Growth Performance and Fecal Microbiota of Dairy Calves Supplemented with Autochthonous Lactic Acid Bacteria as Probiotics in Mexican Western Family Dairy Farming
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. LAB Strains and Probiotic Preparation
2.2. Animals, Treatments and Feeding
2.3. Growth Performance
2.4. Feces Sampling and DNA Extraction
2.5. Construction of the 16S rRNA Libraries and Sequencing Procedures
2.6. Bioinformatics
2.7. Functional Prediction
2.8. Statistical Analysis
3. Results
3.1. Growth Parameters
3.2. Fecal Microbiota
3.3. Functional Prediction of Bacterial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of Gut Microbes on Nutrient Absorption and Energy Regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef]
- Zommiti, M.; Ferchichi, M. Probiotics and Prebiotics in Animal Feed. In Probiotics and Prebiotics in Foods: Challenges, Innovations, and Advances; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar] [CrossRef]
- Zeineldin, M.; Barakat, R.; Elolimy, A.; Salem, A.Z.M.; Elghandour, M.M.Y.; Monroy, J.C. Synergetic Action between the Rumen Microbiota and Bovine Health. Microb. Pathog. 2018, 124, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Shiels, P.G.; Painer, J.; Natterson-Horowitz, B.; Johnson, R.J.; Miranda, J.J.; Stenvinkel, P. Manipulating the Exposome to Enable Better Ageing. Biochem. J. 2021, 478, 2889–2898. [Google Scholar] [CrossRef]
- Al Omran, Y.; Aziz, Q. The Brain-Gut Axis in Health and Disease. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Lyte, M., Cryan, J.F., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2014; Volumn 817. [Google Scholar] [CrossRef]
- Chang, M.N.; Wei, J.Y.; Hao, L.Y.; Ma, F.T.; Li, H.Y.; Zhao, S.G.; Sun, P. Effects of Different Types of Zinc Supplement on the Growth, Incidence of Diarrhea, Immune Function, and Rectal Microbiota of Newborn Dairy Calves. J. Dairy Sci. 2020, 103, 6100–6113. [Google Scholar] [CrossRef] [PubMed]
- Varada, V.V.; Kumar, S.; Tyagi, N.; Tyagi, A.K. Effects of Compound Lyophilized Probiotics on Selected Faecal Microbiota, Immune Response, and Antioxidant Status in Newborn Buffalo Calves. Curr. Res. Biotechnol. 2022, 4, 493–502. [Google Scholar] [CrossRef]
- Golić, N.; Đokić, J. Farm Animals and Pets—Impact on Gut Microbiota. In Comprehensive Gut Microbiota; Elsevier: Amsterdam, The Netherlands, 2022; pp. 125–138. [Google Scholar] [CrossRef]
- Kimminau, E.A.; Karnezos, T.P.; Berghaus, R.D.; Jones, M.K.; Baxter, J.A.; Hofacre, C.L. Combination of Probiotic and Prebiotic Impacts Salmonella Enteritidis Infection in Layer Hens. J. Appl. Poult. Res. 2021, 30, 100200. [Google Scholar] [CrossRef]
- Liao, S.F.; Nyachoti, M. Using Probiotics to Improve Swine Gut Health and Nutrient Utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef]
- Xu, H.; Huang, W.; Hou, Q.; Kwok, L.; Sun, Z.; Ma, H.; Zhao, F.; Lee, Y.-K.; Zhang, H. The Effects of Probiotics Administration on the Milk Production, Milk Components and Fecal Bacteria Microbiota of Dairy Cows. Sci. Bull. 2017, 62, 767–774. [Google Scholar] [CrossRef]
- Dankwa, A.S.; Humagain, U.; Ishaq, S.L.; Yeoman, C.J.; Clark, S.; Beitz, D.C.; Testroet, E.D. Bacterial Communities in the Rumen and Feces of Lactating Holstein Dairy Cows Are Not Affected When Fed Reduced-Fat Dried Distillers’ Grains with Solubles. Animal 2021, 15, 100281. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Y.; Yu, Z.; Xu, Q.; Zheng, N.; Zhao, S.; Huang, G.; Wang, J. Ruminal Microbiota–Host Interaction and Its Effect on Nutrient Metabolism. Anim. Nutr. 2021, 7, 49–55. [Google Scholar] [CrossRef]
- Rey, M.; Enjalbert, F.; Combes, S.; Cauquil, L.; Bouchez, O.; Monteils, V. Establishment of Ruminal Bacterial Community in Dairy Calves from Birth to Weaning Is Sequential. J. Appl. Microbiol. 2014, 116, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R. Probiotics for Farm Animals. In Probiotics: A Critical Review; Tannock, G.W., Ed.; Horizon Scientific Press: Wymondham, UK, 1999; pp. 15–22. [Google Scholar]
- Terzić-Vidojević, A.; Veljović, K.; Tolinački, M.; Živković, M.; Lukić, J.; Lozo, J.; Fira, Đ.; Jovčić, B.; Strahinić, I.; Begović, J.; et al. Diversity of Non-Starter Lactic Acid Bacteria in Autochthonous Dairy Products from Western Balkan Countries—Technological and Probiotic Properties. Food Res. Int. 2020, 136, 109494. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Pradhan, S.; Chakrabarti, S.; Mondal, K.C.; Ghosh, K. Current Status of Probiotic and Related Health Benefits. Appl. Food Res. 2022, 2, 100185. [Google Scholar] [CrossRef]
- Gopal, P.K. Bacteria, Beneficial: Probiotic Lactic Acid Bacteria: An Overview. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2022; pp. 32–33. [Google Scholar]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2002.
- Reyes-Leos, J.R. Identificación de Cepas de Lactobacillus a Partir de Muestras de Estiércol Bovino de Granjas Productoras de Leche de La Región Altos Sur Del Estado de Jalisco. Bachelor Thesis, Centro Universitario Lagos de Moreno, Universidad de Guadalajara, Lagos de Moreno, Mexico, 2020. [Google Scholar]
- Ruvalcaba-Gómez, J.M.; Ruiz-Espinosa, H.; Méndez-Robles, M.D.; Arteaga-Garibay, R.I.; Anaya-Esparza, L.M.; Villagrán, Z.; Delgado-Macuil, R.J. Use of Autochthonous Lactic Acid Bacteria as Starter Culture of Pasteurized Milk Adobera Cheese. Fermentation 2022, 8, 234. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle; National Academies Press: Washington, DC, USA, 2001; ISBN 978-0-309-06997-7.
- Dingwell, R.T.; Wallace, M.M.; McLaren, C.J.; Leslie, C.F.; Leslie, K.E. An Evaluation of Two Indirect Methods of Estimating Body Weight in Holstein Calves and Heifers. J. Dairy Sci. 2006, 89, 3992–3998. [Google Scholar] [CrossRef]
- Villaseñor González, F.; Estrada Cortés, E.; Montes Oceguera, L.D.R.; Vera Ávila, H.R.; Montiel Olguín, L.J.; Jiménez Severiano, H.; Espinosa Martínez, M.A. Factores Asociados a Indicadores de Crianza de Reemplazos Bovinos Durante El Periodo de Lactancia En Unidades Lecheras de Pequeña Escala. Rev. Mex. Cienc. Pecu. 2022, 13, 64–81. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Ewels, P.A.; Peltzer, A.; Fillinger, S.; Patel, H.; Alneberg, J.; Wilm, A.; Garcia, M.U.; Di Tommaso, P.; Nahnsen, S. The Nf-Core Framework for Community-Curated Bioinformatics Pipelines. Nat. Biotechnol. 2020, 38, 276–278. [Google Scholar] [CrossRef]
- Straub, D.; Blackwell, N.; Langarica-Fuentes, A.; Peltzer, A.; Nahnsen, S.; Kleindienst, S. Interpretations of Environmental Microbial Community Studies Are Biased by the Selected 16S RRNA (Gene) Amplicon Sequencing Pipeline. Front. Microbiol. 2020, 11, 550420. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.; Billington, R.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P.D. The MetaCyc Database of Metabolic Pathways and Enzymes—A 2019 Update. Nucleic Acids Res 2020, 48, D445–D453. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Ptak, C.P.; Chang, C.-Y.; Ian, M.-K.; Chia, M.-Y.; Chen, T.-H.; Kuo, C.-J. Autochthonous Lactic Acid Bacteria Isolated From Dairy Cow Feces Exhibiting Promising Probiotic Properties and in Vitro Antibacterial Activity Against Foodborne Pathogens in Cattle. Front. Vet. Sci. 2020, 7, 239. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, S.; Vinay, V.V.; Tyagi, B.; Choudhury, P.K.; Rashmi, H.M.; Banakar, P.S.; Tyagi, N.; Tyagi, A.K. Autochthonous Lactobacillus spp. Isolated from Murrah Buffalo Calves Show Potential Application as Probiotic. Curr. Res. Biotechnol. 2021, 3, 109–119. [Google Scholar] [CrossRef]
- Karamzadeh-Dehaghani, A.; Towhidi, A.; Zhandi, M.; Mojgani, N.; Fouladi-Nashta, A. Combined Effect of Probiotics and Specific Immunoglobulin Y directed against Escherichia coli on Growth Performance, Diarrhea Incidence, and Immune System in Calves. Animal 2021, 15, 100124. [Google Scholar] [CrossRef]
- Górka, P.; Budzińska, K.; Budziński, W.; Jankowiak, T.; Kehoe, S.; Kański, J. Effect of Probiotic and Nucleotide Supplementation in Milk Replacer on Growth Performance and Fecal Bacteria in Calves. Livest. Sci. 2021, 250, 104556. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, H.J.; Cui, Z.Q.; Zhang, Y.G. Effects of Supplementation with Lactobacillus plantarum 299v on the Performance, Blood Metabolites, Rumen Fermentation and Bacterial Communities of Preweaning Calves. Livest. Sci. 2020, 239, 104120. [Google Scholar] [CrossRef]
- Huuki, H.; Tapio, M.; Mäntysaari, P.; Negussie, E.; Ahvenjärvi, S.; Vilkki, J.; Vanhatalo, A.; Tapio, I. Long-Term Effects of Early-Life Rumen Microbiota Modulation on Dairy Cow Production Performance and Methane Emissions. Front. Microbiol. 2022, 13, 983823. [Google Scholar] [CrossRef]
- Signorini, M.L.; Soto, L.P.; Zbrun, M.V.; Sequeira, G.J.; Rosmini, M.R.; Frizzo, L.S. Impact of probiotic administration on the health and fecal microbiota of young calves: A meta-analysis of randomized controlled trials of lactic acid bacteria. Res. Vet. Sci. 2012, 93, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, H.; Gao, H.; Xia, Y.; Zan, L.; Zhao, C. A meta-analysis on the effects of probiotics on the performance of pre-weaning dairy calves. J. Anim. Sci. Biotechnol. 2023, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, B.M.; Rajaei-Sharifabadi, H.; Vazirigohar, M. A Meta-Analysis of the Effect of Probiotics Administration on Growth Performance of Suckling Calves in Iran. Iran. J. Appl. Anim. Sci. 2020, 10, 213–219. [Google Scholar]
- Frizzo, L.S.; Zbrun, M.V.; Soto, L.P.; Signorini, M.L. Effects of probiotics on growth performance in young calves: A meta-analysis of randomized controlled trials. Anim. Feed. Sci. Technol. 2011, 169, 147–156. [Google Scholar] [CrossRef]
- Uyeno, Y.; Shigemori, S.; Shimosato, T. Effect of Probiotics/Prebiotics on Cattle Health and Productivity. Microbes Environ. 2015, 30, 126–132. [Google Scholar] [CrossRef]
- Zeineldin, M.; Aldridge, B.; Lowe, J. Dysbiosis of the Fecal Microbiota in Feedlot Cattle with Hemorrhagic Diarrhea. Microb. Pathog. 2018, 115, 123–130. [Google Scholar] [CrossRef]
- Xiang, K.; Hu, X.; Mu, R.; Animal, L.; Bureau, H.; Li, S.; Wang, Y.; Zhao, C.; Zhang, N.; Fu, Y. Rumen Microbiota Alterations During Ketosis is Associated with the development of Mastitis in Dairy Cows. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Chang, M.; Wang, F.; Ma, F.; Jin, Y.; Sun, P. Supplementation with Galacto-Oligosaccharides in Early Life Persistently Facilitates the Microbial Colonization of the Rumen and Promotes Growth of Preweaning Holstein Dairy Calves. Anim. Nutr. 2022, 10, 223–233. [Google Scholar] [CrossRef]
- Gomez, D.E.; Arroyo, L.G.; Costa, M.C.; Viel, L.; Weese, J.S. Characterization of the Fecal Bacterial Microbiota of Healthy and Diarrheic Dairy Calves. J. Vet. Intern. Med. 2017, 31, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Auffret, M.D.; Dewhurst, R.J.; Duthie, C.A.; Rooke, J.A.; John Wallace, R.; Freeman, T.C.; Stewart, R.; Watson, M.; Roehe, R. The Rumen Microbiome as a Reservoir of Antimicrobial Resistance and Pathogenicity Genes Is Directly Affected by Diet in Beef Cattle. Microbiome 2017, 5, 159. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Xu, H.; Xin, H.; Zhang, Y. Metagenomic Analyses of Microbial and Carbohydrate-Active Enzymes in the Rumen of Holstein Cows Fed Different Forage-to-Concentrate Ratios. Front. Microbiol. 2019, 10, 649. [Google Scholar] [CrossRef]
- Kim, E.-T.; Lee, S.-J.; Kim, T.-Y.; Lee, H.-G.; Atikur, R.M.; Gu, B.-H.; Kim, D.-H.; Park, B.-Y.; Son, J.-K.; Kim, M.-H. Dynamic Changes in Fecal Microbial Communities of Neonatal Dairy Calves by Aging and Diarrhea. Animals 2021, 11, 1113. [Google Scholar] [CrossRef]
- Poudel, P.; Froehlich, K.; Casper, D.P.; St-Pierre, B. Feeding Essential Oils to Neonatal Holstein Dairy Calves Results in Increased Ruminal Prevotellaceae Abundance and Propionate Concentrations. Microorganisms 2019, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, X.; Liu, X.; Zhao, X.; Liu, S.; Li, Y.; Zhang, Y. Growth, Health, Rumen Fermentation, and Bacterial Community of Holstein Calves Fed Lactobacillus rhamnosus GG during the Preweaning Stage. J. Anim. Sci. 2019, 97, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, Y.; Huang, K.; Yang, B.; Zhang, Y.; Yu, Z.; Wang, J. Fecal Microbiota Dynamics and Its Relationship to Diarrhea and Health in Dairy Calves. J. Anim. Sci. Biotechnol. 2022, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, H.; Hua, D.; Zhang, F.; Wang, Y.; Liu, J.; Yao, J.; et al. Dietary Supplementation with Inulin Improves Lactation Performance and Serum Lipids by Regulating the Rumen Microbiome and Metabolome in Dairy Cows. Anim. Nutr. 2021, 7, 1189–1204. [Google Scholar] [CrossRef]
- Kodithuwakku, H.; Maruyama, D.; Owada, H.; Watabe, Y.; Miura, H.; Suzuki, Y.; Hirano, K.; Kobayashi, Y.; Koike, S. Alterations in Rumen Microbiota via Oral Fiber Administration during Early Life in Dairy Cows. Sci. Rep. 2022, 12, 10798. [Google Scholar] [CrossRef]
- Bu, D.; Zhang, X.; Ma, L.; Park, T.; Wang, L.; Wang, M.; Xu, J.; Yu, Z. Repeated Inoculation of Young Calves With Rumen Microbiota does not Significantly Modulate the Rumen Prokaryotic Microbiota Consistently but Decreases Diarrhea. Front. Microbiol. 2020, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Zhao, C.; Hu, P.; Chen, H.; Liu, Z.; Liu, G.; Wang, Z. Correlation between Composition of the Bacterial Community and Concentration of Volatile Fatty Acids in the Rumen during the Transition Period and Ketosis in Dairy Cows. Appl. Environ. Microbiol. 2012, 78, 2386–2392. [Google Scholar] [CrossRef]
- Oikonomou, G.; Teixeira, A.G.V.; Foditsch, C.; Bicalho, M.L.; Machado, V.S.; Bicalho, R.C. Fecal Microbial Diversity in Pre-Weaned Dairy Calves as Described by Pyrosequencing of Metagenomic 16S RDNA. Associations of Faecalibacterium Species with Health and Growth. PLoS ONE 2013, 8, e63157. [Google Scholar] [CrossRef]
- Rice, W.C.; Galyean, M.L.; Cox, S.B.; Dowd, S.E.; Cole, N.A. Influence of Wet Distillers Grains Diets on Beef Cattle Fecal Bacterial Community Structure. BMC Microbiol. 2012, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Shanks, O.C.; Kelty, C.A.; Archibeque, S.; Jenkins, M.; Newton, R.J.; McLellan, S.L.; Huse, S.M.; Sogin, M.L. Community Structures of Fecal Bacteria in Cattle from Different Animal Feeding Operations. Appl. Environ. Microbiol. 2011, 77, 2992–3001. [Google Scholar] [CrossRef]
- Du, W.; Wang, X.; Hu, M.; Hou, J.; Du, Y.; Si, W.; Yang, L.; Xu, L.; Xu, Q. Modulating Gastrointestinal Microbiota to Alleviate Diarrhea in Calves. Front. Microbiol. 2023, 14, 1181545. [Google Scholar] [CrossRef]
- Virginio, G.F.; Reis, M.E.; da Silva, A.P.; de Toledo, A.F.; Cezar, A.M.; Mendes, L.W.; Greco, L.; Montenegro, H.; Coutinho, L.L.; Bittar, C.M.M. Does Algae β-Glucan Affect the Fecal Bacteriome in Dairy Calves? PLoS ONE 2021, 16, e0258069. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; van Niekerk, J.K.; Zhou, M.; Steele, M.A. PSIX-32 Assessment of Mucosa-Associated Microbiota in the Colon and Rumen of Dairy Calves Fed High Plane of Milk and during Weaning Transition. J. Anim. Sci. 2020, 98 (Suppl. S4), 311. [Google Scholar] [CrossRef]
- Dong, C.; Wei, M.; Sun, F.; Bao, H.; Bao, M.; Ju, J.; Du, L. Effect of Lactic Acid Bacteria Preparations on Calf Fecal Flora. Rev. Bras. Zootec. 2023, 52, e20210199. [Google Scholar] [CrossRef]
- Wei, X.; Zou, J.; Zhang, Y.; Yang, J.; Wang, J.; Wang, Y.; Wang, C. Effects of Milk, Milk Replacer, and Milk Replacer plus Ethoxyquin on the Growth Performance, Weaning Stress, and the Fecal Microbiota of Holstein Dairy Calves. Front. Microbiol. 2023, 14, 1113518. [Google Scholar] [CrossRef]
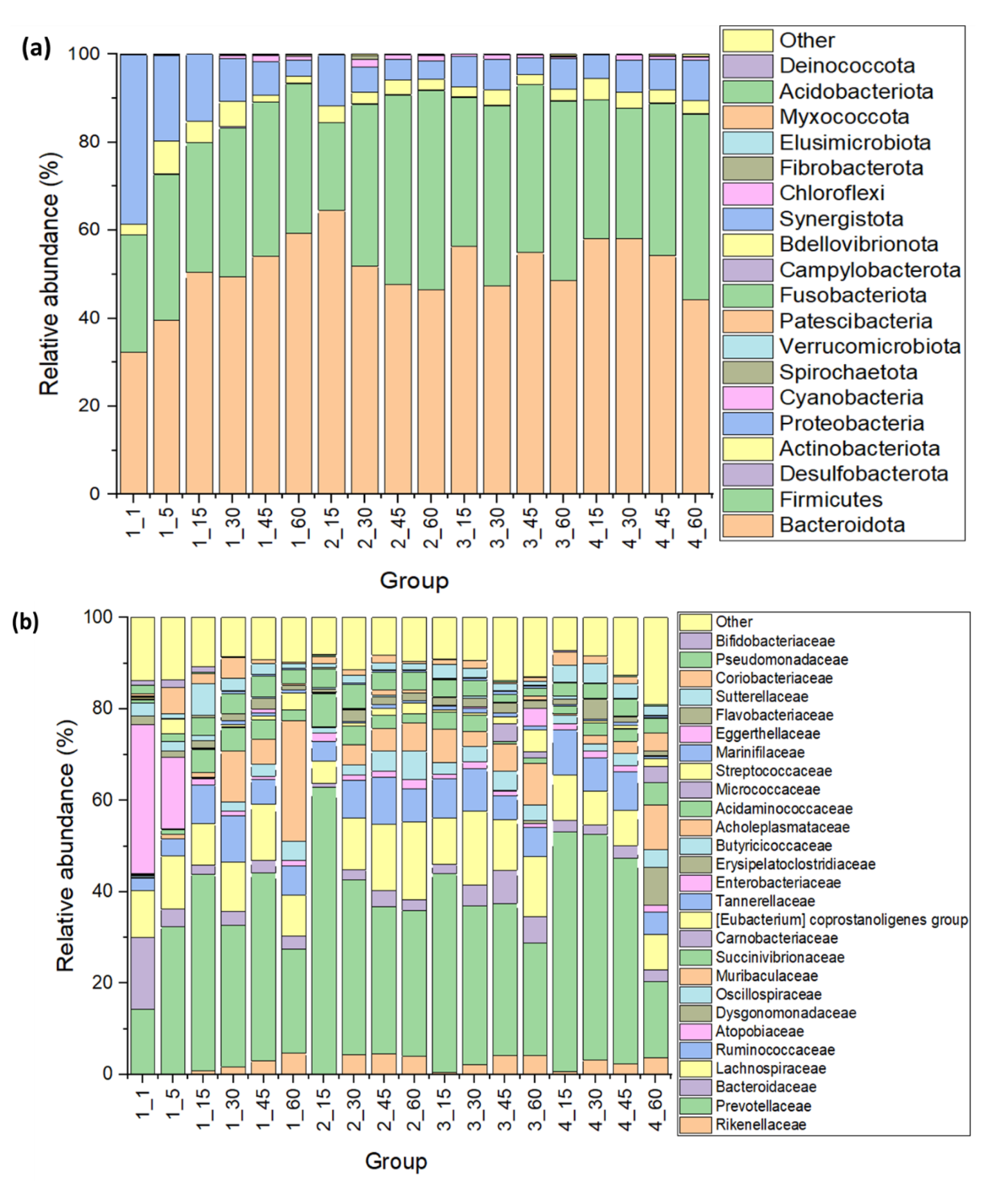

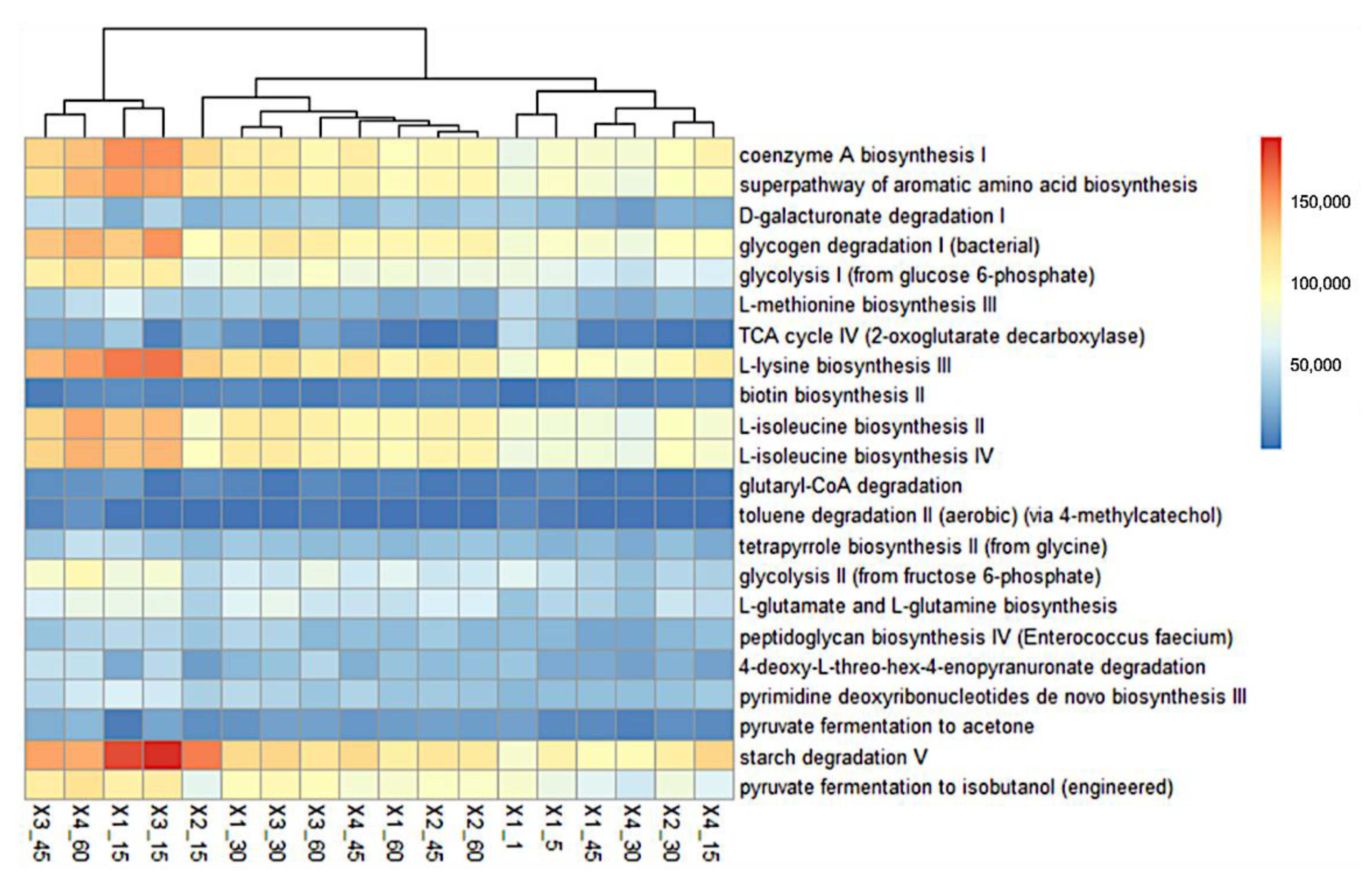
| Parameter/Age (Week) | Calves’ Groups (Teatment) | Significance | |||||
|---|---|---|---|---|---|---|---|
| Control | 6BZ | 6BY | 6BY + 6BZ | T | A | T × A | |
| Body weight (kg) | |||||||
| 0 | 39.55 ± 0.37 | 42.12 ± 0.76 | 40.62 ± 1.22 | 40.23 ± 0.64 | 0.4308 | <0.0001 | 0.7198 |
| 1 | 41.11 ± 0.78 | 42.87 ± 1.02 | 42.50 ± 1.42 | 42.23 ± 0.92 | |||
| 2 | 43.33 ± 1.05 | 45.62 ± 1.06 | 44.00 ± 1.80 | 44.29 ± 1.18 | |||
| 3 | 47.11 ± 1.33 | 48.87 ± 1.43 | 45.75 ± 2.20 | 47.58 ± 1.15 | |||
| 4 | 50.88 ± 1.52 | 54.75 ± 1.67 | 49.25 ± 2.45 | 52.76 ± 1.47 | |||
| 5 | 54.88 ± 1.78 | 59.50 ± 2.28 | 55.00 ± 3.21 | 57.47 ± 1.67 | |||
| 6 | 61.11 ± 2.07 | 64.12 ± 2.18 | 58.87 ± 3.51 | 62.82 ± 2.01 | |||
| 7 | 67.77 ± 3.13 | 71.00 ± 2.64 | 66.25 ± 2.51 | 69.82 ± 2.51 | |||
| 8 | 72.55 ± 3.01 | 78.25 ± 3.78 | 73.12 ± 3.30 | 77.52 ± 2.92 | |||
| Wither height (cm) | |||||||
| 0 | 78.66 ± 0.91 | 80.50 ± 0.53 | 80.50 ± 0.90 | 78.70 ± 0.98 | 0.8233 | <0.0001 | 0.4377 |
| 1 | 80.11 ± 1.05 | 80.62 ± 0.94 | 81.12 ± 1.07 | 79.58 ± 0.74 | |||
| 2 | 81.88 ± 1.00 | 82.50 ± 0.88 | 82.50 ± 0.80 | 81.47 ± 0.73 | |||
| 3 | 84.22 ± 0.79 | 84.25 ± 0.95 | 84.12 ± 0.85 | 83.41 ± 0.71 | |||
| 4 | 85.77 ± 0.74 | 86.50 ± 0.84 | 84.87 ± 0.93 | 85.70 ± 0.64 | |||
| 5 | 87.88 ± 0.77 | 87.87 ± 1.04 | 86.75 ± 1.17 | 87.52 ± 0.79 | |||
| 6 | 89.22 ± 0.86 | 90.62 ± 1.01 | 88.50 ± 1.21 | 89.23 ± 0.82 | |||
| 7 | 91.88 ± 1.47 | 92.00 ± 1.19 | 89.25 ± 1.91 | 91.11 ±0.75 | |||
| 8 | 93.77 ± 0.87 | 93.50 ± 1.23 | 92.25 ± 1.91 | 93.29 ± 0.98 | |||
| Parameter/Age (Months) | Calves’ Groups (Teatment) | Significance | |||||
|---|---|---|---|---|---|---|---|
| Control | 6BZ | 6BY | 6BY + 6BZ | T | A | T × A | |
| Body weight (kg) | |||||||
| 0 | 39.55 ± 0.78 | 42.12 ± 1.02 | 40.62 ± 1.22 | 40.23 ± 0.64 | 0.0480 | <0.0001 | <0.0001 |
| 1 | 101.11 ± 4.48 | 107.8 ± 4.20 | 99.50 ± 2.85 | 96.61 ± 2.61 | |||
| 2 | 123.55 ± 5.77 | 135.4 ± 3.16 | 130.37 ± 5.02 | 119.94 ± 3.46 | |||
| 3 | 145.66 ± 4.98 | 155.28 ± 3.16 | 157.37 ± 7.97 | 152.33 ± 5.15 | |||
| 4 | 166.55 ± 4.92 | 180.14 ± 4.65 | 195.87 ± 17.63 | 159.66 ± 4.44 | |||
| 5 | 186.66 ± 4.57 | 200.28 ± 5.97 | 202.00 ± 12.73 | 184.66 ± 5.90 | |||
| Wither height (cm) | |||||||
| 0 | 78.66 ± 0.91 | 80.50 ± 0.53 | 80.50 ± 0.90 | 78.70 ± 0.98 | 0.3541 | <0.0001 | 0.5826 |
| 1 | 98.33 ± 1.01 | 96.42 ± 2.10 | 97.62 ± 1.22 | 95.38 ± 0.74 | |||
| 2 | 102.00 ± 1.01 | 101.42 ± 1.19 | 102.62 ± 1.71 | 95.38 ± 0.63 | |||
| 3 | 107.88 ± 0.88 | 107.57 ± 1.26 | 108.00 ± 2.35 | 100.72 ± 0.74 | |||
| 4 | 111.33 ± 0.84 | 111.28 ± 1.40 | 113.00 ± 2.14 | 107.44 ± 0.76 | |||
| 5 | 116.55 ± 1.00 | 118.28 ± 1.24 | 117.50 ± 1.76 | 110.38 ± 0.66 | |||
| Age | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weeks | Months | ||||||||||
| Treatment | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 3 | 4 | 5 | |
| Control | P | 0.8955 | 0.590 | 0.590 | 0.514 | 0.380 | 0.382 | 0.498 | 0.528 | 0.601 | 0.681 |
| p | 0.001 | 0.094 | 0.094 | 0.156 | 0.313 | 0.310 | 0.172 | 0.143 | 0.086 | 0.04 | |
| 6BZ | P | 0.889 | 0.881 | 0.872 | 0.735 | 0.724 | 0.695 | 0.858 | 0.255 | 0.217 | 0.149 |
| p | 0.003 | 0.003 | 0.004 | 0.037 | 0.042 | 0.055 | 0.006 | 0.542 | 0.606 | 0.724 | |
| 6BY | P | 0.907 | 0.655 | 0.766 | 0.782 | 0.743 | 0.612 | 0.609 | 0.190 | 0.519 | 0.277 |
| p | 0.001 | 0.077 | 0.026 | 0.021 | 0.034 | 0.106 | 0.109 | 0.652 | 0.187 | 0.507 | |
| 6BY + 6BZ | P | 0.914 | 0.813 | 0.631 | 0.633 | 0.652 | 0.488 | 0.526 | 0.579 | 0.653 | 0.801 |
| p | <0.0001 | <0.0001 | 0.006 | 0.006 | 0.004 | 0.046 | 0.03 | 0.014 | 0.004 | 0.0001 | |
| Age | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weeks | Months | ||||||||||
| Treatment | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 3 | 4 | 5 | |
| Control | P | 0.939 | 0.639 | 0.782 | 0.579 | 0.741 | 0.492 | 0.481 | 0.547 | 0.478 | 0.465 |
| p | 0.000 | 0.064 | 0.013 | 0.102 | 0.022 | 0.178 | 0.190 | 0.127 | 0.193 | 0.207 | |
| 6BZ | P | 0.879 | 0.231 | 0.269 | 0.455 | 0.413 | 0.428 | 0.581 | 0.502 | 0.689 | 0.527 |
| p | 0.004 | 0.583 | 0.520 | 0.257 | 0.309 | 0.290 | 0.131 | 0.205 | 0.059 | 0.180 | |
| 6BY | P | 0.822 | 0.725 | 0.795 | 0.683 | 0.872 | 0.712 | 0.786 | 0.358 | 0.320 | 0.430 |
| p | 0.012 | 0.042 | 0.018 | 0.062 | 0.005 | 0.047 | 0.021 | 0.384 | 0.440 | 0.287 | |
| 6BY + 6BZ | P | 0.886 | 0.820 | 0.664 | 0.661 | 0.601 | 0.541 | 0.626 | 0.538 | 0.472 | 0.457 |
| p | <0.000 | <0.000 | 0.004 | 0.004 | 0.011 | 0.025 | 0.007 | 0.026 | 0.056 | 0.065 | |
| Treatment | ||||
|---|---|---|---|---|
| Average Relative Abundance (%, at Day 60) | ||||
| Control | 6BZ | 6BY | 6BY + 6BZ | |
| Phylum | ||||
| Bacteroidota | 59.3 | 46.5 | 48.6 | 44.1 |
| Firmicutes | 34.0 | 45.3 | 40.6 | 42.2 |
| Actinobacteriota | 1.7 | 2.5 | 2.6 | 3.1 |
| Proteobacteria | 3.6 | 4.1 | 7.0 | 9.1 |
| Class | ||||
| Bacteroidia | 59.3 | 46.5 | 48.6 | 44.1 |
| Bacilli | 3.6 | 3.5 | 6.5 | 12.3 |
| Clostridia | 26.9 | 37.5 | 32.1 | 26 |
| Negativicutes | 3.3 | 4.0 | 1.8 | 3.2 |
| Desulfovibrionia | 0.0 | 0.0 | 0.1 | 0.1 |
| Coriobacteriia | 1.6 | 2.4 | 1.7 | 1.7 |
| Gammaproteobacteria | 3.4 | 3.9 | 6.6 | 8.7 |
| Family | ||||
| Enterobacteriaceae | 0.0 | 0.0 | 3.85 | 0.09 |
| Prevotellaceae | 22.7 | 31.9 | 24.8 | 16.7 |
| Bacteroidaceae | 2.9 | 2.3 | 5.6 | 2.6 |
| Lachnospiraceae | 8.9 | 17.1 | 13.2 | 7.7 |
| Muribaculaceae | 26.4 | 6.1 | 9.1 | 9.8 |
| Succinivibrionaceae | 2.3 | 2.1 | 1.2 | 5.0 |
| Carnobacteriaceae | 0.0 | 0.1 | 1.3 | 3.5 |
| Acholeplasmataceae | 0.3 | 0.8 | 0.8 | 3.9 |
| Genus | ||||
| Bacteroides | 2.9 | 2.3 | 5.6 | 2.6 |
| Alistipes | 0.3 | 0.1 | 0.6 | 0.5 |
| Prevotella | 17.7 | 21.7 | 18.5 | 10.3 |
| Alloprevotella | 3.7 | 9.1 | 5.8 | 5.0 |
| Ruminococcus | 1.7 | 2.0 | 2.5 | 2.2 |
| Faecalibacterium | 0.7 | 1.0 | 1.1 | 1.1 |
| Pseudobutyrivibrio | 0.07 | 0.34 | 0.23 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruvalcaba-Gómez, J.M.; Villaseñor-González, F.; Espinosa-Martínez, M.A.; Gómez-Godínez, L.J.; Rojas-Anaya, E.; Villagrán, Z.; Anaya-Esparza, L.M.; Buendía-Rodríguez, G.; Arteaga-Garibay, R.I. Growth Performance and Fecal Microbiota of Dairy Calves Supplemented with Autochthonous Lactic Acid Bacteria as Probiotics in Mexican Western Family Dairy Farming. Animals 2023, 13, 2841. https://doi.org/10.3390/ani13182841
Ruvalcaba-Gómez JM, Villaseñor-González F, Espinosa-Martínez MA, Gómez-Godínez LJ, Rojas-Anaya E, Villagrán Z, Anaya-Esparza LM, Buendía-Rodríguez G, Arteaga-Garibay RI. Growth Performance and Fecal Microbiota of Dairy Calves Supplemented with Autochthonous Lactic Acid Bacteria as Probiotics in Mexican Western Family Dairy Farming. Animals. 2023; 13(18):2841. https://doi.org/10.3390/ani13182841
Chicago/Turabian StyleRuvalcaba-Gómez, José Martín, Fernando Villaseñor-González, Mario Alfredo Espinosa-Martínez, Lorena Jacqueline Gómez-Godínez, Edith Rojas-Anaya, Zuamí Villagrán, Luis Miguel Anaya-Esparza, Germán Buendía-Rodríguez, and Ramón Ignacio Arteaga-Garibay. 2023. "Growth Performance and Fecal Microbiota of Dairy Calves Supplemented with Autochthonous Lactic Acid Bacteria as Probiotics in Mexican Western Family Dairy Farming" Animals 13, no. 18: 2841. https://doi.org/10.3390/ani13182841
APA StyleRuvalcaba-Gómez, J. M., Villaseñor-González, F., Espinosa-Martínez, M. A., Gómez-Godínez, L. J., Rojas-Anaya, E., Villagrán, Z., Anaya-Esparza, L. M., Buendía-Rodríguez, G., & Arteaga-Garibay, R. I. (2023). Growth Performance and Fecal Microbiota of Dairy Calves Supplemented with Autochthonous Lactic Acid Bacteria as Probiotics in Mexican Western Family Dairy Farming. Animals, 13(18), 2841. https://doi.org/10.3390/ani13182841







