Genomic Epidemiology and Evolution of Fowl Adenovirus 1
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Strains
2.2. Sequencing
2.3. Sequence Analysis
3. Results
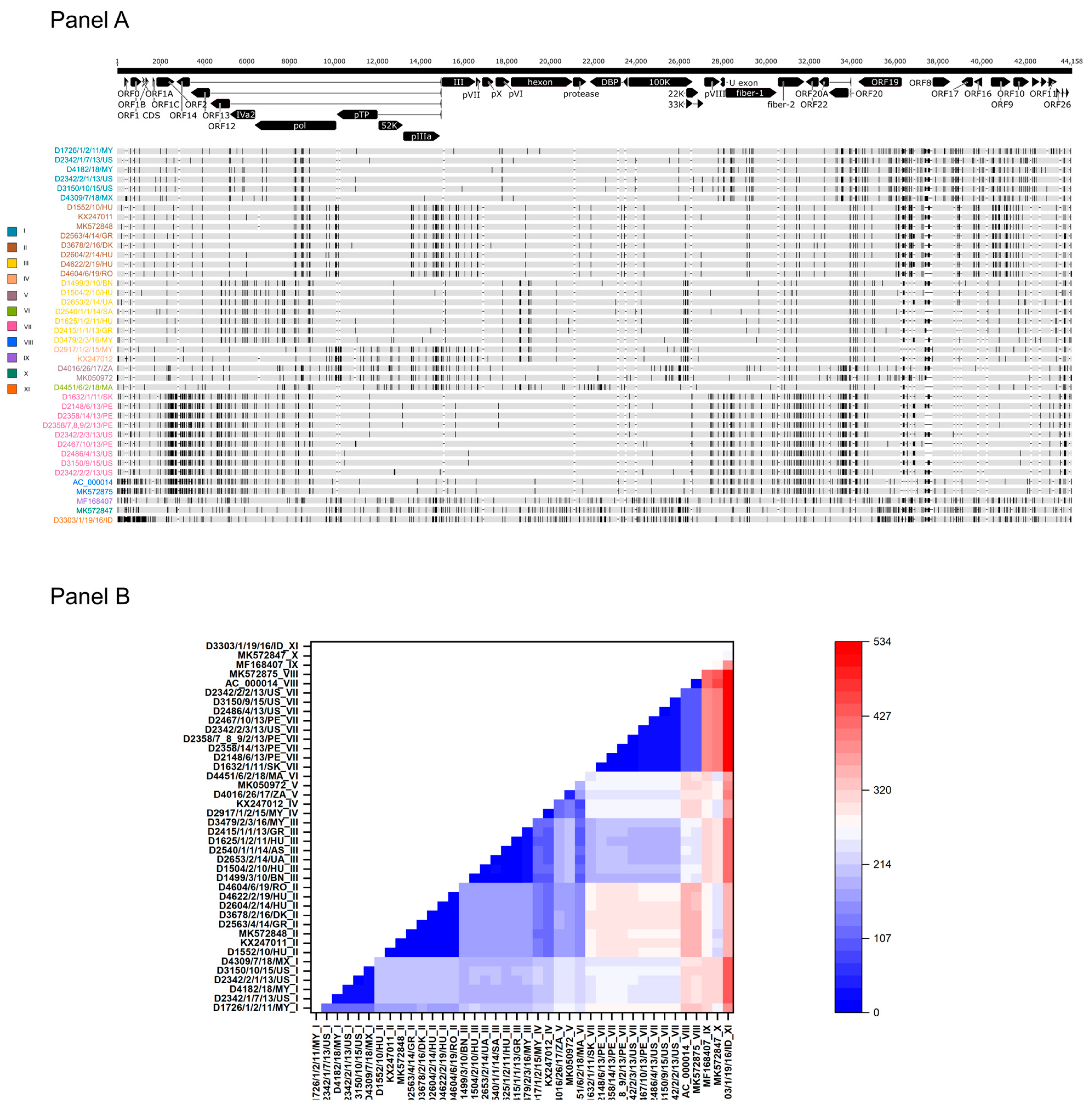
3.1. Features of FAdV-1 Genomes
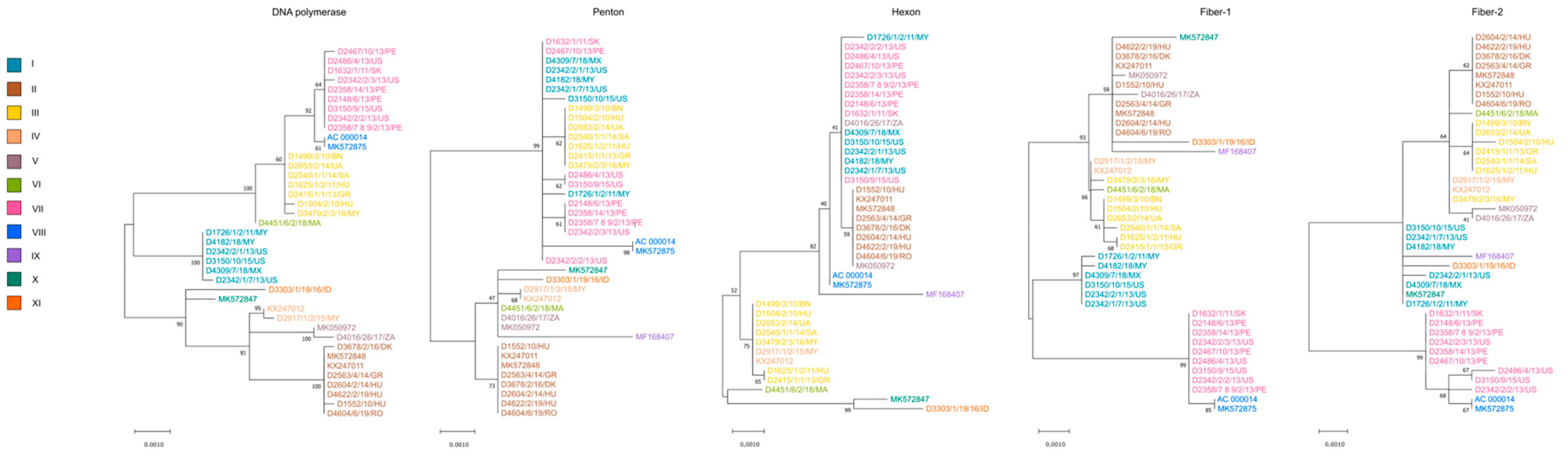
3.2. Genetic Features and Pathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davison, A.J.; Benko, M.; Harrach, B. Genetic content and evolution of adenoviruses. J. Gen. Virol. 2003, 84, 2895–2908. [Google Scholar] [CrossRef] [PubMed]
- Adair, B.; Fitzgerald, S. Group I adenovirus infections. In Diseases of Poultry, 12th ed.; Saif, Y., Fadly, A., Glisson, J., McDougald, L., Nolan, L., Swayne, D., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; pp. 252–266. [Google Scholar]
- Benkő, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarría, M.; Hess, M.; Jones, M.S.; Kaján, G.L.; Kajon, A.E.; ICTV Report Consortium; et al. ICTV virus taxonomy profile: Adenoviridae 2022. J. Gen. Virol. 2022, 103, 001721. [Google Scholar] [CrossRef]
- Chiocca, S.; Kurzbauer, R.; Schaffner, G.; Baker, A.; Mautner, V.; Cotton, M. The complete DNA sequence and genomic organization of the avian adenovirus CELO. J. Virol. 1996, 70, 2939–2949. [Google Scholar] [CrossRef]
- Schachner, A.; Gonzalez, G.; Endler, L.; Ito, K.; Hess, M. Fowl Adenovirus (FAdV) recombination with intertypic crossovers in genomes of FAdV-D and FAdV-E, displaying hybrid serological phenotypes. Viruses 2019, 11, 1094. [Google Scholar] [CrossRef] [PubMed]
- Matczuk, A.K.; Niczyporuk, J.S.; Kuczkowski, M.; Woźniakowski, G.; Nowak, M.; Wieliczko, A. Whole genome sequencing of Fowl aviadenovirus A—A causative agent of gizzard erosion and ulceration, in adult laying hens. Infect. Genet. Evol. 2017, 48, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Thanasut, K.; Fujino, K.; Taharaguchi, M.; Taharaguchi, S.; Shimokawa, F.; Murakami, M.; Takase, K. Genome sequence of fowl aviadenovirus A strain JM1/1, which caused gizzard erosions in Japan. Genome Announc. 2017, 5, e00749-17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, Y.; Zhang, C.; Guo, M.; Cao, Y.; Zhang, X.; Wu, Y. Nearly complete genome sequence of a serotype 1 fowl adenovirus strain isolated in Jiangsu, China. Microbiol. Resour. Announc. 2019, 8, e00310-19. [Google Scholar] [CrossRef] [PubMed]
- Domanska-Blicharz, K.; Tomczyk, G.; Smietanka, K.; Kozaczynski, W.; Minta, Z. Molecular characterization of fowl adenoviruses isolated from chickens with gizzard erosions. Poult. Sci. 2011, 90, 983–989. [Google Scholar] [CrossRef]
- Grafl, B.; Garcia-Rueda, C.; Cargill, P.; Wood, A.; Schock, A.; Liebhart, D.; Schachner, A.; Hess, M. Fowl aviadenovirus serotype 1 confirmed as the aetiological agent of gizzard erosions in replacement pullets and layer flocks in Great Britain by laboratory and in vivo studies. Avian Pathol. 2018, 47, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kiss, I.; Homonnay, Z.G.; Mató, T.; Bányai, K.; Palya, V. Research Note: An overview on distribution of fowl adenoviruses. Poult. Sci. 2021, 100, 101052. [Google Scholar] [CrossRef] [PubMed]
- Olasz, F.; Mészáros, I.; Marton, S.; Kaján, G.L.; Tamás, V.; Locsmándi, G.; Magyar, T.; Bálint, Á.; Bányai, K.; Zádori, Z. A simple method for sample preparation to facilitate efficient whole-genome sequencing of African swine fever virus. Viruses 2019, 11, 1129. [Google Scholar] [CrossRef] [PubMed]
- Bali, K.; Bálint, Á.; Farsang, A.; Marton, S.; Nagy, B.; Kaszab, E.; Belák, S.; Palya, V.; Bányai, K. Recombination events shape the genomic evolution of infectious bronchitis virus in Europe. Viruses 2021, 13, 535. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.P.; Varsani, A.; Roumagnac, P.; Botha, G.; Maslamoney, S.; Schwab, T.; Kelz, Z.; Kumar, V.; Murrell, B. RDP5: A computer program for analyzing recombination in, and removing signals of recombination from, nucleotide sequence datasets. Virus Evol. 2021, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Mase, M.; Nakamura, K. Phylogenetic analysis of fowl adenoviruses isolated from chickens with gizzard erosion in Japan. J. Vet. Med. Sci. 2014, 76, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Mirzazadeh, A.; Grafl, B.; Abbasnia, M.; Emadi-Jamali, S.; Abdi-Hachesoo, B.; Schachner, A.; Hess, M. Reduced performance due to adenoviral gizzard erosion in 16-day-old commercial broiler chickens in Iran, confirmed experimentally. Front. Vet. Sci. 2021, 8, 635186. [Google Scholar] [CrossRef] [PubMed]
- Marek, A.; Schulz, E.; Hess, C.; Hess, M. Comparison of the fibers of Fowl adenovirus A serotype 1 isolates from chickens with gizzard erosions in Europe and apathogenic reference strains. J. Vet. Diagn. Investig. 2010, 22, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Homonnay, Z.; Jakab, S.; Bali, K.; Kaszab, E.; Mató, T.; Kiss, I.; Palya, V.; Bányai, K. Genome sequencing of a novel variant of fowl adenovirus B reveals mosaicism in the pattern of homologous recombination events. Arch. Virol. 2021, 166, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zaheer, I.; Saleemi, M.K.; Qi, X.; Gao, Y.; Cui, H.; Li, K.; Gao, L.; Fayyaz, A.; Hussain, A.; et al. The first complete genome sequence and pathogenicity characterization of fowl adenovirus 11 from chickens with inclusion body hepatitis in Pakistan. Vet. Microbiol. 2020, 244, 108670. [Google Scholar] [CrossRef] [PubMed]
- Kaján, G.L.; Schachner, A.; Gellért, Á.; Hess, M. Species Fowl aviadenovirus B consists of a single serotype despite genetic distance of FAdV-5 isolates. Viruses 2022, 14, 248. [Google Scholar] [CrossRef] [PubMed]
- Ojkic, D.; Nagy, E. The long repeat region is dispensable for fowl adenovirus replication in vitro. Virology 2001, 283, 197–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Slaine, P.D.; Ackford, J.G.; Kropinski, A.M.; Kozak, R.A.; Krell, P.J.; Nagy, É. Molecular characterization of pathogenic and nonpathogenic fowl aviadenovirus serotype 11 isolates. Can. J. Microbiol. 2016, 62, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Absalón, A.E.; Morales-Garzón, A.; Vera-Hernández, P.F.; Cortés-Espinosa, D.V.; Uribe-Ochoa, S.M.; García, L.J.; Lucio-Decanini, E. Complete genome sequence of a non-pathogenic strain of Fowl Adenovirus serotype 11: Minimal genomic differences between pathogenic and non-pathogenic viruses. Virology 2017, 501, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Vera-Hernández, P.F.; Morales-Garzón, A.; Cortés-Espinosa, D.V.; Galiote-Flores, A.; García-Barrera, L.J.; Rodríguez-Galindo, E.T.; Toscano-Contreras, A.; Lucio-Decanini, E.; Absalón, A.E. Clinicopathological characterization and genomic sequence differences observed in a highly virulent fowl Aviadenovirus serotype 4. Avian Pathol. 2016, 45, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, R.; Tian, K.; Wang, Z.; Yang, X.; Gao, D.; Zhang, Y.; Fu, J.; Wang, H.; Zhao, J. Fiber2 and hexon genes are closely associated with the virulence of the emerging and highly pathogenic fowl adenovirus 4. Emerg. Microbes Infect. 2018, 7, 199. [Google Scholar] [CrossRef]
- Grafl, B.; Prokofieva, I.; Wernsdorf, P.; Steinborn, R.; Hess, M. Infection with an apathogenic fowl adenovirus serotype-1 strain (CELO) prevents adenoviral gizzard erosion in broilers. Vet. Microbiol. 2014, 172, 177–185. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakab, S.; Bali, K.; Homonnay, Z.; Kaszab, E.; Ihász, K.; Fehér, E.; Mató, T.; Kiss, I.; Palya, V.; Bányai, K. Genomic Epidemiology and Evolution of Fowl Adenovirus 1. Animals 2023, 13, 2819. https://doi.org/10.3390/ani13182819
Jakab S, Bali K, Homonnay Z, Kaszab E, Ihász K, Fehér E, Mató T, Kiss I, Palya V, Bányai K. Genomic Epidemiology and Evolution of Fowl Adenovirus 1. Animals. 2023; 13(18):2819. https://doi.org/10.3390/ani13182819
Chicago/Turabian StyleJakab, Szilvia, Krisztina Bali, Zalán Homonnay, Eszter Kaszab, Katalin Ihász, Enikő Fehér, Tamás Mató, István Kiss, Vilmos Palya, and Krisztián Bányai. 2023. "Genomic Epidemiology and Evolution of Fowl Adenovirus 1" Animals 13, no. 18: 2819. https://doi.org/10.3390/ani13182819
APA StyleJakab, S., Bali, K., Homonnay, Z., Kaszab, E., Ihász, K., Fehér, E., Mató, T., Kiss, I., Palya, V., & Bányai, K. (2023). Genomic Epidemiology and Evolution of Fowl Adenovirus 1. Animals, 13(18), 2819. https://doi.org/10.3390/ani13182819









