Cerebral Vascularization and the Remaining Area Supply of the Internal Carotid Artery Derivatives of the Red Kangaroo (Osphranter rufus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Methods
2.2.1. Corrosion Casting
2.2.2. Latex Preparations
2.2.3. CBCT Scanning
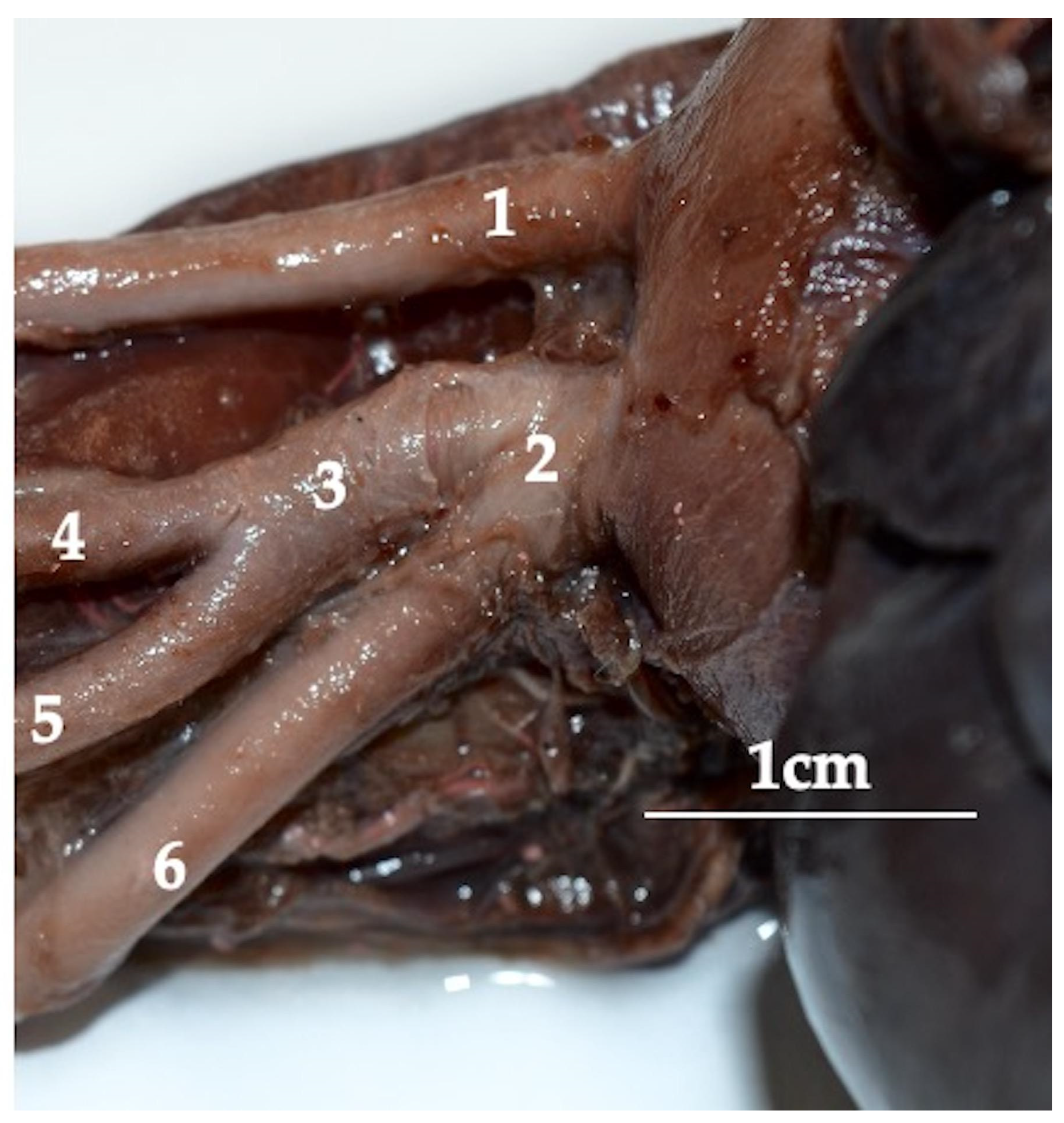
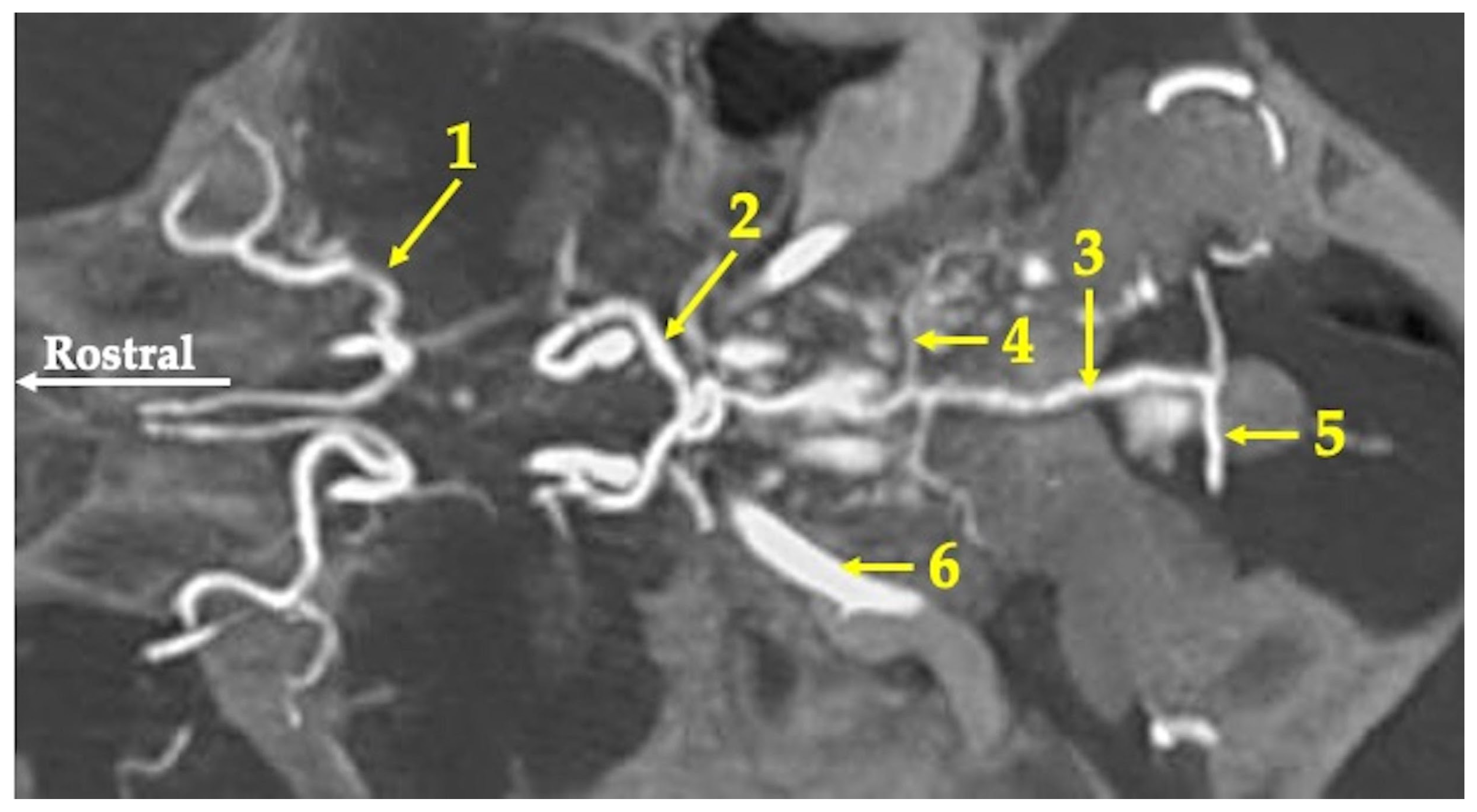
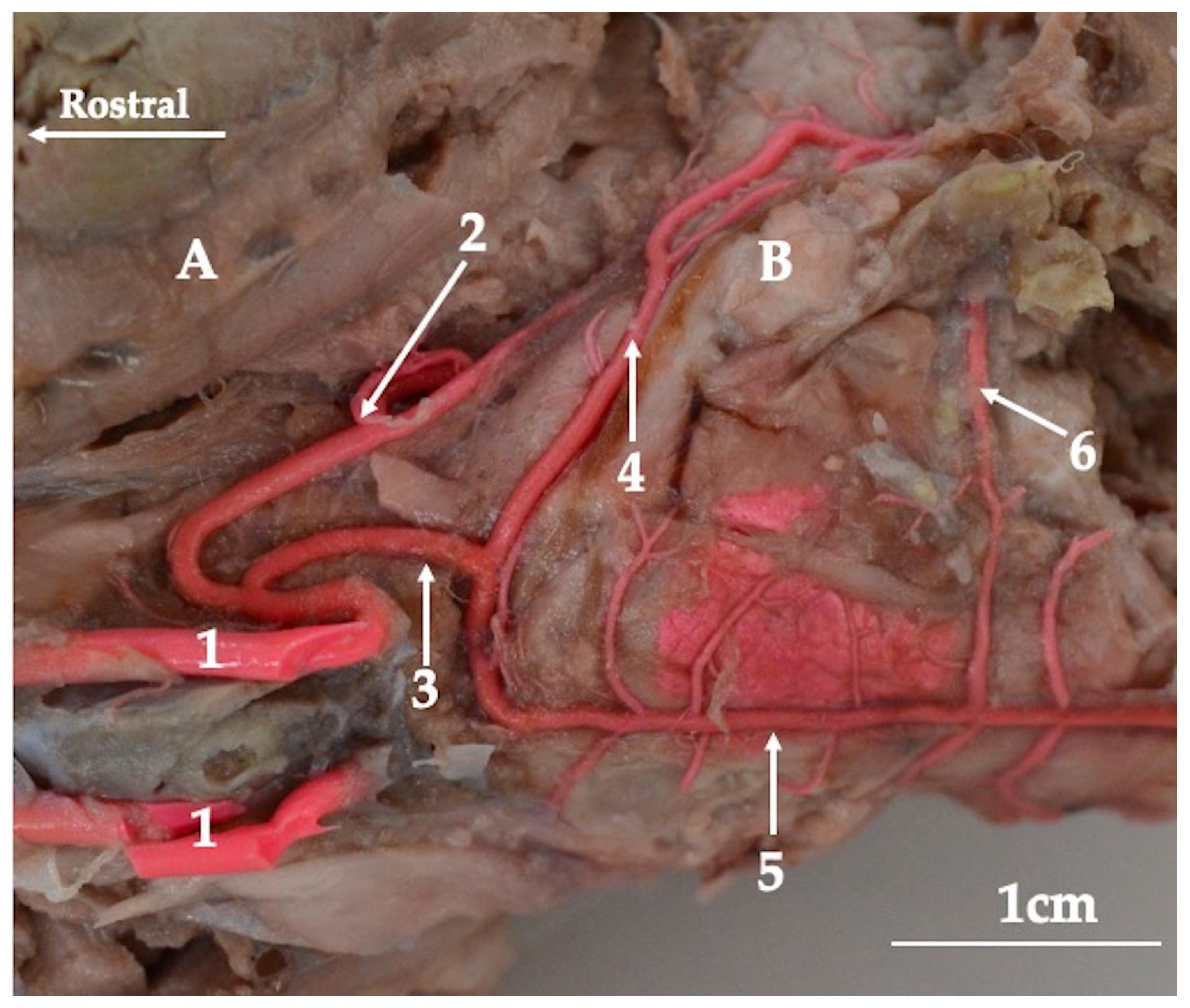
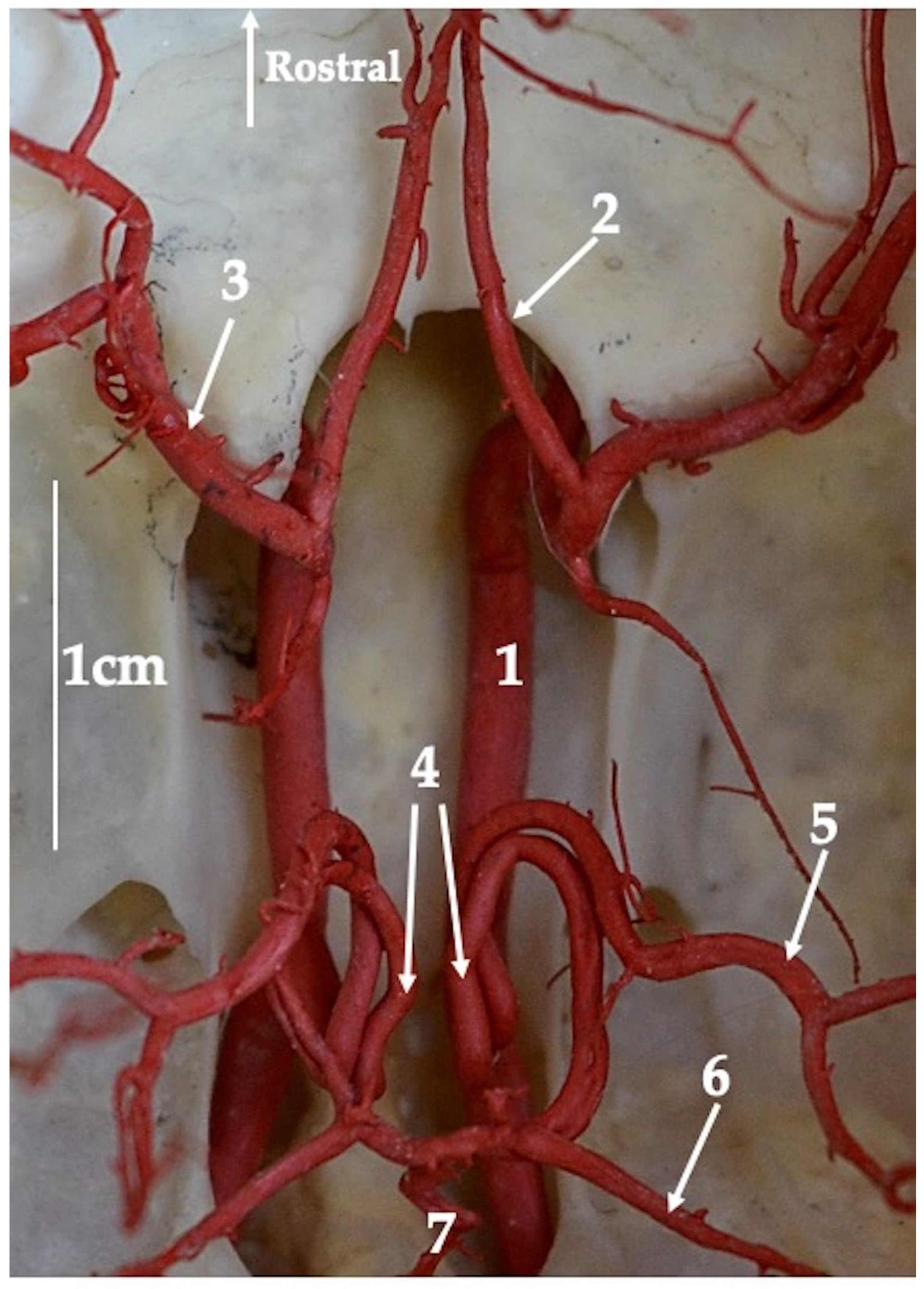
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Croft, D.B.; Clancy, T.G.F. Red kangaroo Macropus rufus. In The Mammals of Australia, 3rd ed.; Van Dyck, S., Strahan, R., Eds.; New Holland: Sydney, NSW, Australia, 2008; pp. 352–354. [Google Scholar]
- Eldridge, M.D.B.; Coulson, G.M.; Wilson, D.E.; Mittermeier, R.A. Family Macropodidae (kangaroos and wallabies). In Handbook of the Mammals of the world; Lynx Edicions: Barcelona, Spain, 2015; Volume 5, pp. 630–735. [Google Scholar]
- Johnson, J.H.; Jensen, J.M. Hepatotoxicity and secondary photosensitization in a red kangaroo (Megaleia rufus) due to ingestion of Lantana camara. J. Zoo Wildl. Med. 1998, 29, 203–207. [Google Scholar]
- Suedmeyer, W.K.; Johnson, G. Survey of neoplasia in red kangaroos (Macropus rufus), 1992–2002, in a zoological collection. J. Zoo Wildl. Med. 2007, 38, 231–239. [Google Scholar] [CrossRef]
- Lindemann, D.M.; Gamble, K.C.; Corner, S. Calcium carbonate obstructive urolithiasis in a red kangaroo (Macropus rufus). J. Zoo Wildl. Med. 2013, 44, 196–199. [Google Scholar] [CrossRef]
- Knafo, S.E.; Rosenblatt, A.J.; Morrisey, J.K.; Flanders, J.A.; Thompson, M.S.; Knapp-Hoch, H.M. Diagnosis and treatment of mesenteric volvulus in a red kangaroo (Macropus rufus). J. Am. Vet. Med. Assoc. 2014, 244, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Takle, G.L.; Suedmeyer, W.K.; Hunkeler, A. Selected diagnostic ophthalmic tests in the red kangaroo (Macropus rufus). J. Zoo Wildl. Med. 2010, 41, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Ballor, K.L.; Gazzola, K.M.; Perry, K.L. Bilateral radial and ulnar fractures in a red kangaroo (Macropus rufus). J. Am. Vet. Med. Assoc. 2019, 255, 942–948. [Google Scholar] [CrossRef]
- Lott, M.J.; Hose, G.C.; Power, M.L. Parasitic nematode communities of the red kangaroo, Macropus rufus: Richness and structuring in captive systems. Parasitol. Res. 2015, 114, 2925–2932. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.W.; Smith, S.; Snider, T.A. Hypertrophic cardiomyopathy in two captive Bennett’s wallabies (Macropus rufogriseus rufogriseus). J. Vet. Diagn. Investig. 2009, 21, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Kagan, R.A.; Kinsel, M.; Gloor, K.; Mylniczenko, N.D.; Langan, J.N.; Farina, L.L.; Terio, K.A. Morphologic evidence suggestive of hypertension in western gray kangaroos (Macropus fuliginosus). Vet. Pathol. 2009, 46, 977–984. [Google Scholar] [CrossRef]
- Ijiri, A.; Yoshiki, K.; Tsuboi, S.; Shimazaki, H.; Akiyoshi, H.; Nakade, T. Surgical resection of twenty-three cases of brain meningioma. J. Vet. Med. Sci. 2014, 76, 331–338. [Google Scholar] [CrossRef]
- de Laforcade, A. Diseases Associated with Thrombosis. Top. Companion Anim. Med. 2012, 27, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Maloney, S.K.; Fuller, A.; Meyer, L.C.; Kamerman, P.R.; Mitchell, G.; Mitchell, D. Brain thermal inertia, but no evidence for selective brain cooling, in free-ranging western grey kangaroos (Macropus fuliginosus). J. Comp. Physiol. 2009, 179, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Windle, B.C.; Parsons, F.G. On the anatomy of Macropus rufus. J. Anat. Physiol. 1897, 32 Pt 1, 119. [Google Scholar] [PubMed]
- Sran, F. Gross anatomy of the digestive system in eastern grey kangaroo (Macropodidae family). Lucr. Ştiinţ. Ser. Med. Vet. Univ. Ştiinţe Agric. Med. Vet. Banat. Timiş. 2015, XLVIII, 173. [Google Scholar]
- Warburton, N.M.; Bateman, P.W.; Fleming, P.A. Anatomy of the cavernous muscles of the kangaroo penis highlights marsupial–placental dichotomy. J. Anat. 2019, 234, 306–315. [Google Scholar] [CrossRef]
- Dawson, R.; Milne, N.; Warburton, N.M. Muscular anatomy of the tail of the western grey kangaroo, Macropus fuliginosus. Aust. J. Zool. 2014, 62, 166–174. [Google Scholar] [CrossRef]
- Lima, M.; Méndez, V.; Pérez, W.; Lima, M.; Méndez, V.; Pérez, W. Gross anatomy of the heart in the western grey kangaroo (Macropus fuliginosus). Int. J. Morphol. 2009, 27, 1099–1104. [Google Scholar] [CrossRef]
- International Committee on Veterinary Gross Anatomical Nomenclature. Nomina Anatomica Veterinária, 6th ed.; Editorial Committee: Hanover, Germany, 2017; pp. 73–147. [Google Scholar]
- Mazensky, D.; Flesarova, S.; Sulla, I. Arterial blood supply to the spinal cord in animal models of spinal cord injury. A review. Anat. Rec. 2017, 300, 2091–2106. [Google Scholar] [CrossRef]
- Nickiel, R.; Schwarz, R. Vergleichende Betrachtung der Kopfarterien der Haussaugetiere (Katze, Hund, Schwein, Rind, Schaf, Ziege, Pferd). Comparative analysis of the head arteries of domestic mammals (cat, dog, pig, cattle, sheep, goat, horse). Zentralblatt Veterinärmedizin Reihe A 1963, 10, 89–120. (In German) [Google Scholar] [CrossRef]
- Goździewska-Harłajczuk, K.; Sokołowski, W.; Klećkowska-Nawrot, J.; Jańczak, D.; Bełkot, Z.; Barszcz, K.; Czubaj, N.; Gappa, M. Morphology and morphometry of ramification of the aortic arch in domestic shorthair cats in the clinical aspect. Med. Weter. 2017, 73, 295–298. [Google Scholar]
- Schorn, C.; Hildebrandt, N.; Schneider, M.; Schaub, S. Anomalies of the aortic arch in dogs: Evaluation with the use of multidetector computed tomography angiography and proposal of an extended classification scheme. BMC Vet. Res. 2021, 17, 387. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A. The arteries originating from the aortic arch and the branches of these arteries in red squirrels (Sciurus vulgaris). Vet. Med. 2011, 56, 131–134. [Google Scholar] [CrossRef]
- Aydin, A.; Ozkan, Z.E.; Yilmaz, S.; Ilgun, R. The arteries originating from the aortic arch and the patterns of their branches in ground squirrels (Spermophilus citellus). Vet. Med. 2011, 56, 469–472. [Google Scholar] [CrossRef]
- Akbari, G.; Asadiahranjani, B.; Goodarzi, N.; Shokrollahi, S. The Branching Pattern of the Brachiocephalic Trunk in the Donkey (Equus asinus). Anat. Histol. Embryol. 2017, 46, 359–364. [Google Scholar] [CrossRef] [PubMed]
- te Shin, S.; Sim, J.H.; Kim, J.T.; Oh, H.S.; Tae, H.J.; Park, B.Y.; Kim, I.S.; Ahn, D. Branching patterns of the aortic arch in the Siberian roe deer (Capreolus pygargus Pallas, 1771). J. Vet. Med. Sci. 2018, 80, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Pérez, W.; Erdoğan, S. Arterial thoracic vascularization in some deer species: Pampas deer (Ozotoceros bezoarticus), brown brocket deer (Mazama gouazoubira) and axis deer (Axis axis). Anat. Histol. Embryol. 2014, 43, 490–494. [Google Scholar] [CrossRef]
- Atalar, Ö.; Yilmaz, S.; Burma, O.; Ilkay, E. The macroanatomical investigations on the aortic arch in porcupines (Hystrix cristata). Anat. Histol. Embryol. 2003, 32, 367–369. [Google Scholar] [CrossRef]
- Aydin, A.; Ozkan, Z.E.; Ilgun, R. The morphology of the arteries originating from the arcus aorta and the branches of these arteries in mole-rats (Spalax leucodon). Vet. Med. 2013, 58, 373–376. [Google Scholar] [CrossRef]
- Santos, R.; Fernandes, M.; De Souza, S.F.; dos Santos-Sousa, C.A.; De Carvalho, Y.K. Anatomical description of the aortic arch of a rare species “the giant armadillo”(Priodontes maximus; Kerr, 1792). Folia Morphol. 2020, 79, 168–171. [Google Scholar] [CrossRef]
- Vučurević, G.; Marinković, S.; Puškaš, L.; Kovačević, I.; Tanasković, S.; Radak, D.; Ilić, A. Anatomy and radiology of the variations of aortic arch branches in 1266 patients. Folia Morphol. 2013, 72, 113–122. [Google Scholar] [CrossRef]
- Zdun, M.; Ruszkowski, J.J.; Gogulski, M.; Józefiak, A.; Hetman, M. Arterial Circle of the Brain of the Red-Necked Wallaby (Notamacropus rufogriseus). Animals 2022, 12, 2796. [Google Scholar] [CrossRef] [PubMed]
- Brudnicki, W.; Nowicki, W.; Skoczylas, B.; Brudnicki, A.; Kirkiłło-Stacewicz, K.; Wach, J. Arteries of the brain in wild European rabbit Oryctolagus cuniculus (Linnaeus, 1758). Folia Biol. 2012, 60, 189–194. [Google Scholar] [CrossRef]
- Brudnicki, W.; Kirkiłło-Stacewicz, K.; Skoczylas, B.; Nowicki, W.; Jabłoński, R.; Brudnicki, A.; Wach, J. The arteries of the brain in hare (Lepus europaeus Pallas, 1778). Anat. Rec. 2015, 298, 1774–1779. [Google Scholar] [CrossRef]
- Habermehl, K.H. Zur Topographie der Gehirngefäße des Hundes. Anat. Histol. Embryol. 1973, 2, 327–353. [Google Scholar] [CrossRef]
- Bugge, J. The cephalic arterial system in carnivores, with special reference to the systematic classification. Acta Anat. 1978, 101, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Frąckowiak, H.; Zawidzka, B. Tętnice głowy u lisa srebrzystego. Rocz. Akad. Rol. W Poznaniu. Zootech. 1990, 220, 27–36. [Google Scholar]
- Skoczylas, B.; Brudnicki, W.; Kirkiłło-Stacewicz, K.; Nowicki, W.; Wach, J. Cortical branches of the middle cerebral artery in silver fox (Vulpes vulpes). Pesqui. Veterinária Bras. 2016, 36, 1053–1057. [Google Scholar] [CrossRef]
- Frąckowiak, H.; Giejdasz, K. Przebieg i zmienność tętnic na podstawie mózgowia u gatunków z rzędu Perissodactyla. Rocz. Akad. Rol. W Poznaniu. Zootech. 1998, 50, 109–117. [Google Scholar]
- Kiełtyka-Kurc, A.; Frackowiak, H.; Zdun, M.; Nabzdyk, M.; Kowalczyk, K.; Tołkacz, M. The arteries on the base of the brain in the camelids (Camelidae). Ital. J. Zool. 2014, 81, 215–220. [Google Scholar] [CrossRef]
- Al Aiyan, A.; Menon, P.; AlDarwich, A.; Almuhairi, F.; Alnuaimi, S.; Bulshawareb, A.; Qablan, M.; Shehab, S. Descriptive analysis of cerebral arterial vascular architecture in dromedary camel (Camelus dromedarius). Front. Neuroanat. 2019, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Jerbi, H.; Khaldi, S.; Pérez, W. Morphometric Study of the rostral epidural rete mirabile in the dromedary (Camelus dromedarius, Linnaeus 1758). Int. J. Morphol. 2016, 34, 1429–1435. [Google Scholar] [CrossRef]
- Da Silva, R.S.B.; De Oliveira, G.B.; Oliveira, C.M.; Bezerra, F.V.F.; Câmara, F.V.; De Oliveira, R.E.M.; De Oliveira, M.F. Arterial vascularization of the brain of the agouti (Dasyprocta aguti Linnaeus, 1766). Semin. Ciências Agrárias 2016, 37, 773–784. [Google Scholar] [CrossRef][Green Version]
- Kuchinka, J.; Nowak, E.; Szczurkowski, A.; Kuder, T. Arteries supplying the base of the brain in the mongolian gerbil (Meriones unguiculatus). Pol. J. Vet. Sci. 2008, 11, 295–299. [Google Scholar] [PubMed]
- Frąckowiak, H.; Śmiełowski, J. Cephalic arteries in the european beaver Castor fiber. Acta Theriol. 1998, 43, 219–224. [Google Scholar] [CrossRef]
- Szczurkowski, A.; Kuchinka, J.; Nowak, E.; Kuder, T. Topography of arterial circle of the brain in Egyptian spiny mouse (Acomys cahirinus, Desmarest). Anat. Histol. Embryol. 2007, 36, 147–150. [Google Scholar] [CrossRef]
- Komatsu, T.; Ohta, H.; Motegi, H.; Hata, J.; Terawaki, K.; Koizumi, M.; Muta, K.; Okano, H.J.; Iguchi, Y. A novel model of ischemia in rats with middle cerebral artery occlusion using a microcatheter and zirconia ball under fluoroscopy. Sci. Rep. 2021, 11, 12806. [Google Scholar] [CrossRef]
- Zdun, M.; Melnyk, O.O.; Ruszkowski, J.J.; Hetman, M. Arterial circle of the brain in the common wildebeest (Connochaetes taurinus). Anat. Rec. 2023, 306, 2052–2058. [Google Scholar] [CrossRef]
- Frąckowiak, H.; Kulawik, M. Przebieg i zmiennosc tetnic na podstawie mozgowia u gatunkow z podrodziny Caprinae. Rocz. AR Pozn. 2001, 53, 37–48. [Google Scholar]
- Frąckowiak, H.; Zdun, M.; Kowalczyk, K.; Komosa, M.; Kiełtyka-Kurc, A. Comparison of cerebral base arteries in antelopes of Tragelaphus, Taurotragus and Boselaphus genera. Zoomorphology 2014, 133, 351–357. [Google Scholar] [CrossRef]
- Frąckowiak, H.; Dębiński, D.; Komosa, M.; Zdun, M. The arterial circle of the brain, its branches and connections in selected representatives of the Antilopinae. J. Morphol. 2015, 276, 766–771. [Google Scholar] [CrossRef]
- Frąckowiak, H.; Jakubowski, H. Arterial vascularization in the giraffe brain. In Annales Zoologici Fennici; Finnish Zoological and Botanical Publishing Board: Helsinki, Finland, 2008; Volume 4, pp. 353–359. [Google Scholar]
- Frąckowiak, H.; Godynicki, S. Brain basal arteries in various species of Felidae. Pol. J. Vet. Sci. 2003, 6, 195–200. [Google Scholar]
- Aydin, A. The morphology of circulus arteriosus cerebri in the red squirrel (Sciurus vulgaris). Vet. Med. 2008, 53, 272. [Google Scholar] [CrossRef]
- Aydın, A.; Yılmaz, S.; Dinç, G.; Özdemir, D.; Karan, M. The morphology of circulus arteriosus cerebri in the porcupine (Hystrix cristata). Vet. Med. 2005, 50, 131–135. [Google Scholar] [CrossRef]
- Azambuja, R.; Goltz, L.; Campos, R. Systematization of the brain base arteries in nutria (Myocastor coypus). Acta Sci. Vet. 2018, 46, 1580. [Google Scholar] [CrossRef]
- Brudnicki, W.; Skoczylas, B.; Jabłoński, R.; Nowicki, W.; Brudnicki, A.; Kirkiłło-Stacewicz, K.; Wach, J. The arteries of the brain base in the degu (Octodon degus Molina 1782). Vet. Med. 2014, 7, 343–348. [Google Scholar] [CrossRef]
- Kuchinka, J. Internal ophtalmic arteries within the brain-base arteria system in guinea pig. Anat. Rec. 2018, 5, 887–891. [Google Scholar] [CrossRef]
- Kuchinka, J. Morphometry and variability of the brain arterial circle in chinchilla (Chinchilla laniger, Molina). Anat. Rec. 2017, 8, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Fenrich, M.; Habjanovic, K.; Kajan, J.; Heffer, M. The circle of Willis revisited: Forebrain dehydration sensing facilitated by the anterior communicating artery: How hemodynamic properties facilitate more efficient dehydration sensing in amniotes. BioEssays 2021, 43, 2000115. [Google Scholar] [CrossRef] [PubMed]
- Trangmar, S.J.; González-Alonso, J. Heat, hydration and the human brain, heart and skeletal muscles. Sports Med. 2019, 49, 69. [Google Scholar] [CrossRef]
- Fantini, S.; Sassaroli, A.; Tgavalekos, K.T.; Kornbluth, J. Cerebral blood flow and autoregulation: Current measurement techniques and prospects for noninvasive optical methods. Neurophotonics 2016, 3, 031411. [Google Scholar] [CrossRef]
- Trangmar, S.J.; Chiesa, S.T.; Llodio, I.; Garcia, B.; Kalsi, K.K.; Secher, N.H.; González-Alonso, J. Dehydration accelerates reductions in cerebral blood flow during prolonged exercise in the heat without compromising brain metabolism. Am. J. Physiol.-Heart Circ. Physiol. 2015, 309, H1598. [Google Scholar] [CrossRef]
- Zdun, M.; Frąckowiak, H.; Kiełtyka-Kurc, A.; Kowalczyk, K.; Nabzdyk, M.; Timm, A. The arteries of brain base in species of Bovini tribe. Anat. Rec. 2013, 296, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- König, H.E. Anatomie und Entwicklung der Blutgefässe in der Schädelhöhle der Hauswiederkäuer (Rind, Schaf und Ziege); Ferdinand Enke Verl: Stuttgart, Germany, 1979; pp. 1–215. [Google Scholar]
- Reckziegel, S.H.; Lindemann, T. A systematic study of the brain base arteries in capybara (Hydrochoerus hydrochaeris). J. Morphol. Sci. 2017, 18, 103–110. [Google Scholar]
- Majewska-Michalska, E. Vascularization of the brain in guinea pig. I. Gross anatomy of the arteries and veins. Folia Morphol. 1994, 53, 249–268. [Google Scholar]
- Aydin, A.; Yilmaz, S.; Ozkan, Z.E.; Ilgun, R. The morphology of the circulus arteriosus cerebri in the ground squirrel (Spermophilus citellus). Vet. Med. 2009, 54, 537–542. [Google Scholar] [CrossRef]
- Green, E.C. Anatomy of the Rat; Hafner Publishing Company: New York, NY, USA; London, UK, 1968; pp. 178–187. [Google Scholar]
- Wiland, C. Comparative study on structure and variation in basal arteries of the brain in laboratory mouse. Anat. Anz. 1974, 135, 455–464. [Google Scholar]
- Nanda, B. Blood supply to the brain. In Sisson and Grossman’s the Anatomy of the Domestic Animals; Getty, R., Ed.; Sounders Company: Philadelphia, PA, USA, 1975; Volume 1, pp. 973–1011. [Google Scholar]
- Zdun, M.; Jabłonski, R.; Dębinski, D.; Frąckowiak, H. The Eurasian elk’s (Alces alces) brain base arteries in view of vascular variation. Anat. Rec. 2019, 302, 339–345. [Google Scholar] [CrossRef]
- Skoczylas, B.; Brudnicki, W.; Nowicki, W.; Kirkillo-Stacewicz, K.; Jablonski, R.; Wach, J. The cortical branches of the middle cerebral artery in the otter (Lutra lutra). Vet. Med. 2012, 57, 282–286. [Google Scholar] [CrossRef]
- Skoczylas, B.; Brudnicki, W.; Kirkiłło-Stacewicz, K.; Nowicki, W.; Wach, J. Ramifications of the middle cerebral artery in polar fox (Alopex lagopus). Electron. J. Pol. Agric. Univ. Ser. Vet. Med. 2017, 20, 3. [Google Scholar] [CrossRef]
- Zdun, M.; Melnyk, O.P.; Melnyk, O.O.; Nabzdyk, M. Blood supply to the cranial cavity in the patagonian mara (Dolichotis patagonum). Vet. Res. Commun. 2023, 1–7. [Google Scholar] [CrossRef]
- Zdun, M.; Ruszkowski, J.J.; Hetman, M.; Melnyk, O.O.; Frąckowiak, H. Strategies of vascularization of the ethmoid labyrinth in selected even-toed ungulates (Artiodactyla) and carnivores (Carnivora). J. Anat. 2023, 242, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Godynicki, S.; Wiland, C. Tętnice podstawy mózgowia u sarny. Rocz. AR Poznań. 1971, 54, 47–54. [Google Scholar]
- Baldwin, B.A.; Bell, F.R. The contribution of the carotid and cerebral arteries to the blood pressure in sheep. J. Physiol. 1960, 151, 9–10. [Google Scholar]
- Baldwin, B.A.; Bell, F.R. Effect of clamping cartotid and vertebral arteries on the blood pressure in sheep. J. Physiol. 1960, 151, 39–40. [Google Scholar]
- Baldwin, B.A.; Bell, F.R. The anatomy of the cerebral circulation in the sheep and ox. J. Anat. 1963, 97, 203–215. [Google Scholar] [PubMed]
- Freitas, L.M.; Pereira, K.F.; De Melo, F.R.; Silveira, L.; Dos Santos, O.P.; Sabec Pereira, D.K.; Alves de Melo, F.C.S.; Jácomo, A.; Lima, F.C. Gross anatomy and vascularization of the brain of Pacarana (Dinomys branickii). Acta Sci. Vet. 2018, 46, 6. [Google Scholar] [CrossRef]
- Bugge, J. The contribution of the stapedial artery to the cephalic arterial supply in muroid rodents. Cells Tissues Organs 1970, 76, 313–336. [Google Scholar] [CrossRef]
- De Vriese, B. Sur la signification morfologique des artères cérébrales. Arch. Biol. 1905, 21, 357–457. [Google Scholar]
- Lima, E.M.M.D.; Prada, I.L.D.S.; Silva, F.O.C.; Severino, R.S.; Santos, A.L.Q.; Borges, B.O.; Paim, T.D.P.; Vianna, A.R.D.C.B. Sistematização da origem, da distribuição e dos territórios da artéria cerebral caudal na superfície do encéfalo em gatos. Ciência Rural 2010, 40, 1961–1965. [Google Scholar] [CrossRef]
- de Alcântara, M.A.; de Santis Prada, I.L. Artérias da base do encéfalo de cães (Canis familiaris, Linnaeus, 1758). I. Estudo Anatômico de suas Origens e Comportamento. Braz. J. Vet. Res. Anim. Sci. 1996, 33, 67–71. [Google Scholar] [CrossRef][Green Version]
- Campos, A.; Prada, I.L.D.S.; Santos Junior, I.D.; Santos, D.D. Artérias da base do encéfalo de eqüinos: Sistema occipito-basilar. Braz. J. Vet. Res. Anim. Sci. 2003, 40, 107–117. [Google Scholar] [CrossRef]
- Lindemann, T.; Reckziegel, S.; Campos, R. A systematic study of brain base arteries in the opossum Didelphis albiventris. Braz. J. Morphol. Sci. 2000, 17, 35–41. [Google Scholar]
- Souza, F.D.; Campos, R. A systematic study of the brain base arteries in the rabbit (Oryctolagus cuniculus). Pesqui. Veterinária Bras. 2013, 33, 796–806. [Google Scholar] [CrossRef]
- Barreiro, J.R.; Carvalho, A.F.D.; Franciolli, A.L.R.; Ferreira, G.J.B.; Ferreira, J.R.; Ambrósio, C.E.; Miglino, M.A. Morfologia dos vasos da base do encéfalo do quati (Nasua nasua). Pesqui. Veterinária Bras. 2012, 32, 567–572. [Google Scholar] [CrossRef]
- do Amaral, S.; Passos, N.C.; Hernandez, J.M.F.; Palhano, H.B.; Antunes, M.S.; Scherer, P.O. Estudo morfológico da vascularização arterial da base do encéfalo de suínos (Sus scrofa domesticus, Linnaeus, 1758) mestiços. Braz. J. Vet. Med. 2013, 35, 365–370. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdun, M.; Ruszkowski, J.J.; Gogulski, M. Cerebral Vascularization and the Remaining Area Supply of the Internal Carotid Artery Derivatives of the Red Kangaroo (Osphranter rufus). Animals 2023, 13, 2744. https://doi.org/10.3390/ani13172744
Zdun M, Ruszkowski JJ, Gogulski M. Cerebral Vascularization and the Remaining Area Supply of the Internal Carotid Artery Derivatives of the Red Kangaroo (Osphranter rufus). Animals. 2023; 13(17):2744. https://doi.org/10.3390/ani13172744
Chicago/Turabian StyleZdun, Maciej, Jakub Jędrzej Ruszkowski, and Maciej Gogulski. 2023. "Cerebral Vascularization and the Remaining Area Supply of the Internal Carotid Artery Derivatives of the Red Kangaroo (Osphranter rufus)" Animals 13, no. 17: 2744. https://doi.org/10.3390/ani13172744
APA StyleZdun, M., Ruszkowski, J. J., & Gogulski, M. (2023). Cerebral Vascularization and the Remaining Area Supply of the Internal Carotid Artery Derivatives of the Red Kangaroo (Osphranter rufus). Animals, 13(17), 2744. https://doi.org/10.3390/ani13172744






