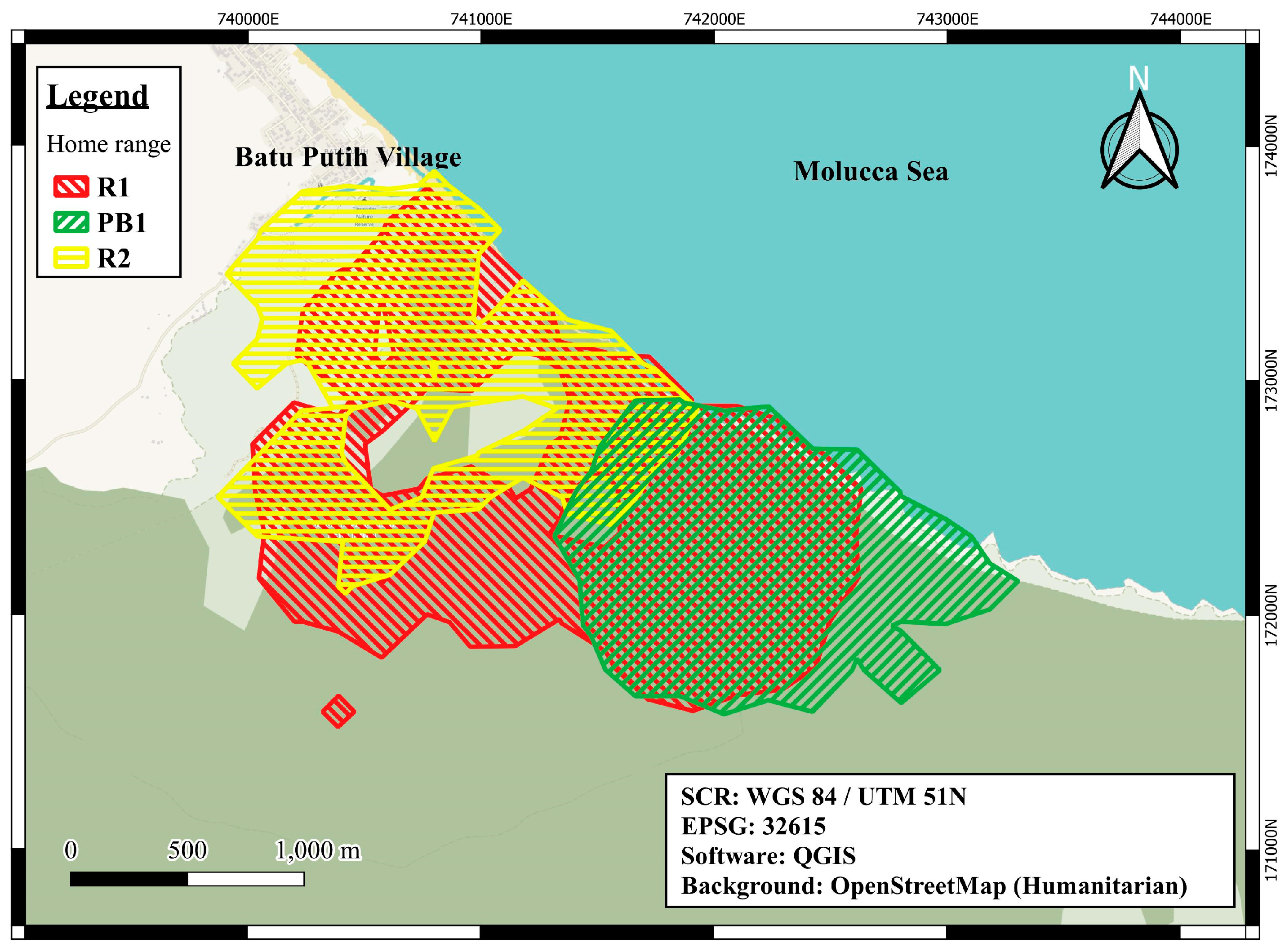
M. nigra in TNR named R1, R2, and PB1. These habituated groups represent a natural experiment, each exposed to different frequencies of tourist visits (study group names: R2 = frequently, R1 = moderately, and PB1 = rarely/research only). The natural experiment is due to their ranging patterns (see Methods,
Section 2.2,
Figure 1); R2 was visited by tourist groups almost daily, R2 was visited generally 3–5 days a week, and PB1 generally once every other month.
1.6. Specific Hypotheses (Table 1)
Although one study group (PB1) rarely encountered tourists, tourists can be loud. Large groups of tourists can be heard by all three study groups from far away. For this reason, we explored the possible effects on macaque FGCM levels of (1) tourists in the reserve when away from the study groups and (2) tourists while in the presence of the study groups.
4. Discussion
This study aimed to test the general hypothesis that glucocorticoid output in wild M. nigra in Tangkoko Nature Reserve, NE Sulawesi, Indonesia is related to aspects of tourism. We collected fecal samples for the measurement of glucocorticoid metabolite (FGCM) levels from three habituated social groups with varying levels of exposure to tourism. Overall, our results suggest that the two groups habituated for tourism, but not the research-only group, exhibited increased levels of FGCMs as the number of tourists in the forest increased, even though tourists were not present within the study groups. When tourists were in the tourism groups, males also showed higher levels of FGCMs on those days when many tourists were present within the group. While female FGCM levels did not apparently respond to variations in the daily numbers of tourists present within the group, those in one tourism group showed a temporal pattern of FGCM increases and decreases to the presence of any tourists that were consistent with indications of acute stress; fecal FGCMs increased from undisturbed levels 36–48 h after exposure and then returned to undisturbed levels the next day. In contrast, males in the tourism groups showed no significant differences between undisturbed, exposure, or postexposure conditions, suggesting little evidence of an FGCM response. FGCM responses to the number of researchers in the group varied by sex, group, and tourist condition. Overall, the relationships we found between FGCM responses and exposure to tourists were independent of relationships between FGCM levels and sex hormones, food availability, physical activity, and rainfall, as well as other factors we controlled (dominance rank, the frequency of crop defense, and the numbers of infants and estrous females present). As such, our findings cannot be attributed to increased metabolic demand from these sources. Thus, we tentatively suggest that tourism in Tangkoko Nature Reserve is a source of acute anthropogenic stress, but not chronic stress for some M. nigra, despite their being exposed to tourism for decades. Below, we develop this argument in greater detail.
(H1) In those months in which greater numbers of tourists were present in the forest, we saw increases in FGCM levels in both males and females in the tourism groups, suggesting that for tourism-habituated groups, greater numbers of tourists in the forest may increase metabolic demand, at least in the short term (H1a–c). In contrast, in the research-only group, females showed no change, and surprisingly, males showed decreased levels. These results are consistent with findings in Yucatan black howler (
Alouatta pigra) [
87] and in
G.g. gorilla [
11] that showed that tourism-exposed groups tended to have higher FGCM levels than research-only groups. Although the results for our tourism groups were consistent across groups and sexes, it should be noted that the number of undisturbed samples for these groups was modest, given that on most days, the groups experienced tourism and/or crop defense. This was especially the case for R2. While slopes for the tourist groups show marked differences from the slopes for PB1 (females: R2 = 4.0; R1 = 5.0; PB1 = 0; males: R2 = 23.3; R1 = 0.5; PB1 = −0.1), those for R2 in particular should be interpreted cautiously. A follow-up study could collect more undisturbed samples over a longer time period from the two tourism groups to better understand their responses.
It is unclear why males in the research-only group showed decreased FGCM levels in months with more tourists in the forest. Given that stress increases with uncertainty [
88] and decreases when situations become more predictable [
89], it may be that males in the research-only group, which was generally farther from most of the tourism activity, could detect the presence of tourists in the forest from a greater distance than the tourism groups and hence avoid their presence more easily, doing so more often when they detected more tourists in the forest. We observed the research-only group moving away from tourists during accidental encounters, likely to areas farther away from tourists than the other tourism groups. The fact that FGCM levels in females in the research-only group remained constant suggests that they may also have benefited from staying out of the areas perceived to be risky. We previously found that the same groups of
M. nigra responded to tourists in the forest with behavioral inhibition, suggesting increased vigilance (similar to when primates detect predators at a distance) rather than increases in stress-related behavior [
56]. Prior research has shown that low levels of predation risk in a variety of species may increase vigilance, without increasing FGCMs, or may even be accompanied by a decrease (see review by [
90]). See also the discussion of H3 below.
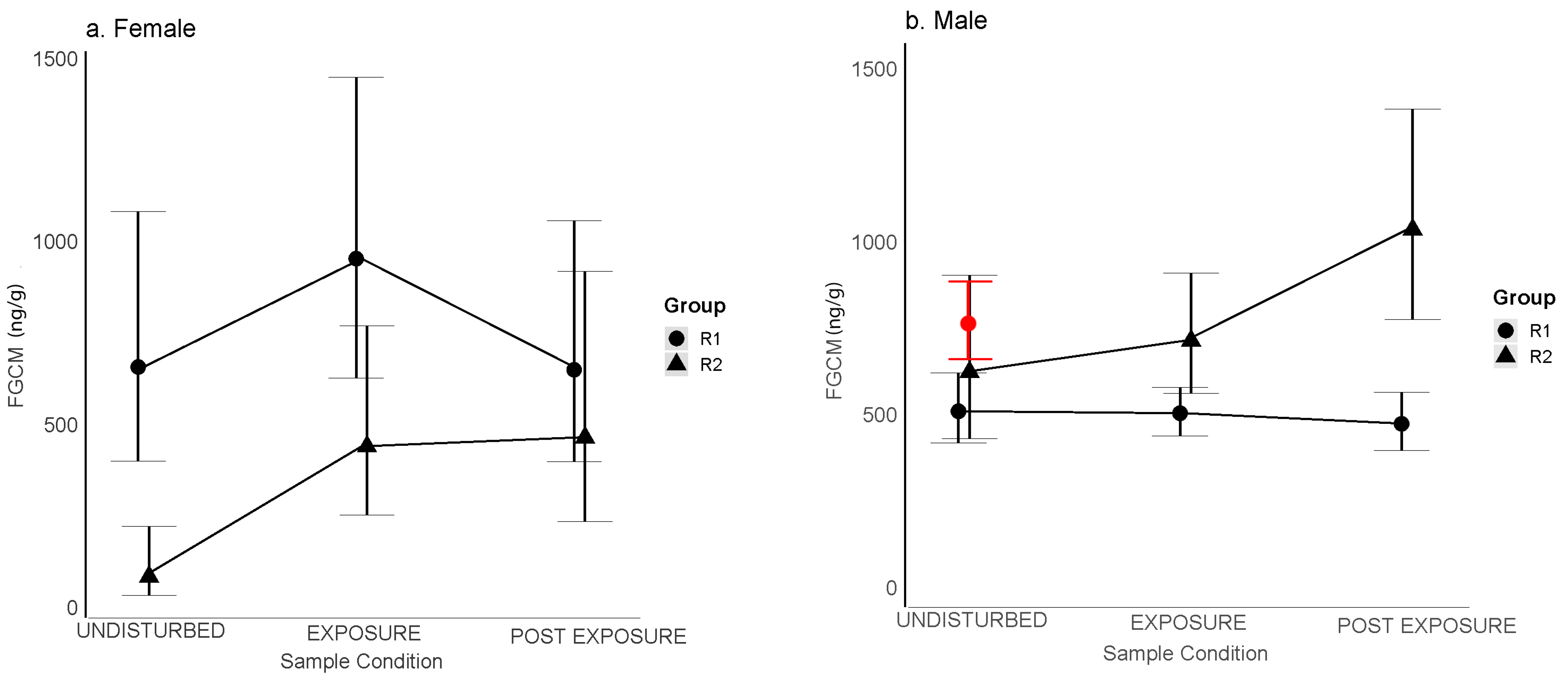
(H2) When tourists were within the two tourism groups, females in R1 experienced a pattern of change in FGCM levels that was consistent with predictions for acute stress; FGCM levels rose from undisturbed levels following exposure to tourists within their groups and then returned to undisturbed levels thereafter (H2a). This result is also consistent with our earlier findings that these females displayed increases in aggression and decreases in sociality when tourists were present within their groups [
56]. A return to undisturbed FGCM levels after exposure to a stressor is generally considered an adaptive response and is often seen as an indication that the stressor is unlikely to cause long-term physiological harm [
91]. While this may be the case for these females, we cannot rule out the possibility that acute stress may be damaging physiologically if it occurs frequently, depending on the reproductive state of the female. Seminal research by Moberg [
92,
93] showed how the stress-induced secretion of cortisol can affect fertility by disrupting the synthesis and secretion of both the follicle-stimulating hormone and the luteinizing hormone. R2 females also showed a significant increase from the undisturbed to the exposure samples, but no significant decrease from exposure to postexposure samples at least within a day. This may be indicative of an inability to return quickly to undisturbed levels after experiencing a stressor (H2b). Although a prolonged return to undisturbed levels can indicate a chronic stress response, we think this is unlikely because subsequent undisturbed samples in this group were often collected after only one more additional day without tourists or crop defense. Hence, it is likely that these females returned to undisturbed levels after about two days postexposure. Males on the other hand showed no significant change and no differences in overall undisturbed FGCM levels (i.e., when not considering numbers of tourists in the forest) from males in the research-only group, results that point to a lack of a stress response to the presence of tourists (H2c) rather than chronic stress. Notably, however, despite the lack of significant differences between tourist conditions, the pattern of changes between conditions for R2 males appeared similar to that for R2 females (
Figure 2a,b), raising the possibility that a larger sample size for R2 males may have yielded a similar significant pattern, and hence a similar possible inability to return quickly to undisturbed levels. Given that R2 males also returned to undisturbed levels often only a day or two after the postexposure samples, it is also unlikely that their responses can be characterized as chronic stress responses. Assuming these interpretations are correct, differences between R1 and R2 may be due to the fact that R2 experienced more frequent tourist visits and may have experienced somewhat more stress as a result.
Our findings for males where undisturbed and exposure levels both increased in those months with more tourists in the forest (H1a) and on days with more tourists within groups (H2d) could be seen as inconsistent with our findings of no significant changes from undisturbed to exposure levels (H2c); however, the fact that undisturbed levels and exposure levels to tourists within groups were similar to each other over the months suggests that these males may have been able to cope with increases in tourist numbers in a healthy manner. Similar increases in females’ FGCM levels in months with more tourists in the forest could be seen as inconsistent with R1 females’ quick recoveries from the presence of tourists within groups. However, the fact that R1 female responses to tourists within groups appeared to recover quickly, and R2 females likely returned to undisturbed levels in another day or so, also suggests that, even if tourists are a source of stress, they may also be able to cope with tourist presence in a healthy manner.
Our findings related to the nature of FGCM responses to tourism are similar to those for
P.p morio subject to tourism. Muehlenbein et al. [
26] found acute but not chronic increases in fecal cortisol metabolites after tourist visits, and in fact lower FGCM levels than in a group subject to research only. Maréchal et al. [
47] also found higher fecal glucocorticoids among
M. sylvanus after tourist encounters in Gibraltar. However, this occurred specifically in samples collected after aggressive interactions with tourists [
47]. Although aggressive interactions with tourists at Tangkoko Nature Reserve are rare enough to be considered almost nonexistent (D.B., personal observations), a renewed examination of FGCM responses would be warranted should this scenario change. Additionally, our finding of increasing FGCM levels in males as the daily number of tourists increased (H2d) suggests that limiting the daily number of tourists allowed in each group may alleviate potential stress responses in the future.
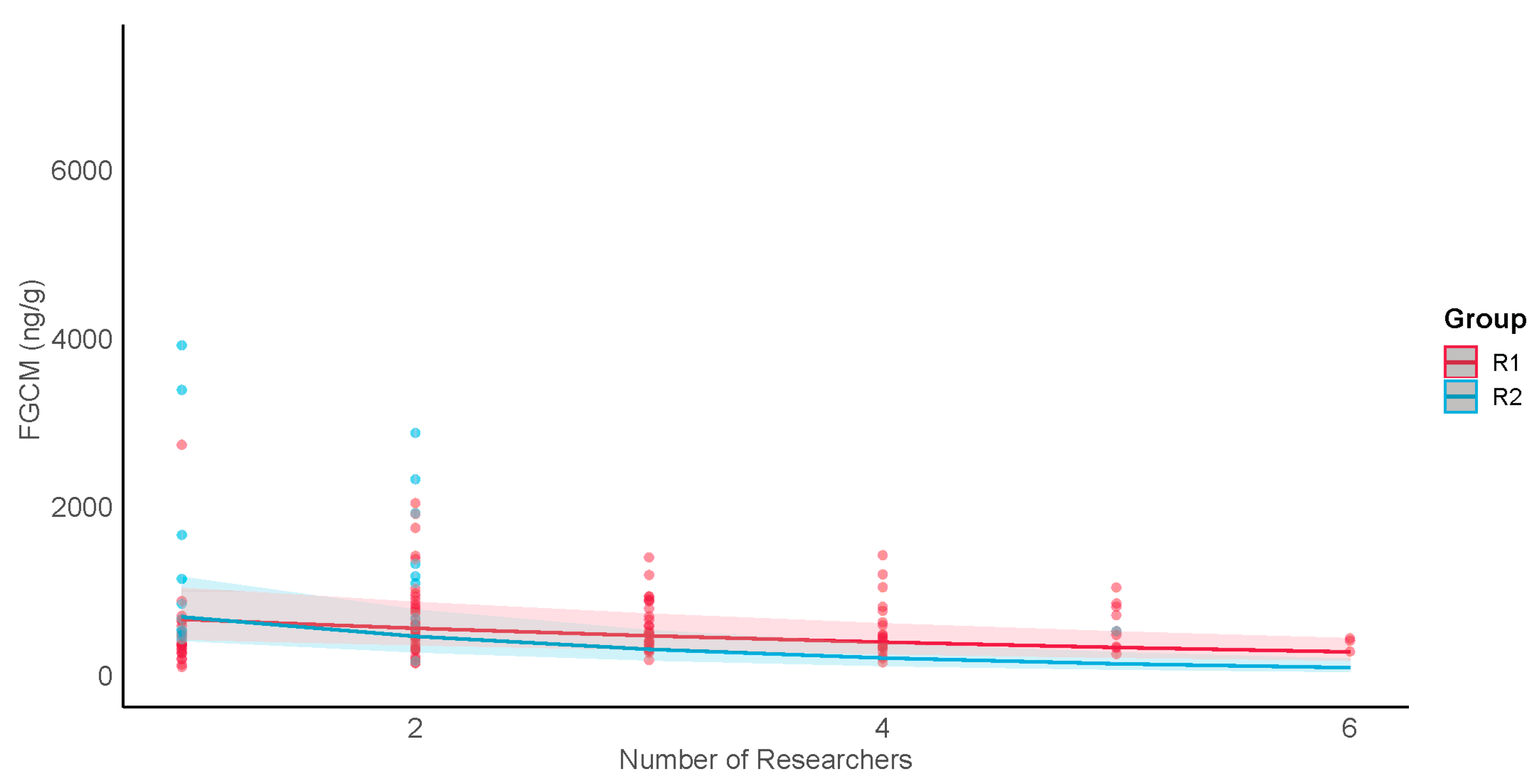
(H3) When no tourists were present (i.e., during undisturbed conditions), FGCM levels increased with the numbers of researchers for females from the research-only group and for both sexes in one tourism group (R1) (H3). This is consistent with previous research showing that females in tourism groups displayed higher levels of in-group aggression on the days when more researchers were present [
56]. In contrast, FGCM levels in the research-only group decreased with the numbers of researchers for males. These differences suggest that more researchers may increase metabolic demand in some groups/sexes but decrease it in others. The reason for this is unclear. Although researchers (who wore identical shirts and received rigorous training to ensure that they behaved in similar ways with the macaques) were easily distinguished from tourists, it may be that researcher gender or time spent with MNP working with these groups specifically may have played a role in our results. A more robust sample size would allow for a finer-tuned understanding of how researcher presence relates to
M. nigra FGCM levels. Nevertheless, it may be beneficial to take a conservative approach and limit the daily number of researchers in the tourism groups to four, even on days when there are no tourists present, and to reduce the number of researchers present in the research-only group.
When tourists were present within groups (i.e., during exposure conditions), males in both tourism groups showed decreased FGCM levels as the numbers of researchers increased, suggesting that researchers may act as buffers to challenges from exposure to tourists. In contrast, females showed no response to different numbers of researchers present. Why females did not respond like males is not clear; however, it may be that a larger sample size is needed.
While there is a paucity of research specifically exploring the FGCM responses of primates to researchers, one such study focused on South African samangos (
Cercopithecus albogularis). LaBarge et al. [
94] found that female FGCM acute responses to predators flattened as observer numbers increased. While it was not possible to determine if this decreased response was due to observers inadvertently deterring predators, it does lend support to the idea that the presence of several familiar humans might affect primate perception of danger [
94]. Future studies that pair FGCM levels with specific tourist/macaque interaction data may clarify this issue.
(H4) Although FGCM levels can be influenced by many internal and environmental factors [
33,
39,
40,
41,
42,
43,
44,
45,
46], we attempted to control for as many such potential confounds. Not surprisingly, we found significant positive relationships between FGCM levels, sex hormones, food availability, physical activity, and rainfall, and significant generally negative associations with dominance rank, crop defense, and infant presence. Nevertheless, these relationships were unable to explain the significant relationships we found between FGCM levels and tourists, adding to the strength of our findings and highlighting the importance of controlling for as many factors as possible.