Stall-Feeding of Sheep on Restricted Grazing: Effects on Performance and Serum Metabolites, Ruminal Fermentation, and Fecal Microbiota
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Growth Performance Traits
2.3. Serum Collection and Serum Analysis
2.4. Ruminal Fermentation Analysis
2.5. Fecal Sampling, Microbiota Sequencing and Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Performances
3.2. Serum Biochemical Parameters
3.3. Serum Antioxidant and Immunity Indices
3.4. Ruminal Fermentation Parameters
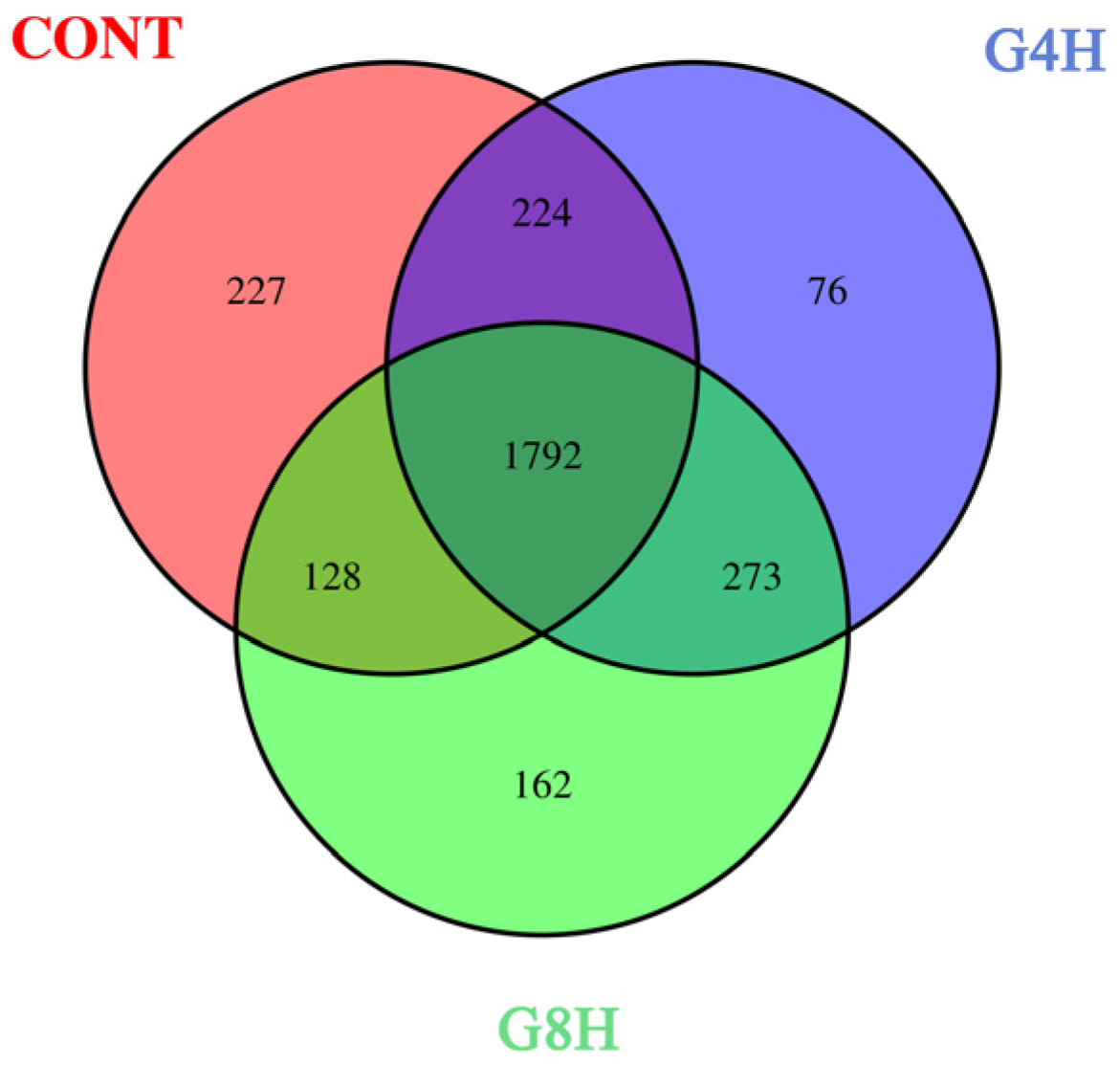
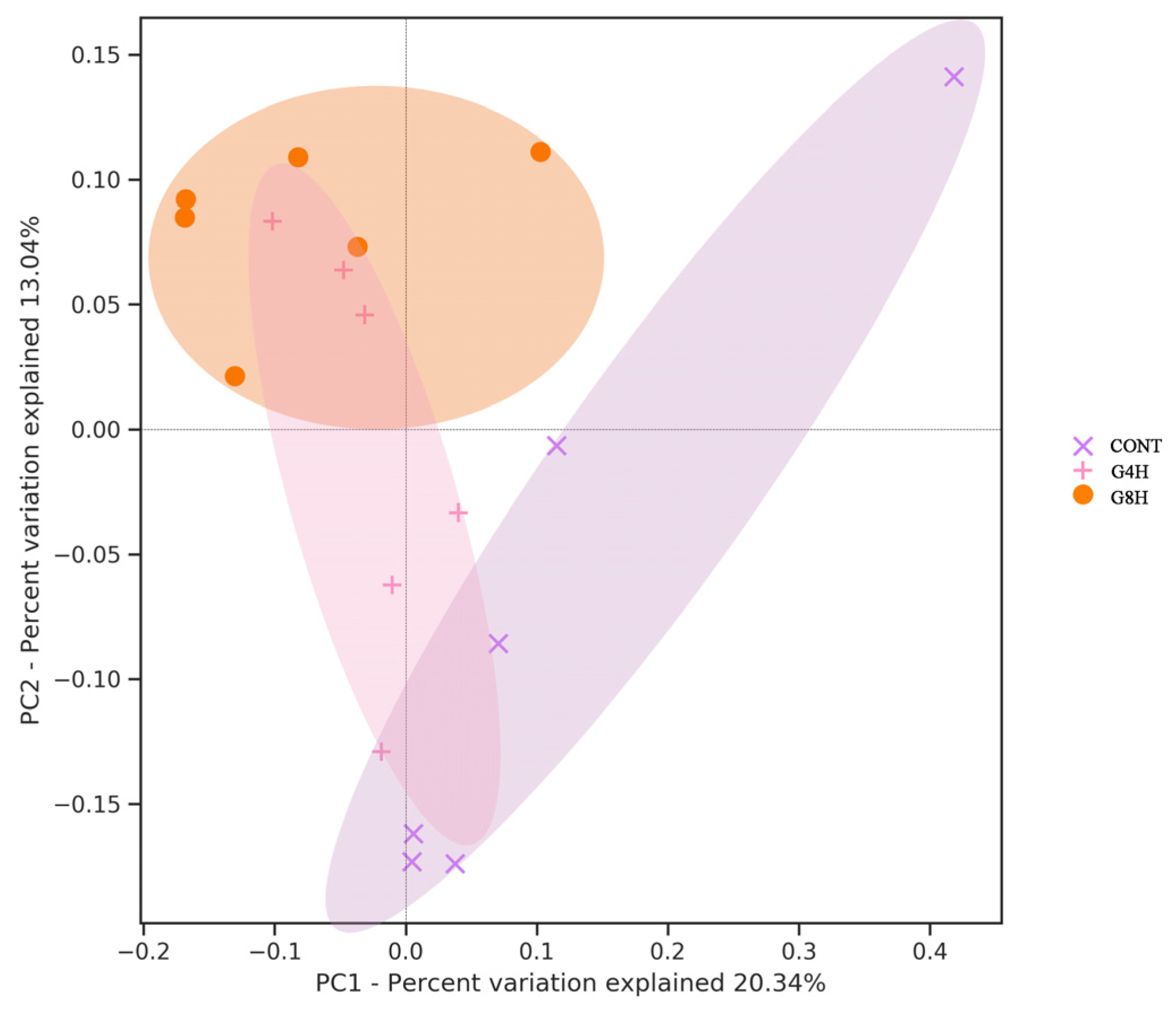
3.5. Fecal Bacterial Microbiome
3.5.1. Bacterial OTUs and Alpha Diversity
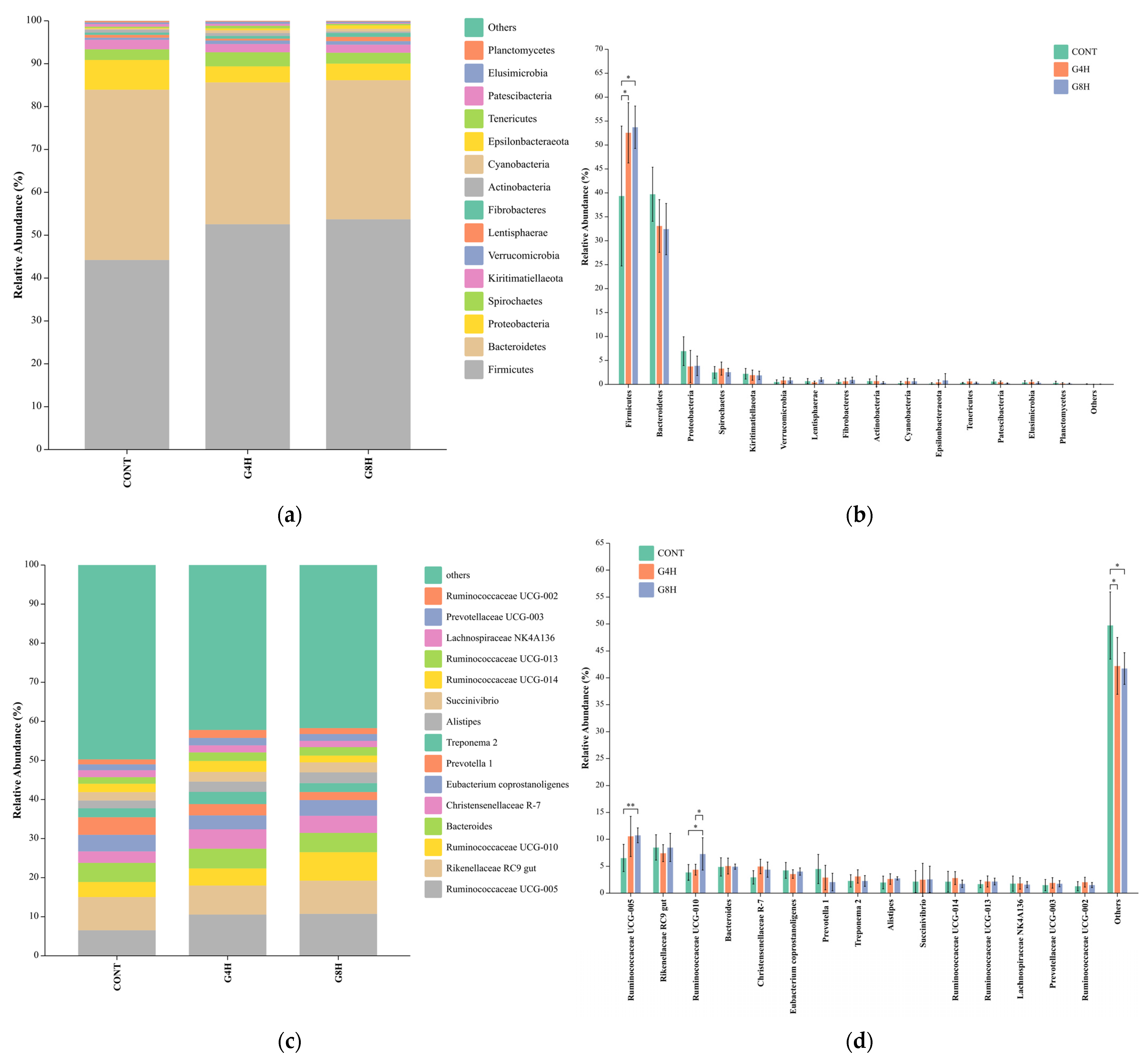
3.5.2. Relative Abundance of Bacteria in Lamb Feces at the Phylum and Genus Level
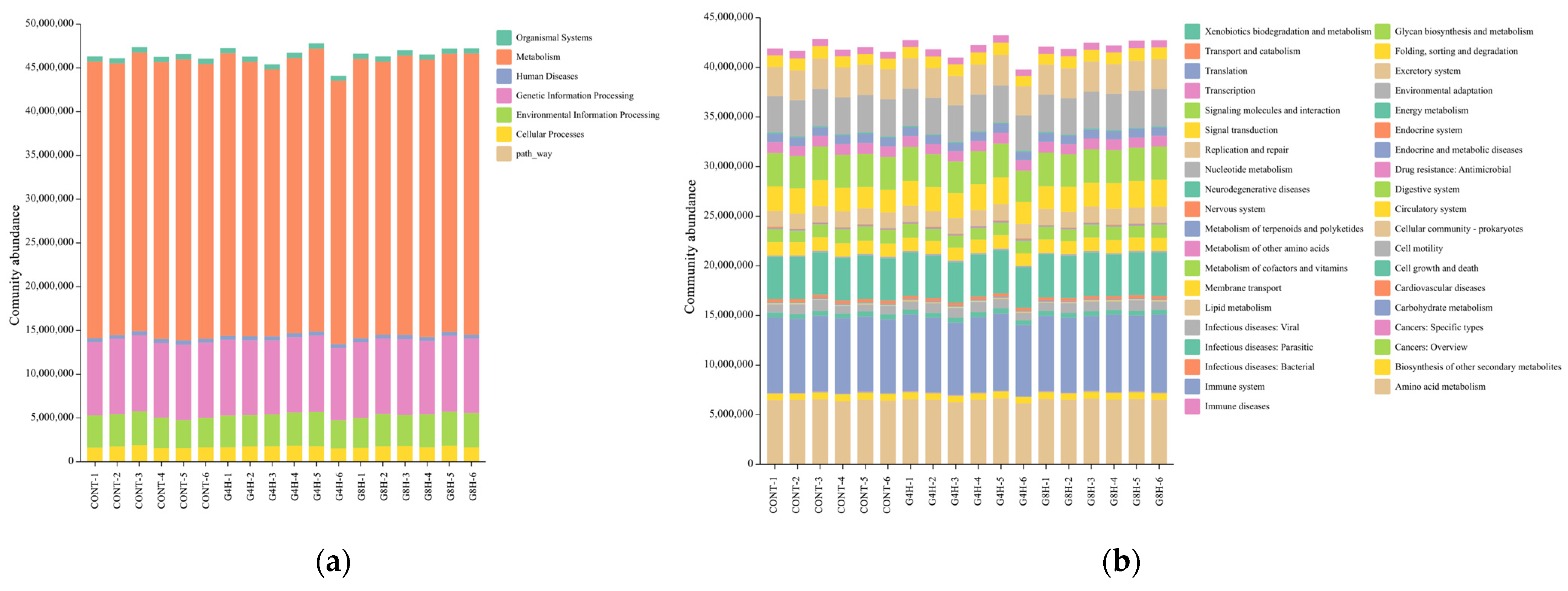
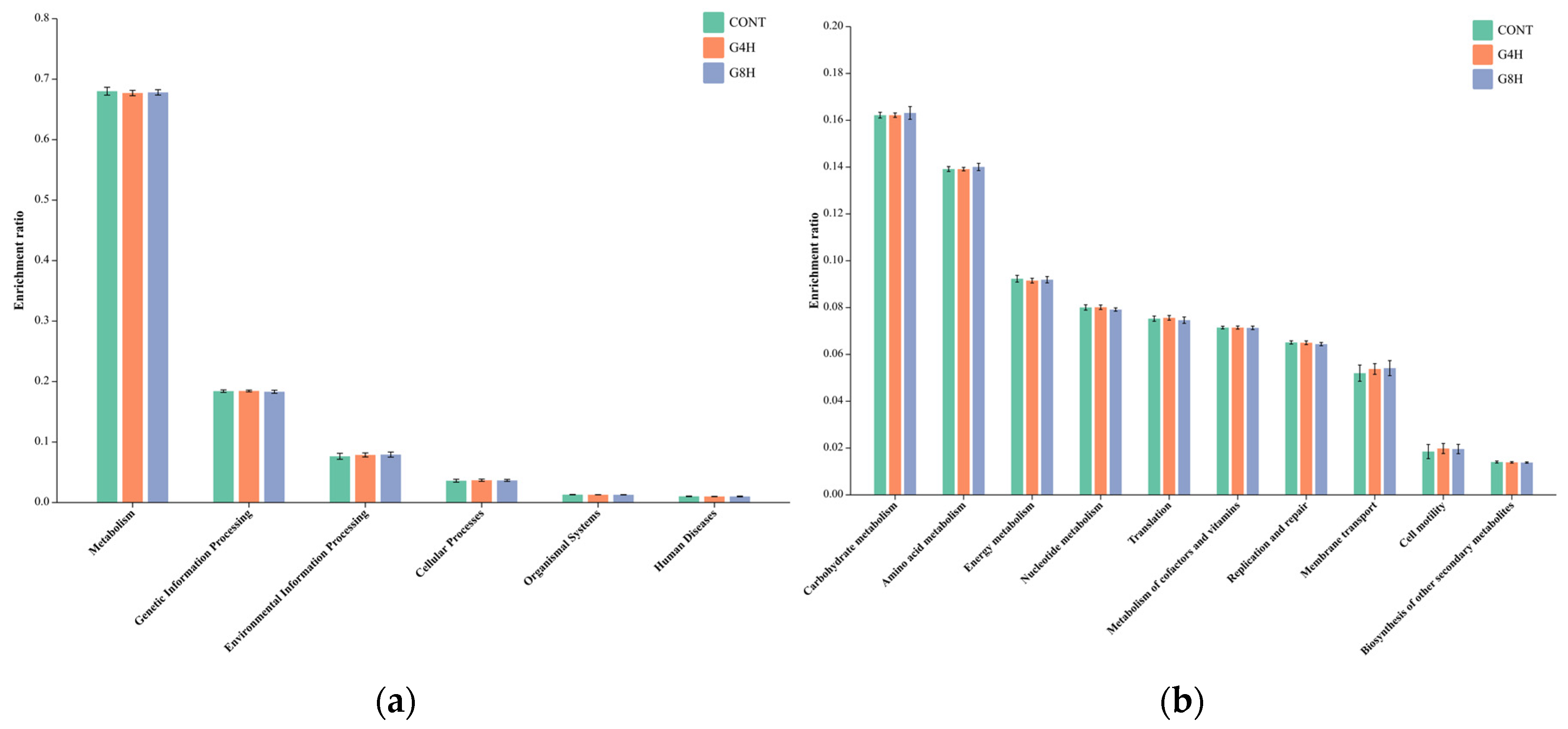
3.5.3. Prediction of Bacterial Gene Functions
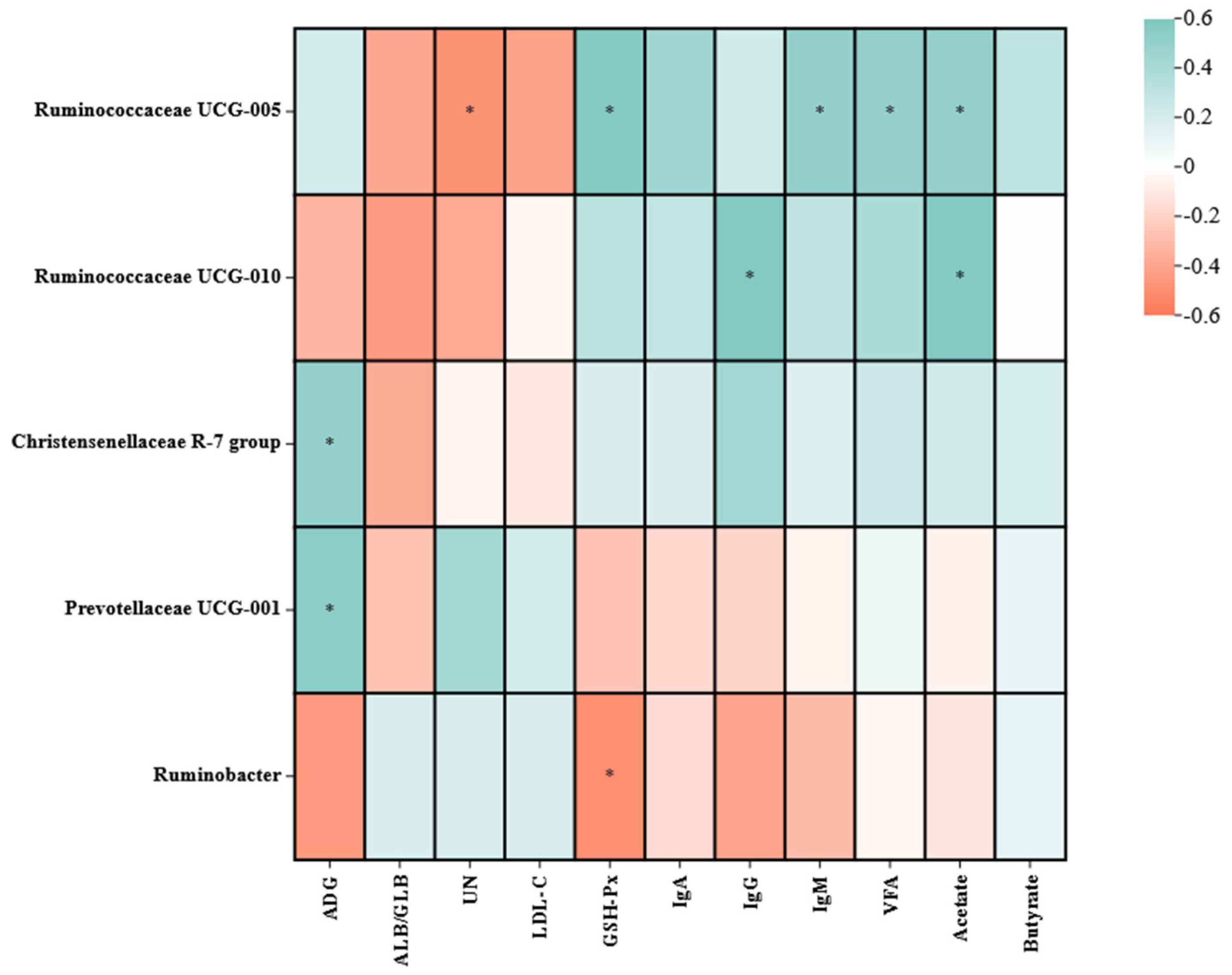
3.5.4. Correlation Analysis
4. Discussion
4.1. Growth Performances
4.2. Serum Biochemical Parameters
4.3. Serum Antioxidant and Immunity Indices
4.4. Ruminal Fermentation Parameters
4.5. Fecal Bacterial Microbiome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.R.; Liu, X.M.; Song, P.K.; Zhao, J.M.; Zhang, J.X.; Zhao, J.X. Skeletal muscle mass, meat quality and antioxidant status in growing lambs supplemented with guanidinoacetic acid. Meat Sci. 2022, 192, 108906. [Google Scholar] [CrossRef]
- Sheng, Y.; Song, L.G. Agricultural production and food consumption in China: A long-term projection. China Econ. Rev. 2019, 53, 15–29. [Google Scholar] [CrossRef]
- Fan, Y.X.; Liang, Y.X.; Deng, K.P.; Zhang, Z.; Wang, G.M.; Zhang, Y.L.; Wang, F. Analysis of dna methylation profiles during sheep skeletal muscle development using whole-genome bisulfite sequencing. BMC Genomics 2020, 21, 497–507. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Y.Q.; Wang, H.B.; Xi, B.; He, X.N.; Yang, X.L.; Li, W.H. Analysis of volatile compounds and flavor fingerprint in Jingyuan lamb of different ages using gas chromatography–ion mobility spectrometry (GC–IMS). Meat Sci. 2021, 175, 108449. [Google Scholar] [CrossRef]
- Aguayo-Ulloa, L.A.; Lama, G.C.M.L.; Pascual-Alonso, M.; Fuchs, K.; Olleta, J.L.; Campo, M.M.; Alierta, S.; Villarroel, M.; María, G.A. Effect of feeding regime during finishing on lamb welfare, production performance and meat quality. Small Rumin. Res. 2013, 111, 147–156. [Google Scholar] [CrossRef]
- Dutta, T.K.; Das, A.K.; Tripathi, P.; Dular, R.K. Effect of concentrate supplementation on growth, nutrient availability, carcass traits and meat quality in barbari kids reared under semi-intensive and intensive systems. Anim. Nutr. Feed Technol. 2020, 2, 20. [Google Scholar] [CrossRef]
- Zheng, M.L.; Mao, P.H.; Tian, X.X.; Meng, L. Effects of grazing mixed-grass pastures on growth performance, immune responses, and intestinal microbiota in free-range Beijing-you chickens. Poult. Sci. 2021, 100, 1049–1058. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, W.; Tian, F.; Li, J.; Wang, Y.; Qi, J.; Xue, S. Corn straw total mix dietary supplementation of bacillus subtilis-enhanced growth performance of lambs by favorably modulating rumen bacterial microbiome. Fermentation 2023, 9, 32. [Google Scholar] [CrossRef]
- Carrasco, S.; Ripoll, G.; Sanz, A.; Álvarez-Rodríguez, J.; Panea, B.; Revilla, R.; Joy, M. Effect of feeding system on growth and carcass characteristics of Churra Tensina light lambs. Livest. Sci. 2009, 121, 56–63. [Google Scholar] [CrossRef]
- Gabryszuk, M.; Kuźnicka, E.; Horbańczuk, K.; Oprządek, J. Effects of housing systems and the diet supplements on the slaughter value and concentration of mineral elements in the loin muscle of lambs. Asian Austral. J. Anim. 2014, 27, 726–732. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Chen, Y.; Luo, H.L.; Liu, X.L.; Liu, K. Influence of restricted grazing time systems on productive performance and fatty acid composition of longissimus dorsi in growing lambs. Asian Austral. J. Anim. 2015, 28, 1105–1115. [Google Scholar] [CrossRef]
- Da Silva, P.C.G.; Ítavo, C.C.B.F.; Ítavo, L.C.V.; Gomes, M.N.B.; Feijó, G.L.D.; Ferelli, K.L.S.M.; Heimbach, N.S.; da Silva, J.A.; de Melo, G.K.A.; Pereira, M.W.F. Carcass traits and meat quality of Texel lambs raised in Brachiaria pasture and feedlot systems. Anim. Sci. J. 2020, 91, e13394. [Google Scholar] [CrossRef]
- Priolo, A.; Micol, D.; Agabriel, J. Effects of grass feeding systems on ruminant meat colour and flavour. A review. Anim. Res. 2001, 50, 185–200. [Google Scholar] [CrossRef]
- Khan, M.L.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef]
- Peng, J.T.; Liang, C.Z.; Niu, Y.M.; Jiang, W.; Wang, W.; Wang, L.X. Moderate grazing promotes genetic diversity of Stipa species in the Inner Mongolian steppe. Landsc. Ecol. 2015, 30, 1783–1794. [Google Scholar] [CrossRef]
- Qi, K.K.; Men, X.M.; Wu, J.; Deng, B.; Xu, Z.W. Effects of feeding methods on carcass traits, blood parameters and meat quality of Lvjiahei black pigs. J. Anim. Sci. 2023, 59, 296–301. [Google Scholar]
- Valentini, J.; Silva, A.S.D.; Fortuoso, B.F.; Reis, J.H.; Gebert, R.R.; Griss, L.G.; Boiago, M.M.; Lopes, L.Q.S.; Santos, R.C.V.; Wagner, R.; et al. Chemical composition, lipid peroxidation, and fatty acid profile in meat of broilers fed with glycerol monolaurate additive. Food Chem. 2020, 330, 127187. [Google Scholar] [CrossRef]
- Rehemujiang, H.; Jie, G.L.; Rehemujiang, K.; Tuoxunjiang, H.; Lin, Z.; Yimamu, A. Winter grazing on cotton stubble affects grazing behavior, feed intake, production, and health of small ruminants. Small Rumin. Res. 2022, 209, 106635. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Luo, H.L.; Hou, X.Y.; Badgery, W.B.; Zhang, Y.J.; Jiang, C. Effect of restricted time at pasture and indoor supplementation on ingestive behaviour, dry matter intake and weight gain of growing lambs. Livest. Sci. 2014, 167, 137–143. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, H.L.; Liu, X.L.; Wang, Z.Z.; Liu, Y.H. Effect of restricted-time grazing on desert grassland vegetation. Heilongjiang Anim. Sci. Vet. Med. 2014, 13, 37–40. [Google Scholar]
- Wang, B.; Wang, Z.; Chen, Y.; Liu, X.; Liu, K.; Zhang, Y.; Luo, H. Carcass traits, meat quality, and volatile compounds of lamb meat from different restricted grazing time and indoor supplementary feeding systems. Foods 2021, 10, 2822. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, X.A.; Li, Z.; Luo, H.L.; Zhang, H.; Guo, Y.P.; Zhang, C.; Ma, Q. Changes of metabolites and gene expression under different feeding systems associated with lipid metabolism in lamb meat. Foods 2021, 10, 2612. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Z.X.; Na, R.H.; Wen, Q.; Li, J.Q. Effects of different feeding patterns and varieties on rumen fermentation characteristics of cashmere goat. J. Domest. Anim. Ecol. 2018, 39, 36–40. [Google Scholar]
- Liu, Y.; Liu, J.H.; Hao, L.Z.; Sun, P.; Degen, A. Effect of substituting steam-flaked corn for course ground corn on in vitro digestibility, average daily gain, serum metabolites and ruminal volatile fatty acids, and bacteria diversity in growing yaks. Anim. Feed Sci. Tech. 2023, 296, 115553. [Google Scholar] [CrossRef]
- Durso, L.M.; Harhay, G.P.; Smith, P.L.T.; Bono, L.J.; Desantis, Z.T.; Harhay, D.M.; Andersen, J.M.; Keen, J.E.; Laegreid, W.W.; Clawson, M.L. Animal-to-Animal Variation in Fecal Microbial Diversity among Beef Cattle. Appl. Environ. Microb. 2010, 76, 4858. [Google Scholar] [CrossRef]
- Yin, X.; Ji, S.; Duan, C.; Ju, S.; Zhang, Y.; Yan, H.; Liu, Y. Rumen fluid transplantation affects growth performance of weaned lambs by altering gastrointestinal microbiota, immune function and feed digestibility—ScienceDirect. Animal 2020, 15, 100076. [Google Scholar] [CrossRef]
- Yin, X.J.; Ji, S.K.; Duan, C.H.; Tian, P.Z.; Ju, S.S.; Yan, H.; Zhang, Y.J.; Liu, Y.Q. The succession of fecal bacterial community and its correlation with the changes of serum immune indicators in lambs from birth to 4 months. J. Integr. Agric. 2023, 22, 537–550. [Google Scholar] [CrossRef]
- Sciences, C.A.O.A.; Agriculture, M.O. Feeding Standard of Meat-Producing Sheep and Goats, 3rd ed.; China Agri: Beijing, China, 2004; pp. 1–33. [Google Scholar]
- Huang, Y.F.; Matthew, C.; Li, F.; Nan, Z.B. Common vetch varietal differences in hay nutritive value, ruminal fermentation, nutrient digestibility and performance of fattening lambs. Animal 2021, 15, 100244. [Google Scholar] [CrossRef]
- Huang, Y.F.; Matthew, C.; Li, F.; Nan, Z.B. Comparative effects of stovers of four varieties of common vetch on growth performance, ruminal fermentation, and nutrient digestibility of growing lambs. Animals 2020, 10, 596. [Google Scholar] [CrossRef]
- Hristov, A.N.; Ivan, M.; Rode, L.M.; Mcallister, T.A. Fermentation characteristics and ruminal ciliate protozoal populations in cattle fed medium- or high-concentrate barley-based diets. J. Anim. Sci. 2021, 79, 515–524. [Google Scholar] [CrossRef]
- Zhang, H.X.; Zhang, Y.Y.; Ren, H.; Xie, W.; Chen, Q.; Hou, S.Z.; Jia, J.L. Effects of feeding patterns on fecal microorganism structure and function of Tibetan sheep. Southwest China J. Agr. Sci. 2022, 35, 692–699. [Google Scholar]
- Sun, Q.; Li, A.; Li, M.X.; Hou, B.L. Effect of pH on biodiesel production and the microbial structure of glucose-fed activated sludge. Int. Biodeterior. Biodegrad. 2015, 104, 224–230. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; He, Y.; Xia, C.Q.; Rahman, M.A.U.; Qiu, Q.H.; Shao, T.Q.; Liang, Y.X.; Ji, L.B.; Wang, H.B.; Cao, B.H. Effects of replacing Leymus chinensis with whole-crop wheat hay on Holstein bull apparent digestibility, plasma parameters, rumen fermentation, and microbiota. Sci. Rep. 2017, 7, 2114. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Bai, C.; Ao, C.; Qi, S.; Cao, Q.; Erdene, K. Effects of the dietary inclusion of allium mongolicum regel extract on serum index and meat quality in Small-Tailed Han sheep. Animals 2023, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Carlos, M.M.L.; Leite, J.H.G.M.; Chaves, D.F.; Vale, A.M.; Facanha, D.A.E.; Melo, M.M.; Soto-Blanco, B.; Carlos, M.M.L.; Leite, J.H.G.M.; Chaves, D.F.; et al. Blood parameters in the Morada Nova sheep: Influence of age, sex and body condition score. J. Anim. Plant Sci. 2015, 25, 950–955. [Google Scholar]
- Chen, F.; Wei, J.T.; Yang, X.H.; Zhao, N.; Zhang, W.; Huang, S.W.; Yan, N.D.; Guo, W.Z. Effect of pelleted total mixed rations with different levels of intact rapeseed on performance, carcass traits, serum biochemical indices and meat quality of Boer goats. Anim. Prod. Sci. 2018, 59, 82–88. [Google Scholar] [CrossRef]
- Simsek, A.; Yaman, T.; Icen, H.; Kochan, A. Diaphragmatic hernia in a sheep-a case report. Vet. Arhiv. 2018, 88, 271–277. [Google Scholar] [CrossRef]
- Majdoub-Mathlouthi, L.; Saïd, B.; Kraiem, K. Carcass traits and meat fatty acid composition of Barbarine lambs reared on rangelands or indoors on hay and concentrate. Animal 2015, 9, 2065–2071. [Google Scholar] [CrossRef]
- Li, Y.X.; Liu, C.; Gao, Y.F.; Diao, Z.C.; Qu, Y.H.; Xu, C.C.; Luo, H.L. Effect of different fattening strategg on slaughter performance and meat quality of Hulun buir lambs. Chin. J. Anim. Sci. 2018, 54, 97–102. [Google Scholar]
- Vicari, T.; Borne, J.J.G.C.V.D.; Gerrits, W.J.J.; Zbinden, Y.; Blum, J.W. Postprandial blood hormone and metabolite concentrations influenced by feeding frequency and feeding level in veal calves. Domest. Anim. Endocrin. 2008, 34, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Dev, K.; Mir, N.A.; Biswas, A.; Kannoujia, J.; Begum, J.; Kant, R.; Mandal, A. Dietary synbiotic supplementation improves the growth performance, body antioxidant pool, serum biochemistry, meat quality, and lipid oxidative stability in broiler chickens-science direct. Anim. Nutr. 2020, 6, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Gong, X.X.; Li, G.D.; Lin, M.; Huo, Y.J.; Li, S.L.; Zhao, G.Q. Effects of dietary alfalfa flavonoids extraction on growth performance, organ development and blood biochemical indexes of Yangzhou geese aged from 28 to 70 days. Anim. Nutr. 2016, 2, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Ke, T.T.; Zhao, M.Y.; Zhang, X.A.; Cheng, Y.; Sun, Y.M.; Wang, P.H.; Ren, C.H.; Cheng, X.; Zhang, Z.J.; Huang, Y.F. Review of feeding systems affecting production, carcass attributes, and meat quality of ovine and caprine species. Life Sci. 2023, 13, 1215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Z.; Zhang, C.Y.; Li, M.; Lee, Y.; Zhang, G.G. Extract methods, molecular characteristics, and bioactivities of polysaccharide from alfalfa (Medicago sativa L.). Nutrients 2019, 11, 1181. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Gao, X.X.; Yang, T.Y.; Jiang, M.C.; Wang, L.; Zhan, K.; Lin, M.; Zhao, G.Q.; Ginkgo Biloba, L. Residues partially replacing alfalfa hay pellet in pelleted total mixed ration on growth performance, serum biochemical parameters, rumen fermentation, immune function and meat quality in finishing haimen white goats. Animals 2021, 11, 3046. [Google Scholar] [CrossRef]
- Shen, F.; Wang, S.X.; Chai, S.T.; Yang, Y.K.; Wang, X. Effect of different feeding methods on growth performance and serum biochemica. Feed Res. 2021, 44, 16–20. [Google Scholar]
- Su, Y.Y.; Sun, X.; Zhao, S.M.; Hu, M.L.; Li, D.F.; Qi, S.L.; Jiao, X.L.; Sun, Y.; Wang, C.Z.; Zhu, X.Y.; et al. Dietary alfalfa powder supplementation improves growth and development, body health, and meat quality of Tibetan sheep. Food Chem. 2022, 396, 133709. [Google Scholar] [CrossRef]
- Xu, M.; Chen, X.L.; Huang, Z.Q.; Chen, D.W.; Li, M.Z.; He, J.; Chen, H.; Zheng, P.; Yu, J.; Luo, Y.H.; et al. Effects of dietary grape seed proanthocyanidin extract supplementation on meat quality, muscle fiber characteristics and antioxidant capacity of finishing pigs. Food Chem. 2022, 367, 130781. [Google Scholar] [CrossRef]
- Bolisetty, S.; Jaimes, E.A. Mitochondria and reactive oxygen species: Physiology and pathophysiology. Int. J. Mol. Sci. 2013, 14, 6306–6344. [Google Scholar] [CrossRef]
- Yang, R.C.; Guo, Y.X.; Zhang, S.; Hao, Q.H.; Duan, C.H.; Wang, Y.; Ji, S.K.; Yan, H.; Zhang, Y.J.; Liu, Y.Q. Effect of dioscorea opposite waste supplementation on antioxidant capacity, immune response and rumen microbiome in weaned lambs. Fermentation 2023, 9, 25. [Google Scholar] [CrossRef]
- Hu, B.; Wan, W.L.; Gong, Y.Z.; Feng, Y.P.; Tan, W.J.; Liu, Y. Effects of Feeding Models on Slaughter Performance, Serum Biochemical lndexes and Intestinal Morphology of Different Strains of Jingyang Chicken. China Poult. 2018, 40, 29–33. [Google Scholar]
- Nagata, R.Y.H.K.; Ohkubo, A.; Kushibiki, S.; Ichijo, T.; Sato, S. Effects of repeated subacute ruminal acidosis challenges on the adaptation of the rumen bacterial community in Holstein bulls. J. Dairy Sci. 2018, 101, 4424–4436. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Diao, Q.Y.; Zhao, Y.G.; Jiang, C.G.; Li, Y.L.; Tu, Y. Effects of different NFC/NDF diets on rumen pH, ammonia nitrogen and volatile fatty acids in meat sheep. J. Anim. Physiol. Anim. Nutr. 2012, 24, 1069–1077. [Google Scholar]
- Lima, T.J.; Costa, R.G.; Medeiros, G.R.D.; Medeiros, A.N.D.; Ribeiro, N.L.; Oliveira, J.S.D.; Guerra, R.R.; Carvalho, F.F.R.D. Ruminal and morphometric parameters of the rumen and intestines of sheep fed with increasing levels of spineless cactus (Nopalea cochenillifera Salm-Dyck). Trop. Anim. Health Prod. 2019, 51, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.T.; Ma, S.; Chen, X.; Wang, Z.; Zhao, S.; Ren, Y. Effects of different proportions of stevia stalk on nutrient utilization and rumen fermentation in ruminal fluid derived from sheep. PeerJ 2023, 25, e14689. [Google Scholar] [CrossRef] [PubMed]
- Shen, F. Effects of Different Feeding Methods on Growth Performance, Serum Biochemical Indices and Rumen Microflora of Yak Calves. Master’s Thesis, Qinghai University, Xining, China, 2022. [Google Scholar]
- Cui, X.X.; Wang, Z.F.; Fan, Q.S.; Chang, S.H.; Yan, T.H.; Hou, F.J. Ligularia virgaurea improved nutrient digestion, ruminal fermentation, and bacterial composition in Tibetan sheep grazing on the Qinghai–Tibetan plateau in winter. Anim. Feed Sci. Technol. 2023, 299, 115628. [Google Scholar] [CrossRef]
- Penner, G.B.; Steele, M.A.; Aschenbach, J.R.; McBride, B.W. Ruminant nutrition symposium: Molecular adaptation of ruminal epithelia to highly fermentable diets. J. Anim. Sci. 2011, 89, 1108–1119. [Google Scholar] [CrossRef]
- Hartinger, T.; Gresner, N.; Südekum, K.H.; Hartinger, T.; Gresner, N.; Südekum, K.H. Does intra-ruminal nitrogen recycling waste valuable resources? A review of major players and their manipulation. J. Anim. Sci. 2018, 9, 33. [Google Scholar] [CrossRef]
- Xue, D.; Chen, H.; Zhao, X.Q.; Xu, S.X.; Hu, L.Y.; Xu, T.W.; Jiang, L.; Zhan, W. Rumen prokaryotic communities of ruminants under different feeding paradigms on the Qinghai-Tibetan Plateau. Syst. Appl. Microbiol. 2017, 40, 227–236. [Google Scholar] [CrossRef]
- Shen, Z.; Seyfert, H.M.; Löhrke, B.; Schneider, F.; Zitnan, R.; Chudy, A.; Kuhla, S.; Hammon, H.M.; Blum, J.W.; Martens, H. An energy-rich diet causes rumen papillae proliferation associated with more IGF type 1 receptors and increased plasma IGF-1 concentrations in young goats. J. Nutr. 2004, 134, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Lopez, E.; Haselmann, A.; Petri, R.M.; Knaus, W.; Zebeli, Q. Evaluation of fecal fermentation profile and bacterial community in organically fed dairy cows consuming forage-rich diets with different particle sizes. J. Dairy Sci. 2020, 103, 8020–8033. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Bayer, A.E.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Reau, A.J.L.; Suen, G. The Ruminococci: Key symbionts of the gut ecosystem. J. Microbiol. 2018, 56, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Carreño, D.; Toral, P.G.; Pinloche, E.; Belenguer, A.; Yáñez-Ruiz, D.R.; Hervás, G.; McEwan, N.R.; Newbold, C.J.; Frutos, P. Rumen bacterial community responses to DPA, EPA and DHA in cattle and sheep: A comparative in vitro study. Sci. Rep. 2019, 9, 11857. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Villot, C.; Renaud, D.; Skidmore, A.; Chevaux, E.; Steele, M.; Guan, L.L. Linking perturbations to temporal changes in diversity, stability, and compositions of neonatal calf gut microbiota: Prediction of diarrhea. ISME J. 2020, 14, 2223–2235. [Google Scholar] [CrossRef]
| Ingredients | Content (% Dry Matter) | Chemical Composition | Content (% Dry Matter) |
|---|---|---|---|
| Whole corn silage | 45.00 | Dry matter | 46.8 |
| Corn | 30.20 | Crude protein | 16.35 |
| Soybean meal | 14.00 | Neutral detergent fiber | 30.28 |
| Wheat bran | 7.80 | Acid detergent fiber | 18.29 |
| NaHCO3 | 1.00 | Crude ash | 8.28 |
| Salt | 1.00 | Calcium | 0.71 |
| Premix (1) | 1.00 | Phosphorus | 0.40 |
| Total | 100 | ||
| Title 1 | Treatment | Mean | SEM | p-Value | ||
|---|---|---|---|---|---|---|
| CONT 2 | G4H | G8H | ||||
| Initial BW 1, kg 0 d | 29.71 | 30.79 | 30.36 | 30.29 | 1.22 | 0.945 |
| Final BW, kg | ||||||
| 30 d | 36.43 | 39.86 | 37.43 | 37.91 | 1.25 | 0.555 |
| 60 d | 39.43 | 44.59 | 41.57 | 41.86 | 1.51 | 0.416 |
| 90 d | 42.36 | 49.14 | 45.34 | 45.61 | 1.57 | 0.235 |
| ADG, g/day | ||||||
| 0–30 d | 223.81 | 302.38 | 235.71 | 253.97 | 14.89 | 0.069 |
| 30–60 d | 100.00 | 157.62 | 138.10 | 131.91 | 15.29 | 0.328 |
| 60–90 d | 97.62 | 151.90 | 125.71 | 125.08 | 21.44 | 0.625 |
| 0–90 d | 140.48 | 203.97 | 166.51 | 170.32 | 11.51 | 0.080 |
| Title 1 | Treatment | Mean | SEM | p-Value | Normal Reference Range 3 | ||
|---|---|---|---|---|---|---|---|
| CONT 2 | G4H | G8H | |||||
| TP (g/L) | 60.74 | 61.96 | 60.96 | 61.22 | 1.57 | 0.951 | 59.0–78.0 |
| ALB (g/L) | 23.86 | 22.79 | 22.41 | 23.02 | 0.57 | 0.592 | 27.0–37.0 |
| GLB (g/L) | 36.88 | 39.18 | 38.55 | 38.20 | 1.13 | 0.721 | 35.0–57.0 |
| ALB/GLB | 0.65 | 0.59 | 0.59 | 0.61 | 0.01 | 0.087 | _ |
| UN (mmol/L) | 6.36 | 5.56 | 5.37 | 5.76 | 0.19 | 0.096 | 4.6–15.7 |
| ALT (IU/L) | 19.15 | 16.79 | 14.05 | 16.66 | 1.36 | 0.337 | 19.6–44.1 |
| AST (IU/L) | 113.21 | 101.64 | 99.48 | 104.78 | 4.05 | 0.362 | 49.0–123 |
| TC (mmol/L) | 1.68 | 1.84 | 1.90 | 1.81 | 0.09 | 0.623 | 1.4–5.2 |
| TG (mmol/L) | 0.30 | 0.24 | 0.26 | 0.27 | 0.02 | 0.640 | _ |
| HDL-C (mmol/L) | 0.75 | 0.82 | 0.84 | 0.80 | 0.02 | 0.187 | _ |
| LDL-C (mmol/L) | 0.76 a | 0.62 b | 0.63 b | 0.67 | 0.03 | 0.042 | _ |
| Item 1 | Treatment | Mean | SEM | p-Value | ||
|---|---|---|---|---|---|---|
| CONT 2 | G4H | G8H | ||||
| Antioxidant indice | ||||||
| GSH-Px (pg/mL) | 1071.43 b | 1384.35 a | 1544.85 a | 1333.54 | 51.82 | <0.001 |
| Immunoglobulin indices | ||||||
| IgA (ug/mL) | 118.34 b | 157.63 a | 181.71 a | 152.56 | 7.44 | <0.001 |
| IgG (mg/mL) | 22.98 b | 29.84 a | 35.42 a | 29.41 | 1.73 | 0.007 |
| IgM (ug/mL) | 1176.23 b | 1565.52 a | 1681.90 a | 1474.55 | 59.12 | <0.001 |
| Item | Treatment | Mean | SEM | p-Value | ||
|---|---|---|---|---|---|---|
| CONT 1 | 4H | 8H | ||||
| pH | 6.39 | 6.28 | 6.65 | 6.44 | 0.094 | 0.326 |
| NH3-N (mg/dL) | 13.08 | 13.12 | 15.75 | 13.98 | 0.787 | 0.322 |
| Total VFA (mmol L−1) | 44.39 | 53.34 | 48.66 | 48.80 | 1.676 | 0.100 |
| Acetate (mmol L−1) | 27.75 | 33.05 | 31.80 | 30.87 | 0.948 | 0.057 |
| Propionate (mmol L−1) | 7.65 | 9.75 | 8.32 | 8.57 | 0.524 | 0.280 |
| Butyrate (mmol L−1) | 6.95 | 8.57 | 6.36 | 7.29 | 0.404 | 0.071 |
| Valerate (mmol L−1) | 0.88 | 0.84 | 0.99 | 0.90 | 0.108 | 0.862 |
| Isobutyrate (mmol L−1) | 0.88 | 0.93 | 0.86 | 0.89 | 0.045 | 0.825 |
| Isovalerate (mmol L−1) | 0.28 | 0.20 | 0.33 | 0.27 | 0.068 | 0.766 |
| Acetate: propionate | 3.72 | 3.47 | 4.23 | 3.81 | 0.215 | 0.378 |
| Item | Treatment | Mean | SEM | p-Value | ||
|---|---|---|---|---|---|---|
| CONT 1 | G4H | G8H | ||||
| Chao 1 | 1354.79 | 1538.33 | 1551.71 | 1481.61 | 39.81 | 0.144 |
| PD_whole_tree | 78.74 | 86.72 | 87.36 | 84.27 | 2.35 | 0.459 |
| Goods_coverage | 1.00 | 0.99 | 1.00 | 1.00 | 0.0002 | 0.245 |
| Observed_features | 1224.17 | 1360.83 | 1399.17 | 1328.06 | 34.53 | 0.135 |
| Shannon index | 7.90 | 8.28 | 8.43 | 8.20 | 0.098 | 0.182 |
| Simpson index | 0.99 | 0.99 | 0.99 | 0.99 | 0.001 | 0.103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Zhang, X.; Chen, Y.; Ren, C.; Sun, Y.; Wang, P.; Cheng, X.; Zhang, Z.; Chen, J.; Huang, Y. Stall-Feeding of Sheep on Restricted Grazing: Effects on Performance and Serum Metabolites, Ruminal Fermentation, and Fecal Microbiota. Animals 2023, 13, 2644. https://doi.org/10.3390/ani13162644
Zhao M, Zhang X, Chen Y, Ren C, Sun Y, Wang P, Cheng X, Zhang Z, Chen J, Huang Y. Stall-Feeding of Sheep on Restricted Grazing: Effects on Performance and Serum Metabolites, Ruminal Fermentation, and Fecal Microbiota. Animals. 2023; 13(16):2644. https://doi.org/10.3390/ani13162644
Chicago/Turabian StyleZhao, Mengyu, Xiaoan Zhang, Yao Chen, Chunhuan Ren, Yiming Sun, Penghui Wang, Xiao Cheng, Zijun Zhang, Jiahong Chen, and Yafeng Huang. 2023. "Stall-Feeding of Sheep on Restricted Grazing: Effects on Performance and Serum Metabolites, Ruminal Fermentation, and Fecal Microbiota" Animals 13, no. 16: 2644. https://doi.org/10.3390/ani13162644
APA StyleZhao, M., Zhang, X., Chen, Y., Ren, C., Sun, Y., Wang, P., Cheng, X., Zhang, Z., Chen, J., & Huang, Y. (2023). Stall-Feeding of Sheep on Restricted Grazing: Effects on Performance and Serum Metabolites, Ruminal Fermentation, and Fecal Microbiota. Animals, 13(16), 2644. https://doi.org/10.3390/ani13162644





