Simple Summary
Chickens can be infected by several avian blood parasites (Plasmodium, Haemoproteus, Trypanosoma and microfilaria) that can cause a big impact on poultry production. However, some of them are known to cause a high impact on poultry production (Plasmodium and Leucocytozoon), while others still require further investigation. Raising backyard chickens is a common practice in Thailand, and the low-biosecurity system in which they are kept favors the transmission of vector-borne diseases, which include several blood parasites. The spread of such infections can compromise production, resulting in economic impact. This study aimed to report the molecular prevalence, lineage diversity and morphology of blood parasites infecting backyard chickens in three different provinces in Thailand. We found a high prevalence of Plasmodium sp. and Leucocytozoon sp. infections, while Trypanosoma and microfilaria had a lower prevalence. Plasmodium gallinaceum and Leucocytozoon macleani were present in the studied individuals as well as Trypanosoma, which resembles T. calmettei. The buffy-coat method and molecular analysis were shown to be valuable diagnostic tools for blood parasites in chickens. These results can be used to promote awareness of parasite infections in the study area.
Abstract
Avian malaria and leucocytozoonosis can cause fatal diseases, whereas avian trypanosomiasis is reported to be harmless in chickens. Backyard chickens can be infected by several pathogens, including blood parasites, that may shed to industrial poultry production, with a consequently higher economic impact. This study aimed to investigate the presence of several blood parasites (Plasmodium, Leucocytozoon and Trypanosoma) in backyard chickens raised in Southern Thailand, using PCR-based detection and microscopic methods. From June 2021 to June 2022, 57 backyard chickens were sampled. Fresh thin blood smears were prepared from 11 individuals, and buffy coat smears were prepared from 55 of them. Both thin blood smears and buffy coat smears were used for microscopic analysis. Two nested PCR protocols that amplify a fragment of cytochrome b (cytb) and small subunit rRNA (SSU rRNA) genes were used to identify Haemosporida and Trypanosoma parasites, respectively. The number of positive samples was higher with the application of nested PCR than when buffy coat smears were used. Three new Plasmodium lineages (GALLUS47-49) and thirteen Leucocytozoon lineages (GALLUS50-62) were found. Trophozoites, meronts and gametocytes of Plasmodium gallinaceum (GALLUS01) were present in one thin blood smear. All thin blood smears revealed Leucocytozoon infections, but only three samples were a single infection. These three samples revealed the presence of fusiform host cell–parasite complexes, of which the morphological features resembled those of Leucocytozoon macleani (possible synonym is Leucocytozoon sabrazesi), while the cytb showed that this parasite is closely related to the lineage GALLUS06-07, described as Leucocytozoon schouteni. The Trypanosoma prevalence was 33.33%; it was present in only one of the thin blood smears, and it resembles Trypanosoma calmettei. This study showed the prevalence of a high diversity of Plasmodium (64.91%) and Leucocytozoon (89.47%) in Thai chickens. Both nested-PCR and buffy coat smear can be used as the diagnostic tool for the testing of Plasmodium, Leucocytozoon and Trypanosoma for parasitic control in backyard chickens and poultry farms. The information on the parasite species that can be found in chickens raised in Southern Thailand was also considered as the baseline information for further study.
1. Introduction
Avian haemosporidian (Apicomplexa: Haemosporida) includes the genera Haemoproteus, Plasmodium, Fallisia and Leucocytozoon, and these are vector-borne parasites distributed worldwide, except Antarctica [1,2,3]. There are about 277 described species among 23 host orders [4,5,6,7]. In domestic chickens (Gallus gallus domesticus), two Plasmodium species were described: Plasmodium gallinaceum, likely endemic in Asia, and Plasmodium juxtanucleare [4], which has a global distribution [8]. Three Leucocytozoon species were reported in domestic chickens: Leucocytozoon macleani (possible synonym is Leucocytozoon sabrazesi), Leucocytozoon schouteni and Leucocytozoon caulleryi [4]. The Trypanosoma spp. found in domestic chickens include Trypanosoma calmettei, Trypanosoma gallinarum and Trypanosoma numidae [9].
These parasites are transmitted by various vectors. Plasmodium gallinaceum and P. juxtanucleare are transmitted by mosquitoes (Diptera: Culicidae), with P. gallinaceum being transmitted mainly by Mansonia crassipes and Culex quinquefasciatus [4,10], while P. juxtanucleare is mainly transmitted by several species of Culex mosquitoes [4]. Leucocytozoon spp. are transmitted by several species of back flies (Diptera: Simuliidae), but only L. caulleryi is transmitted by insects belonging to the Culicoides genus (Diptera: Ceratopogonidae) [11,12]. For Trypanosoma spp., these parasites are transmitted by blood-sucking arthropods belonging to families Simullidae, Culicidae, Ceratopogonidae, Hippoboscidae (Diptera) and Dermanyssidae (Mesostigmata) [13,14,15,16].
Generally, the clinical signs of Plasmodium infection in chickens (known as avian malaria) are greenish feces, anemia, depression, reduced weight gain, fluffed-out feathers and often death [17]. Leucocytozoon causes a malaria-like disease called leucocytozoonosis [18,19], and its pathogenicity can vary according to the vertebrate host, parasite species and lineage [20,21,22,23]. This important disease was first reported in Vietnam in 1909 [18]. Leucocytozoon caulleryi can cause a lethal hemorrhagic disease [24,25], and infected chickens frequently exhibit anemia, anorexia, ataxia, lethargy, green diarrhea, pallor, decreased egg production and eventually death [9,25]. Leucocytozoon macleani and L. schouteni are less pathogenic but can still cause anemia and greenish droppings, slight emaciation and reduced egg production [4,9]. Avian trypanosomes have been reported as non-pathogenic for domestic chickens, due to their impact in wild birds being rarely reported and understudied [26,27]. Even though about 100 species of avian trypanosomes have been described, they are poorly known [13,28,29].
Backyard chickens is one of the common low biosecurity poultry production systems in Thailand [30]. The animals raised in this system are not kept in areas protected from blood-sucking insects that can transmit these parasites. They can easily be bitten by those insects that can be infected by blood parasites (Plasmodium sp., Leucocytozoon sp. and Trypanosoma sp.) which will be injected during the blood meal. Once infected, these backyard chickens can act as reservoirs of these pathogens that can shed to other households or the bigger sector of poultry production [31], resulting in serious economic impact. A combination of microscopic and PCR-based methods is helpful for the diagnosis of avian blood parasites and can show not only whether the infected animal is a reservoir of avian blood parasites (when the parasite can be seen in the blood) but also the species and lineages (haplotypes) that are circulating in the region [32]. Therefore, this study aimed to investigate the presence of Plasmodium, Leucocytozoon and Trypanosoma in backyard chickens raised in Southern Thailand. We also report microscopic and molecular characteristics of parasites found in backyard chickens. This information might be helpful for parasite prevention, parasite elimination and further studies.
2. Materials and Method
2.1. Sample Collection and Processing and Microscopic Examination
Blood samples were collected during a one-year period, between June 2021 to June 2022. This allowed us to collect samples during the rainy season (June–September) and dry season (April–May). A maximum 1 mL volume of blood was taken from the brachial vein of 57 backyard chickens raised in Nakhon Si Thammarat (8°25′ N, 99°58′ E), Phatthalung (7°37′ N, 100°4′ E) and Surat Thani (9°7′ N, 99°20′ E). The blood was immediately transferred to tubes containing EDTA as an anticoagulant (MediPlusTM, Bangkok, Thailand) and transported to the Laboratory of Hematology, Akkhararatchakumari Veterinary College, Walailak University, where the analyses were processed. During the transport, these samples were kept in icebox until processing. From eleven samples, two to five fresh thin blood smears (without any anticoagulant) were prepared using a few drops of blood (all from native chickens) and dried using an electric fan for further microscopic analysis.
In the laboratory, samples stored in EDTA-tubes were used to prepare buffy coat smears according to Chagas et al. [33] with some modifications. Briefly, EDTA-blood was filled into a microhematocrit capillary tube and centrifuged at 12,000 rpm for 5 min. Then, buffy coat layer was transferred into a glass slide and smeared, allowed to air dry at room temperature, fixed in absolute methanol for one minute and stained with a 10% Giemsa solution for 45 min. The remaining samples in the EDTA-tube were frozen for further molecular analysis, which is described below. The freshly prepared thin blood smears were fixed and stained as indicated for the buffy coat smear.
Microscopic examination for both buffy coat smear (55 samples) and fresh thin blood smears (11 samples) were conducted following the previous report [32]. Briefly, 100 fields were examined at 400× as well as 100 fields at 1000× magnification. Parasitemia was calculated for the samples with thin blood smears available as a percentage of the infected cells per 10,000 red blood cells [34]. A light microscope was used for the examination of blood smears and for collecting images of blood parasites. A microscope Nikon ECLIPSE Ci-L (Nikon, Tokyo, Japan) equipped with a Nikon DS-Fi3 digital camera (Nikon, Tokyo, Japan) together with NIS Elements D imaging software (version 5.01, Nikon, Tokyo, Japan) was used for analysis of buffy coat smears. Blood smears were analyzed using an Olympus BX43 (Olympus, Tokyo, Japan) equipped with an Olympus DP27 digital camera (Olympus, Tokyo, Japan) together with CellSens imaging software (version 1.18, Olympus, Tokyo, Japan).
2.2. DNA Extraction, Nested-PCR and Sequencing
Fifty microliters of each blood sample were used for genomic DNA extraction using the Blood Genomic DNA Extraction Mini Kit (FavorPrep, Pingtung, Taiwan) following the manufacturer’s instructions. A DNA fragment of approximately 479 bp of the cytb gene of Haemosporida parasites and a 770 bp of the small subunit ribosomal RNA (SSU rRNA) of Trypanosoma parasites were amplified using two different nested-PCR protocols with some modification of thermal profile [13,35,36]. In brief, the PCR mix for all parasites was prepared in a total volume of 20 µL containing 10 µL of PCR master mix (OnePCRTM Ultra, Bio-Helix, New Taipei City, Taiwan), 1 µL of each primer (concentration = 10 µM), 6 µL of water and 2 µL of DNA template (concentration < 25 ng/µL in most samples). A negative (ultra-pure water) and several positive controls—Plasmodium sp. cytb lineage GLACUC08 [37], Leucocytozoon isolate SEO-KU483 [38] and Trypanosoma isolate CSO-KU127 [29]—were used in every run. Thermal profile of nested-PCR amplifying cytb and SSU rRNA was followed [38,39]. The amplicons were checked using 1.5% agarose gel prepared from the Agarose Tablets (Bio-Helix, New Taipei City, Taiwan). The amplicons were then submitted to the U2Bio Thailand (Bangkok, Thailand) for gel extraction, DNA purification and Sanger sequencing for both forward and reverse strands.
2.3. Sequence Analysis and Phylogenetics
Plasmodium, Leucocytozoon and Trypanosoma sequences were evaluated using BioEdit version 7.0.5.3 [40]. Forward and reverse strands were aligned and checked to obtain a contig sequence. Sequences were also checked for the presence of co-infections (presence of double peaks in the electropherograms). If such co-infections were seen, they were excluded from the analysis [41,42]. Plasmodium and Leucocytozoon sequences from single infections were compared with the sequence deposited on the MalAvi database [43], using BLAST tool. The sequences showing at least one nucleotide of difference were considered as a new lineage [44,45], and it was named according to MalAvi database [43]; all sequences were deposited in MalAvi and GenBank databases (https://www.ncbi.nlm.nih.gov/nucleotide/, accessed on 21 January 2023).
Hemosporidian lineages isolated from our study were used for Bayesian phylogenetic analysis. In total, 30 Plasmodium lineages and 39 Leucocytozoon lineages were used. Phylogenetic analyses of Plasmodium and Leucocytozoon were conducted separately. A sequence of Leucocytozoon sp. SISKIN2 was used as an outgroup for Plasmodium phylogenetic analysis, whereas Haemoproteus columbae COLIV03 and H. iwa FREMIN01 were used as the outgroup for Leucocytozoon phylogenetic analysis. Missing data in each nucleotide position were replaced with “N” [37,39].
Bayesian phylogenetic of Plasmodium and Leucocytozoon were constructed using MrBayes version 3.2.6 [46]. Application of the model of general time-reversible (GTR) was selected with the mrModeltest 2.3 program [47], based on hierarchical likelihood ratio test (hLRT). Markov chain Monte Carlo (MCMC) was run for three million (Plasmodium) or five million generations (Leucocytozoon), with sampling every 100 generations. The first 25% of three were discarded as “burn-in” step. Then, the consensus tree was calculated using the 22,500 and 37,500 remaining trees for Plasmodium and Leucocytozoon, respectively. The tree was visualized using Figtree version 1.4.3 (http://tree.bio.ed.ac.uk/software/figtree, accessed on 8 November 2022). Jukes–Cantor (JC) model, for which all substitution were equally weighted, was used to calculated the genetic distance between lineages using MEGA version 11 [48].
In total, 49 Trypanosoma sequences were used in the phylogenetic analysis, including the isolates from this study. Two sequences of amphibian trypanosomes were used as an outgroup, including Trypanosoma rotatorium and Trypanosoma mega. The phylogenetics with 1000 replications of bootstrap was constructed using the maximum-likelihood method implemented in MEGA 11 software [48]. Best-fit model was Kimura 2-parameter with gamma distribution (K2 + G), which was selected based on lowest Bayesian information criterion (BIC). Genetic distances were determined using the JC model.
2.4. Statistical Analysis
Prevalence of Plasmodium, Leucocytozoon and Trypanosoma infections in Southern Thailand were calculated based on nested-PCR results from the 57 backyard chickens sampled. The confidence intervals (95% CI) were calculated using the function of ‘binom.approx’ in package ‘epitools’, implemented in R version 4.2.2 [49]. Fisher’s exact test was performed to compare the prevalence of blood parasite infection (based on nested-PCR results) between areas (Nakhon Si Thammarat, Phatthalung and Surat Thani), implemented in R version 4.2.2 [49]. The significance was obtained at p-value ≤ 0.05.
3. Results
A total of 57 backyard chickens’ (Gallus gallus domesticus) blood samples were investigated for the presence of Plasmodium, Leucocytozoon and Trypanosoma infections. This included native chickens (n = 33), hybrid chickens (n = 10), laying hens (n = 5) and fighting roosters (n = 9). These chickens were raised in a backyard production system, with low biosecurity, by local farmer in three provinces located in the central part of Southern Thailand: Nakhon Si Thammarat (NST) where 23 individuals were sampled, Phatthalung (PHL) where 25 individuals were sampled and Surat Thani (SUT) where 9 individuals were sampled. Based on nested-PCR results, 37 samples were positive for Plasmodium parasites showing a prevalence of 64.91% (95% CI: 52.52–77.30); 51 were positive for Leucocytozoon parasites with a prevalence of 89.47% (95% CI: 81.51–97.44); and 19 samples were positive for Trypanosoma parasites showing a prevalence of 33.33% (95% CI: 21.10–45.57) (Table 1). In a comparison of the prevalence of blood parasite infections between areas, the prevalence of Plasmodium in NST (69.56%), PHL (32.00%) and SUT (77.77%) were not significantly different (p = 0.012); the prevalence of Leucocytozoon in NST (78.26%), PHL (96.00%) and PHL (100.00%) were not significantly different (p = 0.118); and the prevalence of Trypanosoma in NST (0.00%), PHL (60.00%) and SUT (22.22%) were significantly different (p < 0.05).

Table 1.
Number of blood parasites found in backyard chickens’ blood examined using buffy coat smear and nested-PCR.
Of these 37 Plasmodium-positive and 51 Leucocytozoon-positive samples, 6 Plasmodium and 24 Leucocytozoon sequences showed double peaks in the electropherogram; considered as co-infections, these sequences were excluded from the sequence and phylogenetic analysis. The remaining sequences, 31 Plasmodium and 27 Leucocytozoon, were considered as single infections and used in the phylogenetic analysis. Altogether, 6 Plasmodium and 16 Leucocytozoon lineages were identified in the studied animals (Table 2).

Table 2.
Plasmodium and Leucocytozoon lineages isolated from backyard chickens (Gallus gallus domesticus) raised in Southern Thailand, during June 2021–June 2022.
Microscopic analysis of the buffy coat smears revealed the presence of Plasmodium in 5 individuals (Figure 1A–E), Leucocytozoon in 48 (Figure 1I,J), and Trypanosoma in 4 (Figure 1F–H) (Table 1). Unfortunately, the PCR-protocol used in the study failed to amplify a few microscopically positive samples: two positives for Plasmodium and Trypanosoma and one sample for Leucocytozoon. In the buffy coat smear analyses, we could also observe that a small number of individuals were infected by microfilaria of filarial nematodes (Figure 1L).
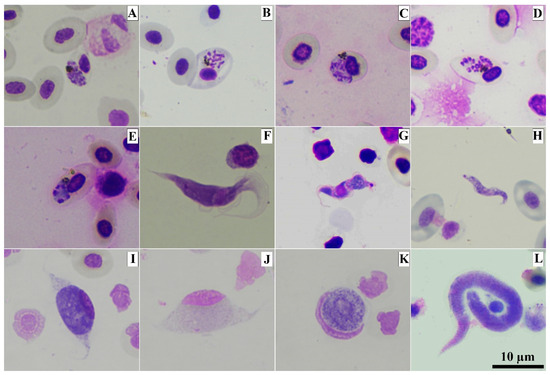
Figure 1.
Photomicrographs of blood parasites present in buffy coat smears from domestic chickens (Gallus gallus domesticus). Erythrocytic meront of Plasmodium gallinaceum GALLUS01 (A–E), trypomastigote of avian Trypanosoma sp. (F–H) and gametocytes of Leucocytozoon sp.: elongate macrogametocyte (I), elongate microgametocyte (J) round macrogametocyte (K) and microfilaria of filarial nematodes (L). Methanol-fixed and stained with 10% Giemsa solution.
Of the eleven fresh thin blood smear analyses, in one individual (AVC52) the presence of trophozoites, erythrocytic meronts, young and mature gametocytes of P. gallinaceum (Figure 2A–F) were verified, the lineage of which was molecularly identified as GALLUS01 (Table 2). The morphological identification was possible due to the presence of the following characteristics: trophozoites were located anywhere in the infected cell (Figure 2A); fully grown erythrocytic meronts markedly displacing the nuclei of erythrocytes occupied more than half of the cytoplasmic space of infected erythrocytes (Figure 2B); growing gametocytes were found in mature erythrocytes, displacing the nuclei of host cells (Figure 2C); and mature gametocytes were varying in shape and markedly deformed host cells and displaced their nuclei laterally (Figure 2D–F). Parasitemia was lower than 0.01%.
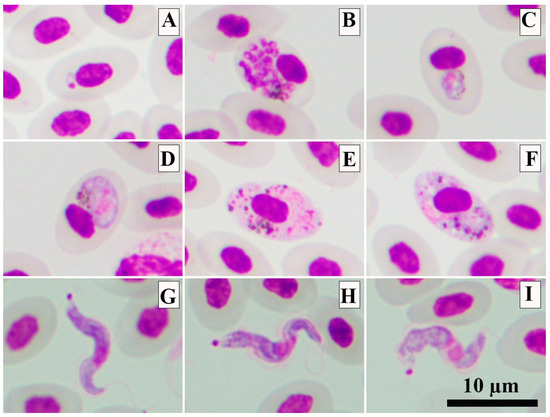
Figure 2.
The photomicrographs of Plasmodium gallinaceum GALLUS01 (A–E) and Trypanosoma sp. (G–I) present in blood smears from domestic chickens. Trophozoite (A), mature erythrocytic meront (B), growing gametocyte (C), mature microgametocyte (D) and mature macrogametocytes (E,F). Trypomastigotes of Trypanosoma sp. (G–I). Methanol-fixed and stained with 10% Giemsa solution.
In the Bayesian phylogenetic analysis, the five Plasmodium lineages identified in the present study were grouped in the P. juxtanucleare clade, and they were separated from P. gallinaceum clade (Figure 3). In these two clades, Plasmodium parasites of Phasianidae birds were separated from avian Plasmodium lineages from other host families. The homology in the P. juxtanucleare clade was 99.33–100%, whereas in the P. gallinaceum it was 100%. Plasmodium lineages GALLUS48 and GALLUS49 were closely related to P. juxtanucleare GALLUS02, with 99.78% and 99.55% similarity. In contrast, the Plasmodium lineage GALLUS47 showed 100% similarity to Plasmodium lineage TSUB01.
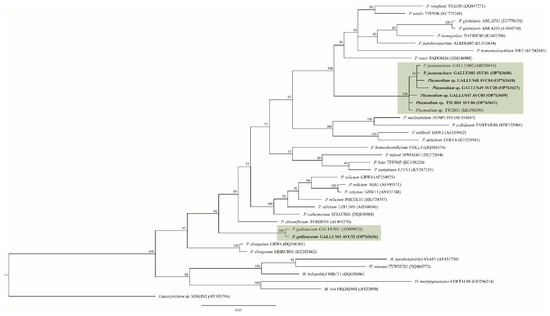
Figure 3.
Plasmodium Bayesian phylogeny based on a fragment of cytochrome b gene (479 bp). The lineages isolated in this study are given in bold. Parasite lineages and isolate number are given after species names. GenBank accession numbers are given between brackets. Node values indicate percentages of posterior probabilities. Plasmodium lineages isolated from chickens are shown in the green boxes. Leucocytozoon sp. lineage SISKIN2 was an outgroup.
All eleven fresh blood smears were positive for Leucocytozoon parasites that developed into fusiform host cells–parasite complexes. Of these eleven samples, seven samples were found with only fusiform host cells–parasite complexes, whereas the other four samples were found with both fusiform host cells–parasite complexes and roundish host cells–parasite complexes (Figure 4). Three different genetic lineages were present in the studied samples: GALLUS55, GALLUS61 and GALLUS62 (Table 2). Based on the microscopic analysis of the thin blood smears, the gametocytes of GALLUS55 and GALLUS62 showed fusiform host cells–parasite complexes and roundish host cells–parasite complexes in (Figure 4I,M,K,O), whereas GALLUS61 developed only in fusiform host cells (Figure 4A). These three parasites were suspected to be L. macleani. However, the Bayesian phylogenetic inference revealed that these three parasites were grouped in the Leucocytozoon schouteni clade (Figure 5), which had the homology ranging between 91.02 and 100%.
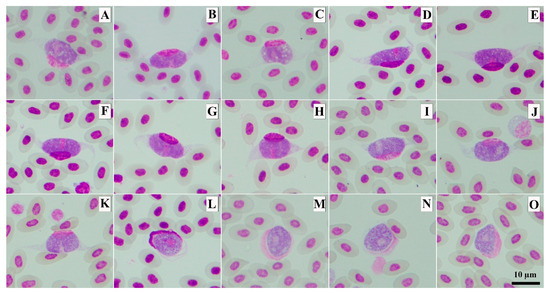
Figure 4.
The photomicrographs of Leucocytozoon sp. macrogametocytes developed into fusiform host cells–parasite complexes (A–K) and roundish host cell–parasite complexes (L). Methanol-fixed and stained with 10% Giemsa solution. Leucocytozoon lineage GALLUS55 (K,O), GALLUS61 (A), GALLUS62 (I,M) and undescribed lineage (B–H,J,L,N).
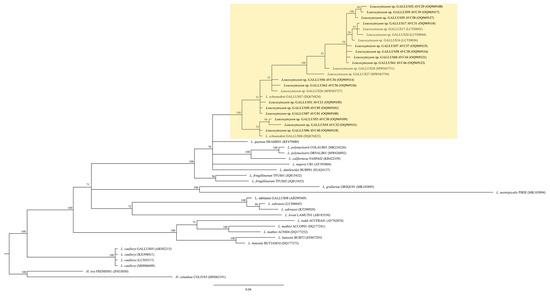
Figure 5.
Leucocytozoon spp. bayesian phylogeny based on a fragment of cytochrome b gene (479 bp). The lineages isolated in this study are given in bold. Parasite lineages and isolation number are given after species names. GenBank accession numbers are given between brackets. Node values indicate percentages of posterior probabilities. L. schouteni clade is highlighted in yellow. Haemoproteus iwa lineage FREMIN01 and H. columbae COLIV03 were the outgroup.
One out of eleven fresh thin blood smears showed the presence of tiny trypomastigotes of Trypanosoma sp. (individual AVC49). This Trypanosoma sp. had a short and slender body (Figure 2G–I). However, due to the presence of only a few trypomastigotes in the sample, it was not possible to morphologically identify it. Unfortunately, the PCR-protocol used failed to amplify the SSU rRNA of this parasite. However, some morphometric parameters were measured from five trypomastigotes. Detailed information can be found in Table 3.

Table 3.
Morphometry of trypomastigotes of Trypanosoma sp. infected in backyard chickens (Gallus gallus domesticus, AVC49) raised in Southern Thailand.
It was possible to amplify two SSU rRNA sequences of Trypanosoma from native chickens (individuals AVC56 and AVC57) raised in Phatthalung. However, due to the lack of fresh thin blood smears, we could not determine their morphological features. These two sequences were used for maximum-likelihood phylogenetic analysis, which revealed that Trypanosoma GGD-AVC56 and Trypanosoma GGD-AVC57 were phylogenetically grouped in the Trypanosoma avium clade (Figure 6). The homology of this clade was 99.74–100%. Trypanosoma GGD-AVC56 and Trypanosoma GGD-AVC57 had 99.74% similarity to others in this clade. However, our two sequences do not belong to any of the described lineages.
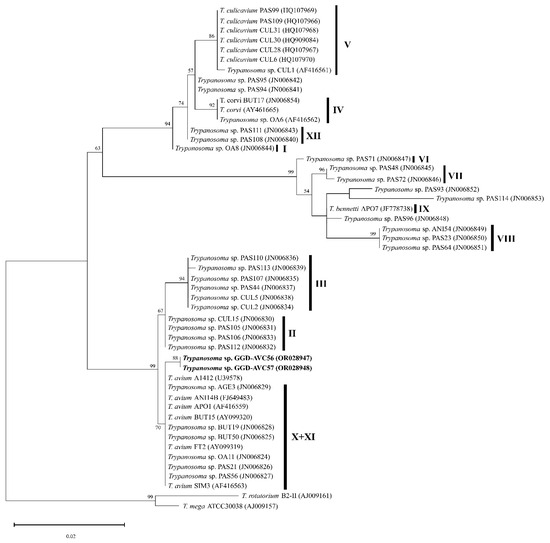
Figure 6.
Trypanosoma spp. Maximun-likelihood phylogeny inference based on a fragment of the small subunit rRNA gene (798 bp). The sequences isolated in this study are given in bold. Vertical bars indicate the group of avian trypanosomes lineages (I–XII) following Zídková et al. (2012) [27]. Bootstrap values > 50% are shown at the branch points.
4. Discussion
The prevalence of Plasmodium spp. and Leucocytozoon spp. in backyard chickens in Southern Thailand was high (64.91% and 89.47%, respectively). The study area was located in the central region of southern Thailand that is close to the coastal area, with water bodies that may support mosquitoes and other blood-sucking insects’ development. The examples of insect vector found in Southern Thailand were Culex mosquitoes [50], Culicoides biting midges [51] and Simuliidae [52]. Additionally, the high prevalence and diversity of these parasites might be related to anthropogenic disturbance and activities in this area [53] or landscape characteristics in this area [54]. The prevalences of Plasmodium spp. and Leucocytozoon spp. between provinces were not significantly different, whereas Trypanosoma spp. in PHL was significantly high. This suggested that serious impacts caused by avian malaria and leucocytozoonosis should be a concern in NST, PHL and SUT. Although avian trypanosomiasis was harmless in domestic chickens, further investigation to maximize the information of these diseases should be considered. Since the molecular prevalence of Trypanosoma spp. was not high (33.33%), PHL might be the suitable area for sample collection.
This study described new genetic lineages of Plasmodium and Leucocytozoon (Table 2), indicating that the diversity of parasites in this area was high and even new species can be involved in the infections. Especially for Leucocytozoon, many of their lineages were found (Table 2), and some samples (AVC46, AVC56 and AVC58) might be undescribed Leucocytozoon. Co-infection and PCR failing to amplify the DNA of parasites were not new phenomena in avian malaria studies [55,56], and our findings reinforce the importance of combining microscopic and PCR-based techniques in the investigation of avian blood parasites. The sequencing of the PCR product helped for the identification of co-infection, whereas microscopic examination of thin blood smears provided the morphologic features for descriptions of the morphospecies. Therefore, further investigations are necessary to better understand whether we were dealing with a co-infection or if these finding can be due to the presence of a cryptic or a new Leucocytozoon species in backyard chickens in Thailand. It is noted that the detection of multiple detections can be difficult sometimes. This is because of the preferential amplification of Plasmodium co-infection with Haemoproteus. Additionally, co-infection between two strains at very different intensities can result in small double peaks [57].
Sixteen individuals were PCR-positive for Plasmodium sp. TSUB01. This lineage was first reported from the Eastern Slaty Thrush (Turdus subalaris, Turdidae, Passeriformes) in Brazil [8], which showed that P. juxtanucleare can spillover from domestic chicken to wild animals. Since P. juxtanucleare has a global distribution, when a suitable temperature is present, not only can the vectors develop but also the parasite can complete its life cycle and be transmitted to the next host, which can be domestic and wild birds [37,58,59]. Southern Thailand may be an excellent environment for the development and transmission of P. juxtanucleare and other vector-borne diseases between wild passerine birds and backyard chickens. The other seven individuals were PCR-positive to P. gallinaceum GALLUS01. This lineage was isolated from chickens (Gallus gallus, Phasianidae, Galliformes) in Vietnam more than 20 years ago [60]. This suggested that P. gallinaceum GALLUS01 can be transmitted among Southeastern Asian countries. Together with their high pathogenicity and high transmission rate [61], it was important to impose the preventive measures to minimize the occurrence of disease and prevent the loss of household incomes.
Three Trypanosoma species were reported to infect domestic chickens: T. calmettei, T. gallinarum and T. numidae [9]. The Trypanosoma sp. present in our samples had a short and slender trypomastigote (Figure 3G–I), which resembled T. calmettei [62]. The Trypanosoma sp. present in our samples can be readily differentiated from T. gallinarum and T. numidae by its smaller size (18.04 ± 1.55 µm), while T. gallinaceum had its length between 54.5 and 76.3 µm, and in T. numidae it is around 53 µm in length [9,63]. Due to the low number of trypomastigotes in the fresh blood smears, morphological identification was not possible. There were only two Trypanosoma SSU rRNA sequences isolated in the present study, and they were phylogenetically close to T. avium clade (Figure 6). However, these two sequences were different from the avian trypanosomes lineages described in the literature [27]. It is noteworthy that the previous article [27] described avian trypanosomes lineages based on the RAPD method. Thus, to define whether our sequences were new lineages, the RAPD analysis might be needed. The result of nested PCR (Table 1) indicates the existence of this parasite in Southern Thailand. Three trypomastigotes of trypanosomes found in buffy coat smears showed variation in their sizes and shapes (Figure 1F–H). Although this method was not recommended for describing morphospecies, this information may be evidence that domestic chickens can be infected by different species of Trypanosoma parasites. Therefore, the present study reinforces the importance of further studies addressing Trypanosoma parasites in the study area.
The rapid development of DNA-based molecular methods allowed us to understand the genetic diversity [64], population genetic structure [65] and parasite taxonomy [66]. The nested-PCR used in this study detected a higher number of Plasmodium spp., Leucocytozoon spp. and Trypanosoma spp. than the buffy-coat smear (Table 1), indicating low sensitivity of buffy-coat smear. However, the observation of Giemsa-stained buffy coat smears (Figure 1) was the evidence that this method can be used for the diagnosis of an infection of Plasmodium sp., Leucocytozoon sp. or Trypanosoma sp. Nevertheless, due to the initiation of the exflagellation process when mature Leucocytozoon gametocytes are in contact with air and the modifications that can be seen in the stained slides, this material is not recommended for descriptions of species—even though it can be used for avian blood parasite diagnosis [33]. On the other hand, the resources and facilities to perform molecular analysis might not be present and/or available in all veterinarian laboratories, and molecular-based diagnosis is more expensive than using microscopy. Therefore, even though molecular techniques were more specific and sensitive for avian haemosporidian diagnosis [32,33], the Giemsa-stained buffy-coat method can be recommended as a useful tool in the diagnosis of such infections.
The buffy coat smear was prepared by breaking the glass capillary tube right below the buffy coat layer, which results in a small portion of red blood cells being transferred to the glass slide [67]. This is a well-known methodology to investigate blood parasites, widely applied in the diagnosis of parasites of medical and veterinary importance [33,68,69,70], for both extra- and intracellular parasites, such as microfilaria, Trypanosoma, Erlichia canis, Haemoproteus and Lankesterella. However, different parasites might require some modification in the protocol to facilitate their diagnosis. For instance, human Plasmodium can be diagnosed with the application of the buffy coat method when blood samples are collected in capillary tubes containing orange acridine [71,72,73,74]. Previously, it was reported that the buffy coat method was not appropriate for the diagnosis of Plasmodium and Leucocytozoon (see [33] for detailed information). Here, we modified the proposed methodology that consisted of the preparation of smears and staining them with Giemsa. This improved the detection of these two parasites, making this methodology useful for the diagnosis of blood parasites in chickens. Additionally, this is a cheap and quick methodology, that can be easily applied in veterinary diagnosis.
5. Conclusions
This study aimed to investigate molecular prevalence and diversity of blood parasites infecting backyard chickens raised in Southern Thailand. This study showed a high molecular prevalence of Plasmodium spp. (64.91%) and Leucocytozoon spp. (89.47%). Trypanossoma parasites were also identified but in a lower prevalence (33.33%). Here we identified three new lineages of Plasmodium sp. (GALLUS47-49) and 13 new lineages of Leucocytozoon spp. (GALLUS50-62), confirming a high diversity of parasites infecting chickens in the study area. This prevalence and diversity might be due to the low biosecurity production system in which these chickens are raised. Plasmodium gallinaceum was identified in the few thin blood smears that were collected. These results highlight the importance of raising awareness to the livestock authorities and local farmers about the presence of these parasites, how they can compromise their production and the influence of poultry production on a bigger scale. Even though it is not recommended for detailed morphological analysis, buffy coat smears can be a useful tool for the diagnosis of avian blood parasites in veterinary clinics and laboratories, being cheap and easy to be performed. If more detailed information about parasite genetic diversity is necessary, then PCR-based methods should be employed. Further studies are necessary to better understand the pathogenicity and patterns of transmission of these parasites in backyard chickens. Additionally, potential vectors should be investigated in the area in order to elaborate and to test prophylactic methods to reduce the impact of infections.
Author Contributions
Methodology, P.P.; Investigation, K.B., T.T. and P.P.; Resources, T.T. and P.P.; Data curation, K.B. and P.P.; Writing—original draft, K.B. and T.T.; Writing—review and editing, C.R.F.C. and P.P.; Visualization, P.P.; Project administration, P.P.; Funding acquisition, P.P. All authors have read and agreed to the published version of the manuscript.
Funding
Walailak University Individual Research Grant, Walailak University, Thailand (Grant number: WU-IRG-64-054).
Institutional Review Board Statement
This study was approved by the Walailak University Institutional Animal Care and Use Committee, Walailak University, Thailand (Approval number: WU-AICUC-64014 and WU-ACUC-65052, day of approval: 30 July 2021 and 30 July 2022) and the Institutional Biosafety and Biosecurity (Approval number: WU-IBC-64-006, day of approval: 30 July 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in GenBank database (https://www.ncbi.nlm.nih.gov/genbank/, accessed on 23 May 2023) (accession numbers: OP763608-OP763638, OQ969100-OQ969127, OR028947-OR028948).
Acknowledgments
This study was fully supported by Walailak University Individual Research Grant, Walailak University, Thailand (Grant number: WU-IRG-64-054). The authors would like to thank Noppharat Thantahathipchai, Sunsaneeya Thaikoed, livestock authorities and local farmers for their cooperation in sample collection.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Braga, E.M.; Silveira, P.; Belo, N.O.; Valkiūnas, G. Recent advances in the study of avian malaria: An overview with an emphasis on the distribution of Plasmodium spp in Brazil. Mem. Inst. Oswaldo. Cruz. 2011, 106, 3–11. [Google Scholar]
- Permin, A.; Juhl, J. The development of Plasmodium gallinaceum infections in chickens following single infections with three different dose levels. Vet. Parasitol. 2002, 105, 1–10. [Google Scholar] [PubMed]
- Valkiūnas, G.; Sehgal, R.N.M.; Iezhova, T.A.; Hull, A.C. Identification of Leucocytozoon toddi group (Haemosporida: Leucocytozoidae), with remarks on the species taxonomy of leucocytozoids. J. Pasasitol. 2010, 96, 170–177. [Google Scholar]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Roton, FL, USA, 2005. [Google Scholar]
- Valkiūnas, G.; Iezhova, T.A. Insights into the biology of Leucocytozoon Species (Haemosporida, Leucocytozoidae): Why is there slow research progress on agents of leucocytozoonosis? Microorganisms 2023, 11, 1251. [Google Scholar]
- Valkiūnas, G.; Iezhova, T.A. Keys to the avian malaria parasites. Malar. J. 2018, 17, 212. [Google Scholar] [PubMed]
- Valkiūnas, G.; Iezhova, T.A. Keys to the avian Haemoproteus parasites (Haemosporida, Haemoproteidae). Malar. J. 2022, 21, 269. [Google Scholar] [PubMed]
- Ferreira-Junior, F.; de Angeli Dutra, D.; Silveira, P.; Pacheco, R.; Witter, R.; de Souza Ramos, D.; Pacheco, M.A.; Escalante, A.; Braga, É. A new pathogen spillover from domestic to wild animals: Plasmodium juxtanucleare infects free-living passerines in Brazil. Parasitol 2016, 145, 1949–1958. [Google Scholar]
- Sehgal, R.N.M.; Valkiunas, G.; Iezhova, T.A.; Smith, T.B. Blood parasites of chickens in Uganda and Cameroon with molecular descriptions of Leucocytozoon schoutedeni and Trypanosoma gallinarum. J. Pasasitol. 2006, 92, 1336–1343. [Google Scholar]
- Pruck-Ngern, M.; Pattaradilokrat, S.; Chumpolbanchorn, K.; Pimnon, S.; Harnyuttanakorn, P.; Buddhirakkul, P.; Saiwichai, T. Refractoriness of the natural malaria vector Culex quinquefasciatus to Plasmodium gallinaceum. J. Trop. Med. Parasitol. 2014, 37, 60–68. [Google Scholar]
- Wiegmann, A.; Springer, A.; Rinaud, T.; Ottensmann, M.; Legler, M.; Krüger, O.; Fehr, M.; Chakarov, N.; Strube, C. The prevalence of Leucocytozoon spp. in nestlings of three wild raptor species including implications on haematological and blood chemistry values. Int. J. Parasitol. Parasites. Wild. 2021, 16, 236–243. [Google Scholar]
- Chawengkirttikul, R.; Junsiri, W.; Watthanadirek, A.; Poolsawat, N.; Minsakorn, S.; Srionrod, N.; Anuracpreeda, P. Molecular detection and genetic diversity of Leucocytozoon sabrazesi in chickens in Thailand. Sci. Rep. 2021, 11, 16686. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Iezhova, T.A.; Carlson, J.S.; Sehgal, R.N.M. Two new Trypanosoma species from African birds, with notes on the taxonomy of avian trypanosomes. J. Pasasitol. 2011, 97, 924–930. [Google Scholar] [CrossRef]
- Molyneux, D.H. Vector relationships in the Trypanosomatidae. Adv. Parasitol. 1977, 15, 1–82. [Google Scholar]
- Miltgen, F.; Landau, I. Culicoides nubeculosus, vecteur expérimental d’un nouveau trypanosome de psittaciforme: Trypanosoma bakeri n. sp. Ann. Parasitol. Hum. Comp 1982, 57, 423–428. [Google Scholar] [CrossRef]
- Votýpka, J.; Svobodová, M. Trypanosoma avium: Experimental transmission from black flies to canaries. Parasitol. Res. 2004, 92, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.B. Avian malaria: Clinical and chemical pathology of Plasmodium gallinaceum in the domesticated fowl Gallus gallus. Avian. Pathol. 2005, 34, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Koo, B.-S.; Jeon, E.-O.; Han, M.-S.; Min, K.-C.; Lee, S.B.; Bae, Y.; Mo, I.-P. Pathology and molecular characterization of recent Leucocytozoon caulleryi cases in layer flocks. J. Biomed. Res. 2016, 30, 517–524. [Google Scholar] [PubMed]
- Prasopsom, P.; Salakij, C.; Lertwatcharasarakul, P.; Pornpranom, P. Hematological and phylogenetic studies of Leucocytozoon spp. in backyard chickens and fighting cocks around Kamphaeng Saen, Thailand. Agr. Nat. Resour. 2020, 54, 595–602. [Google Scholar]
- Desser, S.S.; Stuht, J.; Fallis, A.M. Leucocytozoonosis in Canada geese in upper Michigan,. I. Strain differences among geese from different localities. J. Wildl. Dis. 1978, 14, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Desser, S.S. Schizogony and gametogony of Leucocytozoon simondi and associated reactions in the avian host. J. Protozool. 1967, 14, 244–254. [Google Scholar] [CrossRef]
- Desser, S.S.; Ryckman, A.K. The development and pathogenesis of Leucocytozoon simondi in Canada and domestic geese in Algonquin Park, Ontario. Can. J. Zool. 1976, 54, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Fallis, A.M. Comparison of infections with Leucocytozoon simondi in black ducks (Anas rubripes), mallards (Anas platyrhynchos), and white Pekins (Anas bochas). Can. J. Zool. 1968, 46, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Win, S.Y.; Chel, H.M.; Hmoon, M.M.; Htun, L.L.; Bawm, S.; Win, M.M.; Murata, S.; Nonaka, N.; Nakao, R.; Katakura, K. Detection and molecular identification of Leucocytozoon and Plasmodium species from village chickens in different areas of Myanmar. Acta Trop. 2020, 212, 105719. [Google Scholar] [CrossRef] [PubMed]
- Pohuang, T.; Jittimanee, S.; Junnu, S. Pathology and molecular characterization of Leucocytozoon caulleryi from backyard chickens in Khon Kaen Province, Thailand. Vet. World 2021, 14, 2634–2639. [Google Scholar] [CrossRef] [PubMed]
- Šlapeta, J.; Morin-Adeline, V.; Thompson, P.; McDonell, D.; Shiels, M.; Gilchrist, K.; Votýpka, J.; Vogelnest, L. Intercontinental distribution of a new trypanosome species from Australian endemic Regent Honeyeater (Anthochaera phrygia). Parasitol 2016, 143, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Zídková, L.; Cepicka, I.; Szabová, J.; Svobodová, M. Biodiversity of avian trypanosomes. Infect. Genet. Evol. 2012, 12, 102–112. [Google Scholar] [CrossRef]
- Votýpka, J.; Szabová, J.; Rádrová, J.; Zídková, L.; Svobodová, M. Trypanosoma culicavium sp. nov., an avian trypanosome transmitted by Culex mosquitoes. Int. J. Syst. Evol. Microbiol. 2012, 62, 745–754. [Google Scholar] [CrossRef]
- Pornpanom, P.; Salakij, C.; Prasopsom, P.; Lertwatcharasarakul, P.; Kasorndorkbua, C. Morphological and molecular characterization of avian trypanosomes in raptors from Thailand. Parasitol. Res. 2019, 118, 2419–2429. [Google Scholar] [CrossRef]
- Chantong, W.; Kaneene, J.B. Poultry raising systems and highly pathogenic avian influenza outbreaks in Thailand: The situation, associations, and impacts. Southeast Asian J. Trop. Med. Public Health 2011, 42, 596–608. [Google Scholar]
- Ayala, A.J.; Yabsley, M.J.; Hernandez, S.M. A review of pathogen transmission at the backyard chicken–wild bird interface. Front. Vet. Sci. 2020, 7, 539925. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Iezhova, T.-A.; Krizˇanauskiene˙, A.; Palinauskas, V.; Sehgal, R.N.M.; Bensch, S. A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J. Parasitol. 2008, 94, 1395–1401. [Google Scholar] [CrossRef]
- Chagas, C.R.F.; Binkienė, R.; Ilgūnas, M.; Iezhova, T.; Valkiūnas, G. The buffy coat method: A tool for detection of blood parasites without staining procedures. Parasit. Vectors 2020, 13, 104. [Google Scholar] [CrossRef]
- Godfrey, R.D.; Fedynich, A.M.; Pence, D.B. Quantification of hematozoa in blood smears. J. Wildl. Dis. 1987, 23, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Bensch, S.; Stjerman, M.; Hasselquist, D.; Östman, Ö.; Hansson, B.; Westerdahl, H.; Pinheiro, R.T. Host specificity in avian blood parasites: A study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc. Roy. Soc. Lond. B 2000, 267, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, O.; WaldenstrÖm, J.; Bensch, S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avain blood. J. Pasasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Pornpanom, P.; Chagas, C.R.F.; Lertwatcharasarakul, P.; Kasorndorkbua, C.; Valkiūnas, G.; Salakij, C. Molecular prevalence and phylogenetic relationship of Haemoproteus and Plasmodium parasites of owls in Thailand: Data from a rehabilitation centre. Int. J. Parasitol. Parasites Wildl. 2019, 9, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Lertwatcharasarakul, P.; Salakij, C.; Prasopsom, P.; Kasorndorkbua, C.; Jakthong, P.; Santavakul, M.; Suwanasaeng, P.; Ploypan, R. Molecular and morphological analyses of Leucocytozoon parasites (Haemosporida: Leucocytozoidae) in raptors from Thailand. Acta Parasitol. 2021, 66, 1406–1416. [Google Scholar] [CrossRef]
- Pornpanom, P.; Kasorndocbau, C.; Lertwatcharasarakul, P.; Salakij, C. Prevalence and genetic diversity of Haemoproteus and Plasmodium in raptors from Thailand: Data from rehabilitation center. Int. J. Parasitol. Parasites Wildl. 2021, 16, 75–82. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Harl, J.; Himmel, T.; Valkiūnas, G.; Ilgūnas, M.; Nedorost, N.; Matt, J.; Kübber-Heiss, A.; Alic, A.; Konicek, C.; Weissenböck, H. Avian haemosporidian parasites of accipitriform raptors. Malar. J. 2022, 21, 14. [Google Scholar] [CrossRef]
- Cadena-Ortiz, H.; Mantilla, J.S.; de Aguilar, J.R.; Flores, D.; Bahamonde, D.; Matta, N.E.; Bonaccorso, E. Avian haemosporidian infections in rufous-collared sparrows in an Andean dry forest: Diversity and factors related to prevalence and parasitaemia. Parasitol 2019, 146, 765–773. [Google Scholar] [CrossRef]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef]
- Chagas, C.R.F.; Valkiūnas, G.; de Oliveira Guimarães, L.; Monteiro, E.F.; Vaz Guida, F.J.; Simões, R.F.; Rodrigues, P.T.; de Albuquerque Luna, E.J.; Karin Kirchgatter, K. Diversity and distribution of avian malaria and related haemosporidian parasites in captive birds from a Brazilian megalopolis. Malar. J. 2017, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, K.; Zehtindjiev, P.; Mariaux, J.; Georgiev, B.B. Genetic diversity of avian haemosporidians in Malaysia: Cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Selangor. Infect. Genet. Evol. 2015, 31, 33–39. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J.A.A. MrModeltest 2.3. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Chumsri, A.; Tina, F.W.; Jaroensutasinee, M.; Jaroensutasinee, K. Seasons and water container types affecting Culex spp. in southern Thailand. J. Anim. Behav. Biometeorol. 2020, 8, 55–62. [Google Scholar] [CrossRef]
- Sunantaraporn, S.; Hortiwakul, T.; Kraivichian, K.; Siriyasatien, P.; Brownell, N. Molecular identification of host blood meals and detection of blood parasites in Culicoides Latreille (Diptera: Ceratopogonidae) collected from Phatthalung province, Southern Thailand. Insects 2022, 13, 912. [Google Scholar] [CrossRef]
- Srisuka, W.; Takaoka, H.; Otsuka, Y.; Fukuda, M.; Thongsahuan, S.; Taai, K.; Saeung, K. Biodiversity, seasonal abundance, and distribution of blackflies (Diptera: Simuliidae) in six different regions ofThailand. Parasit. Vectors 2017, 10, 574. [Google Scholar] [CrossRef]
- Muriel, J.; Marzal, A.; Magallanes, S.; García-Longoria, L.; Suarez-Rubio, M.; Bates, P.J.J.; Lin, H.H.; Soe, A.N.; Oo, K.S.; Aye, A.A.; et al. Prevalence and diversity of avian haemosporidians may vary with anthropogenic disturbance in tropical habitats in Myanmar. Diversity 2021, 13, 111. [Google Scholar] [CrossRef]
- Ferraguti, M.; Martínez-de la Puente, J.; Bensch, S.; Roiz, D.; Ruiz, S.; Viana, D.S.; Soriguer, R.C.; Figuerola, J. Ecological determinants of avian malaria infections: An integrative analysis at landscape, mosquito and vertebrate community levels. J. Anim. Ecol. 2018, 87, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Bensch, S.; Iezhova, T.A.; Križanauskienė, A.; Hellgren, O.; Bolshakov, C.V. Nested cytochrome b polymerase chain reaction diagnostics underestimate mixed infections of avian blood haemosporidian parasites: Microscopy is still essential. J. Pasasitol. 2006, 92, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Zehtindjiev, P.; Križanauskienė, A.; Bensch, S.; Palinauskas, V.; Asghar, M.; Dimitrov, D.; Scebba, S.; Valkiūnas, G. A new morphologically distinct avian malaria parasite that fails detection by established polymerase chain reaction–based protocols for amplification of the cytochrome b gene. J. Pasasitol. 2012, 98, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tris, J.; Bensch, S. Diagnosing genetically diverse avian malarial infections using mixed-sequence analysis and TA-cloning. Parasitol 2005, 131, 15–23. [Google Scholar] [CrossRef] [PubMed]
- LaPointe, D.A.; Goff, M.L.; Atkinson, C.T. Thermal constraints to the sporogonic development and altitudinal dstribution of avian malaria Plasmodium relictum in Hawaii. J. Parasitol. 2010, 96, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Chaiphongpachara, T.; Sumruayphol, S. Species diversity and distribution of mosquito vectors in coastal habitats of Samut Songkhram province, Thailand. Trop. Biomed. 2017, 34, 524–532. [Google Scholar]
- Perkins, S.L.; Schall, J.J. A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J. Pasasitol. 2002, 88, 972–978. [Google Scholar] [CrossRef]
- Pattaradilokrat, S.; Tiyamaneea, W.; Simpalipana, P.; Kaewthamasornb, M.; Saiwichai, T.; Li, J.; Harnyuttanakorna, P. Molecular detection of the avian malaria parasite Plasmodium gallinaceum in Thailand. Vet. Parasitol. 2015, 210, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Matis, C.; Léger, M. Trypanosome de la poule. Comptes Rendus Des Se’Ances Socie´Te´ Biol. Ses Fil. 1909, 67, 452–454. [Google Scholar]
- Fallis, A.M.; Jacobson, R.L.; Raybould, J.N. Experimental transmission of Trypanosoma numidae Wenyon to guinea fowl and chickens in Tanzania. J. Protozool. 1973, 20, 436–437. [Google Scholar] [CrossRef]
- Cendron, F.; Perini, F.; Mastrangelo, S.; Tolone, M.; Criscione, A.; Bordonaro, S.; Iaffaldano, N.; Castellini, C.; Marzoni, M.; Buccioni, A.; et al. Genome-wide SNP analysis reveals the population structure and the conservation status of 23 Italian chicken breeds. Animals 2020, 10, 1441. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Kabasakal, B.; Erdoğan, A. Geographic genetic structure of Alectoris chukar in Türkiye: Post-LGM-induced hybridization and human-mediated contaminations. Biology 2023, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- Anjos, C.C.; Chagas, C.R.F.; Fecchio, A.; Schunck, F.; Costa-Nascimento, M.J.; Monteiro, E.F.; Mathias, B.S.; Bell, J.F.; Guimarães, L.O.; Comiche, K.J.M.; et al. Avian malaria and related parasites from resident and migratory birds in the brazilian atlantic forest, with description of a new Haemoproteus species. Pathogens 2021, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.T.; Hargrove, G.H.; Cornbleet, P.J. Preparation of buffy coat smears in leukopenic patients. Lab. Med. 1981, 12, 96–98. [Google Scholar] [CrossRef]
- Marcos, R.; Pereira, C.; Santos, M.; Luzzago, C.; Lauzi, S.; Maia, J.P.; Faustino, A.; Puente-Payo, P. Buffy coat smear or Knott’s test: Which to choose for canine microfilaria screening in field studies? Vet. Clin. Pathol. 2016, 45, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Salakij, C.; Kasorndorkbua, C.; Lertwatcharasarakul, P.; Salakij, J. Ultra-structure of blood cells and molecular characteristics of Haemoproteus sp. in Blyth’s hawk eagle. Comp. Clin. Pathol. 2015, 24, 1293–1299. [Google Scholar] [CrossRef]
- Setiawan, A.; Nurcahyo, W.; Priyowidodo, D.; Budiati, R.T.; Susanti, D.S.R. Genetic and parasitological identification of Trypanosoma evansi infecting cattle in South Sulawesi, Indonesia. Vet. World 2021, 14, 113–119. [Google Scholar] [CrossRef]
- Adeoye, G.O.; Nga, I.C. Comparison of quantitative buffy coat technique (QBC) with Giemsa-stained thick film (GTF) for diagnosis of malaria. Parasitol. Int. 2007, 56, 308–312. [Google Scholar] [CrossRef]
- Kocharekar, M.M.; Sarkar, S.S.; Dasgupta, D. Comparative study of modified quantitative buffy coat and two rapid tests in comparison with peripheral blood smear in malaria diagnosis in Mumbai, India. J. Parasitol. Res. 2014, 2014, 194651. [Google Scholar] [CrossRef]
- Ifeorah, I.K.; Brown, B.J.; Sodeinde, O.O. A comparison of rapid diagnostic testing (by Plasmodium lactate dehydrogenase), and quantitative buffy coat technique in malaria diagnosis in children. Afr. J. Infect. Dis. 2017, 11, 31–38. [Google Scholar]
- Charpentier, E.; Benichou, E.; Pagès, A.; Chauvin, P.; Fillaux, J.; Valentin, A.; Guegan, H.; Guemas, E.; Salabert, A.-S.; Armengol, C.; et al. Performance evaluation of different strategies based on microscopy techniques, rapid diagnostic test and molecular loop-mediated isothermal amplification assay for the diagnosis of imported malaria. Clin. Microbiol. Infect. 2020, 26, 115–121. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).