Breeding Substrate Containing Distillation Residues of Mediterranean Medicinal Aromatic Plants Modulates the Effects of Tenebrio molitor as Fishmeal Substitute on Blood Signal Transduction and WBC Activation of Gilthead Seabream (Sparus aurata)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing of Tenebrio Molitor and Production of Insect Meals
2.2. Fish Diets
2.3. Dietary Experiments, Rearing Facilities, and Experimental Conditions
2.4. Sampling Procedures
2.5. Manual Hematological Analysis
2.6. Determination of HSPs, MAPKs, and Apoptosis
2.7. Statistical Analysis and Formulae
2.8. Experiment—Ethics Aspects
3. Results
3.1. Growth Performance and Relevant Growth Indices
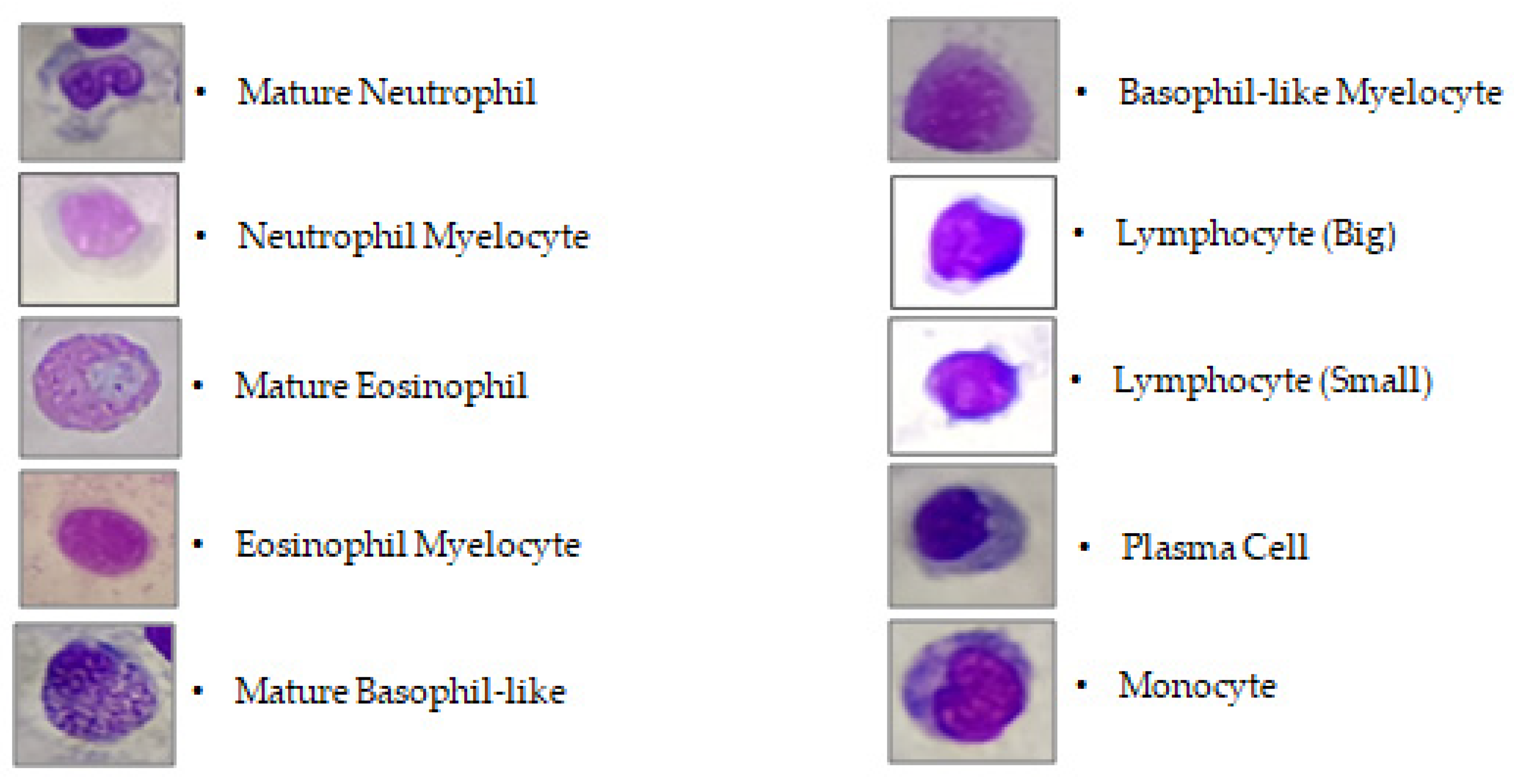
3.2. Differential Leukocyte Count (DLC)
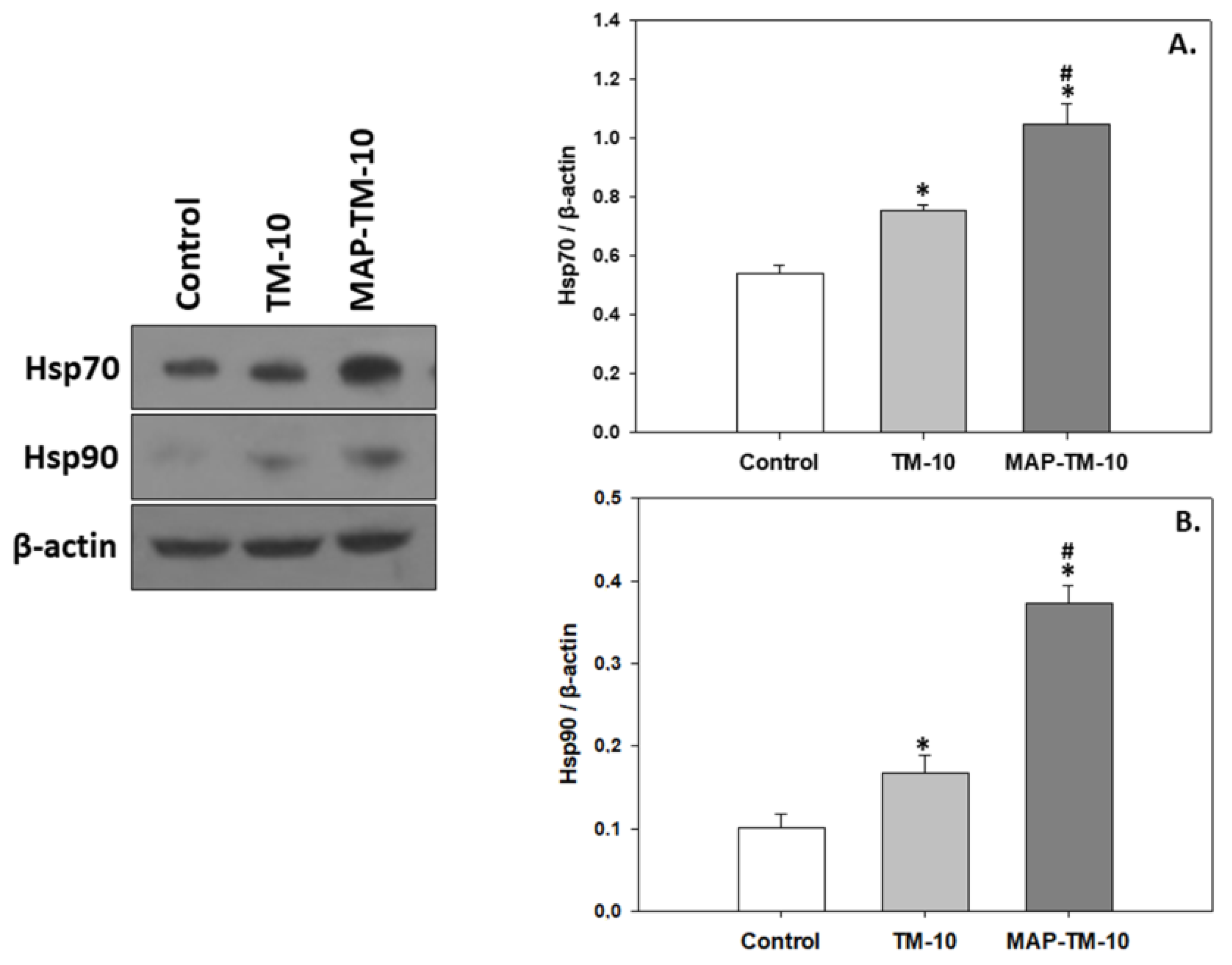
3.3. Heat Shock Induction
3.4. MAPK Signaling
3.5. Apoptosis
4. Discussion
4.1. Gilthead Sea Bream Growth Performance
4.2. White Blood Cell Modultation
4.3. Apoptosis, Heat Shock Proteins, and MAPK Signaling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fabrikov, D.; Vargas-García, M.D.C.; Barroso, F.G.; Sánchez-Muros, M.J.; Cacua Ortíz, S.M.; Morales, A.E.; Cardenete, G.; Tomás-Almenar, C.; Melenchón, F. Effect on Intermediary Metabolism and Digestive Parameters of the High Substitution of Fishmeal with Insect Meal in Sparus aurata Feed. Insects 2021, 12, 965. [Google Scholar] [CrossRef] [PubMed]
- Shafique, L.; Abdel-Latif, H.M.R.; Hassan, F.; Alagawany, M.; Naiel, M.A.E.; Dawood, M.A.O.; Yilmaz, S.; Liu, Q. The Feasibility of Using Yellow Mealworms (Tenebrio molitor): Towards a Sustainable Aquafeed Industry. Animals 2021, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Gasco, L.; Henry, M.; Piccolo, G.; Marono, S.; Gai, F.; Renna, M.; Lussiana, C.; Antonopoulou, E.; Mola, P.; Chatzifotis, S. Tenebrio molitor Meal in Diets for European Sea Bass (Dicentrarchus labrax L.) Juveniles: Growth Performance, Whole Body Composition and in Vivo Apparent Digestibility. Anim. Feed. Sci. Technol. 2016, 220, 34–45. [Google Scholar] [CrossRef]
- Ghosh, S.; Lee, S.-M.; Jung, C.; Meyer-Rochow, V.B. Nutritional Composition of Five Commercial Edible Insects in South Korea. J. Asia-Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Gkinali, A.-A.; Matsakidou, A.; Vasileiou, E.; Paraskevopoulou, A. Potentiality of Tenebrio molitor Larva-Based Ingredients for the Food Industry: A Review. Trends Food Sci. Technol. 2022, 119, 495–507. [Google Scholar] [CrossRef]
- Gasco, L.; Biasato, I.; Dabbou, S.; Schiavone, A.; Gai, F. Animals Fed Insect-Based Diets: State-of-the-Art on Digestibility, Performance and Product Quality. Animals 2019, 9, 170. [Google Scholar] [CrossRef]
- Tippayadara, N.; Dawood, M.A.O.; Krutmuang, P.; Hoseinifar, S.H.; Doan, H.V.; Paolucci, M. Replacement of Fish Meal by Black Soldier Fly (HermetiaiIllucens) Larvae Meal: Effects on Growth, Haematology, and Skin Mucus Immunity of Nile Tilapia, Oreochromis Niloticus. Animals 2021, 11, 193. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.; De Haro, C.; Sanz, A.; Trenzado, C.E.; Villareces, S.; Barroso, F.G. Nutritional Evaluation of Tenebrio molitor Meal as Fishmeal Substitute for Tilapia (Oreochromis niloticus) Diet. Aquacult. Nutr. 2016, 22, 943–955. [Google Scholar] [CrossRef]
- Tubin, J.S.B.; Paiano, D.; Hashimoto, G.S.D.O.; Furtado, W.E.; Martins, M.L.; Durigon, E.; Emerenciano, M.G.C. Tenebrio Molitor Meal in Diets for Nile Tilapia Juveniles Reared in Biofloc System. Aquaculture 2020, 519, 734763. [Google Scholar] [CrossRef]
- Fabrikov, D.; Sánchez-Muros, M.J.; Barroso, F.G.; Tomás-Almenar, C.; Melenchón, F.; Hidalgo, M.C.; Morales, A.E.; Rodriguez-Rodriguez, M.; Montes-Lopez, J. Comparative Study of Growth Performance and Amino Acid Catabolism in Oncorhynchus mykiss, Tinca tinca and Sparus aurata and the Catabolic Changes in Response to Insect Meal Inclusion in the Diet. Aquaculture 2020, 529, 735731. [Google Scholar] [CrossRef]
- Antonopoulou, E.; Nikouli, E.; Piccolo, G.; Gasco, L.; Gai, F.; Chatzifotis, S.; Mente, E.; Kormas, K.A. Reshaping Gut Bacterial Communities after Dietary Tenebrio molitor Larvae Meal Supplementation in Three Fish Species. Aquaculture 2019, 503, 628–635. [Google Scholar] [CrossRef]
- Iaconisi, V.; Secci, G.; Sabatino, G.; Piccolo, G.; Gasco, L.; Papini, A.M.; Parisi, G. Effect of Mealworm (Tenebrio molitor L.) Larvae Meal on Amino Acid Composition of Gilthead Sea Bream (Sparus aurata L.) and Rainbow Trout (Oncorhynchus mykiss W.) Fillets. Aquaculture 2019, 513, 734403. [Google Scholar] [CrossRef]
- Mente, E.; Bousdras, T.; Feidantsis, K.; Panteli, N.; Mastoraki, M.; Kormas, K.A.; Chatzifotis, S.; Piccolo, G.; Gasco, L.; Gai, F.; et al. Tenebrio molitor Larvae Meal Inclusion Affects Hepatic Proteome and Apoptosis and/or Autophagy of Three Farmed Fish Species. Sci. Rep. 2022, 12, 121. [Google Scholar] [CrossRef]
- Terova, G.; Gini, E.; Gasco, L.; Moroni, F.; Antonini, M.; Rimoldi, S. Effects of Full Replacement of Dietary Fishmeal with Insect Meal from Tenebrio molitor on Rainbow Trout Gut and Skin Microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Rema, P.; Saravanan, S.; Armenjon, B.; Motte, C.; Dias, J. Graded Incorporation of Defatted Yellow Mealworm (Tenebrio molitor) in Rainbow Trout (Oncorhynchus mykiss) Diet Improves Growth Performance and Nutrient Retention. Animals 2019, 9, 187. [Google Scholar] [CrossRef]
- Jeong, S.-M.; Khosravi, S.; Mauliasari, I.R.; Lee, S.-M. Dietary Inclusion of Mealworm (Tenebrio molitor) Meal as an Alternative Protein Source in Practical Diets for Rainbow Trout (Oncorhynchus mykiss) Fry. Fish Aquatic. Sci. 2020, 23, 12. [Google Scholar] [CrossRef]
- Melenchón, F.; Larrán, A.M.; De Mercado, E.; Hidalgo, M.C.; Cardenete, G.; Barroso, F.G.; Fabrikov, D.; Lourenço, H.M.; Pessoa, M.F.; Tomás-Almenar, C. Potential Use of Black Soldier Fly (Hermetia illucens) and Mealworm (Tenebrio molitor) Insectmeals in Diets for Rainbow Trout (Oncorhynchus mykiss). Aquacult. Nutr. 2021, 27, 491–505. [Google Scholar] [CrossRef]
- Chemello, G.; Renna, M.; Caimi, C.; Guerreiro, I.; Oliva-Teles, A.; Enes, P.; Biasato, I.; Schiavone, A.; Gai, F.; Gasco, L. Partially Defatted Tenebrio molitor Larva Meal in Diets for Grow-Out Rainbow Trout, Oncorhynchus mykiss (Walbaum): Effects on Growth Performance, Diet Digestibility and Metabolic Responses. Animals 2020, 10, 229. [Google Scholar] [CrossRef]
- Belforti, M.; Gai, F.; Lussiana, C.; Renna, M.; Malfatto, V.; Rotolo, L.; De Marco, M.; Dabbou, S.; Schiavone, A.; Zoccarato, I.; et al. Tenebrio molitor Meal in Rainbow Trout (Oncorhynchus mykiss) Diets: Effects on Animal Performance, Nutrient Digestibility and Chemical Composition of Fillets. Ital. J. Anim. Sci. 2015, 14, 4170. [Google Scholar] [CrossRef]
- Su, J.; Gong, Y.; Cao, S.; Lu, F.; Han, D.; Liu, H.; Jin, J.; Yang, Y.; Zhu, X.; Xie, S. Effects of Dietary Tenebrio Molitor Meal on the Growth Performance, Immune Response and Disease Resistance of Yellow Catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2017, 69, 59–66. [Google Scholar] [CrossRef]
- Roncarati, A.; Gasco, L.; Parisi, G.; Terova, G. Growth Performance of Common Catfish (Ameiurus Melas Raf.) Fingerlings Fed Mealworm (Tenebrio molitor) Diet. J. Insects Food Feed. 2015, 1, 233–240. [Google Scholar] [CrossRef]
- Tran, H.Q.; Kiljunen, M.; Van Doan, H.; Stejskal, V. European Perch (Perca fluviatilis) Fed Dietary Insect Meal (Tenebrio Molitor): From a Stable Isotope Perspective. Aquaculture 2021, 545, 737265. [Google Scholar] [CrossRef]
- Tran, H.Q.; Van Doan, H.; Stejskal, V. Does Dietary Tenebrio molitor Affect Swimming Capacity, Energy Use, and Physiological Responses of European Perch Perca fluviatilis? Aquaculture 2021, 539, 736610. [Google Scholar] [CrossRef]
- Tran, H.Q.; Prokešová, M.; Zare, M.; Matoušek, J.; Ferrocino, I.; Gasco, L.; Stejskal, V. Production Performance, Nutrient Digestibility, Serum Biochemistry, Fillet Composition, Intestinal Microbiota and Environmental Impacts of European Perch (Perca fluviatilis) Fed Defatted Mealworm (Tenebrio molitor). Aquaculture 2022, 547, 737499. [Google Scholar] [CrossRef]
- Ng, W.-K.; Liew, F.-L.; Ang, L.-P.; Wong, K.-W. Potential of Mealworm (Tenebrio Molitor) as an Alternative Protein Source in Practical Diets for African Catfish, Clarias Gariepinus: Mealworm as a Protein Source for Catfish. Aquac. Res. 2001, 32, 273–280. [Google Scholar] [CrossRef]
- Bousdras, T.; Feidantsis, K.; Panteli, N.; Chatzifotis, S.; Piccolo, G.; Gasco, L.; Gai, F.; Antonopoulou, E. Dietary Tenebrio molitor Larvae Meal Inclusion Exerts Tissue-Specific Effects on Cellular, Metabolic, and Antioxidant Status in European Sea Bass (Dicentrarchus labrax) and Gilthead Seabream (Sparus aurata). Aquac. Nutr. 2022, 2022, 9858983. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.A.; Golomazou, E.; Asimaki, A.; Psofakis, P.; Fountoulaki, E.; Mente, E.; Rumbos, C.I.; Athanassiou, C.G.; Karapanagiotidis, I.T. Partial Dietary Fishmeal Replacement with Full-Fat or Defatted Superworm (Zophobas morio) Larvae Meals Modulates the Innate Immune System of Gilthead Seabream, Sparus aurata. Aquac. Rep. 2022, 27, 101347. [Google Scholar] [CrossRef]
- Piccolo, G.; Iaconisi, V.; Marono, S.; Gasco, L.; Loponte, R.; Nizza, S.; Bovera, F.; Parisi, G. Effect of Tenebrio molitor Larvae Meal on Growth Performance, in Vivo Nutrients Digestibility, Somatic and Marketable Indexes of Gilthead Sea Bream (Sparus aurata). Anim. Feed. Sci. Technol. 2017, 226, 12–20. [Google Scholar] [CrossRef]
- Mastoraki, M.; Katsika, L.; Enes, P.; Guerreiro, I.; Kotzamanis, Y.P.; Gasco, L.; Chatzifotis, S.; Antonopoulou, E. Insect Meals in Feeds for Juvenile Gilthead Seabream (Sparus aurata): Effects on Growth, Blood Chemistry, Hepatic Metabolic Enzymes, Body Composition and Nutrient Utilization. Aquaculture 2022, 561, 738674. [Google Scholar] [CrossRef]
- Iaconisi, V.; Marono, S.; Parisi, G.; Gasco, L.; Genovese, L.; Maricchiolo, G.; Bovera, F.; Piccolo, G. Dietary Inclusion of Tenebrio molitor Larvae Meal: Effects on Growth Performance and Final Quality Treats of Blackspot Sea Bream (Pagellus bogaraveo). Aquaculture 2017, 476, 49–58. [Google Scholar] [CrossRef]
- Jeong, S.-M.; Khosravi, S.; Yoon, K.-Y.; Kim, K.-W.; Lee, B.-J.; Hur, S.-W.; Lee, S.-M. Mealworm, Tenebrio molitor, as a Feed Ingredient for Juvenile Olive Flounder, Paralichthys olivaceus. Aquac. Rep. 2021, 20, 100747. [Google Scholar] [CrossRef]
- Ido, A.; Hashizume, A.; Ohta, T.; Takahashi, T.; Miura, C.; Miura, T. Replacement of Fish Meal by Defatted Yellow Mealworm (Tenebrio molitor) Larvae in Diet Improves Growth Performance and Disease Resistance in Red Seabream (Pargus major). Animals 2019, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Motte, C.; Rios, A.; Lefebvre, T.; Do, H.; Henry, M.; Jintasataporn, O. Replacing Fish Meal with Defatted Insect Meal (Yellow Mealworm Tenebrio molitor) Improves the Growth and Immunity of Pacific White Shrimp (Litopenaeus vannamei). Animals 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-G.; Chi, S.-Y.; Tan, B.-P.; Liang, G.-L.; Lu, B.-Q.; Dong, X.-H.; Yang, Q.-H.; Liu, H.-Y.; Zhang, S. Effects of Fishmeal Replacement by Tenebrio molitor Meal on Growth Performance, Antioxidant Enzyme Activities and Disease Resistance of the Juvenile Pearl Gentian Grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus ♀). Aquac. Res. 2018, 49, 2210–2217. [Google Scholar] [CrossRef]
- Khosravi, S.; Kim, E.; Lee, Y.-S.; Lee, S.-M. Dietary Inclusion of Mealworm (Tenebrio Molitor) Meal as an Alternative Protein Source in Practical Diets for Juvenile Rockfish (Sebastes schlegeli). Entomol. Res. 2018, 48, 214–221. [Google Scholar] [CrossRef]
- Akinyemi, O.; Oyewole, S.O.; Jimoh, K.A. Medicinal Plants and Sustainable Human Health: A Review. HIJ 2018, 2, 194–195. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, A. Review on Essential Oil Extraction from Aromatic and Medicinal Plants: Techniques, Performance and Economic Analysis. Sustainable Chem. Pharm. 2022, 30, 100829. [Google Scholar] [CrossRef]
- Aqeel, U.; Aftab, T.; Khan, M.M.A.; Naeem, M. Regulation of Essential Oil in Aromatic Plants under Changing Environment. J. Appl. Res. Med. Aromat. Plants 2022, 32, 100441. [Google Scholar] [CrossRef]
- Taghouti, I.; Cristobal, R.; Brenko, A.; Stara, K.; Markos, N.; Chapelet, B.; Hamrouni, L.; Buršić, D.; Bonet, J.-A. The Market Evolution of Medicinal and Aromatic Plants: A Global Supply Chain Analysis and an Application of the Delphi Method in the Mediterranean Area. Forests 2022, 13, 808. [Google Scholar] [CrossRef]
- Zuazo, V.H.D.; García-Tejero, I.F.; Gálvez Ruiz, B.; Cermeño Sacristán, P.; Cuadros Tavira, S. Response of Essential-Oil Yield of Aromatic and Medicinal Plants to Different Harvesting Strategies. Com. Sci. 2019, 10, 429–437. [Google Scholar] [CrossRef]
- Andreadis, S.S.; Panteli, N.; Mastoraki, M.; Rizou, E.; Stefanou, V.; Tzentilasvili, S.; Sarrou, E.; Chatzifotis, S.; Krigas, N.; Antonopoulou, E. Towards Functional Insect Feeds: Agri-Food By-Products Enriched with Post-Distillation Residues of Medicinal Aromatic Plants in Tenebrio molitor (Coleoptera: Tenebrionidae) Breeding. Antioxidants 2021, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- de Elguea-Culebras, G.O.; Bravo, E.M.; Sánchez-Vioque, R. Potential Sources and Methodologies for the Recovery of Phenolic Compounds from Distillation Residues of Mediterranean Aromatic Plants. An Approach to the Valuation of by-Products of the Essential Oil Market–A Review. Ind. Crops Prod. 2022, 175, 114261. [Google Scholar] [CrossRef]
- Mandalakis, M.; Anastasiou, T.I.; Martou, N.; Keisaris, S.; Greveniotis, V.; Katharios, P.; Lazari, D.; Krigas, N.; Antonopoulou, E. Antibacterial Effects of Essential Oils of Seven Medicinal-Aromatic Plants Against the Fish Pathogen Aeromonas Veronii Bv. Sobria: To Blend or Not to Blend? Molecules 2021, 26, 2731. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, T.I.; Mandalakis, M.; Krigas, N.; Vézignol, T.; Lazari, D.; Katharios, P.; Dailianis, T.; Antonopoulou, E. Comparative Evaluation of Essential Oils from Medicinal-Aromatic Plants of Greece: Chemical Composition, Antioxidant Capacity and Antimicrobial Activity against Bacterial Fish Pathogens. Molecules 2019, 25, 148. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant Activity of Medicinal and Aromatic Plants. A Review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Oliva-Teles, A. Nutrition and Health of Aquaculture Fish. J. Fish Dis. 2012, 35, 83–108. [Google Scholar] [CrossRef]
- Feidantsis, K.; Soumalevris, A.; Panteli, N.; Chatzifotis, S.; Antonopoulou, E. Synergistic Effect of Long-Term Feed Deprivation and Temperature on the Cellular Physiology of Meagre (Argyrosomus regius). J. Therm. Biol. 2022, 105, 103207. [Google Scholar] [CrossRef]
- Craig, E.A. Chaperones: Helpers along the Pathways to Protein Folding. Science 1993, 260, 1902–1903. [Google Scholar] [CrossRef]
- Jäättelä, M. Heat Shock Proteins as Cellular Lifeguards. Ann. Med. 1999, 31, 261–271. [Google Scholar] [CrossRef]
- Iwama, G.K.; Vijayan, M.M.; Forsyth, R.B.; Ackerman, P.A. Heat Shock Proteins and Physiological Stress in Fish. Am. Zool. 1999, 39, 901–909. [Google Scholar] [CrossRef]
- Antonopoulou, E.; Chouri, E.; Feidantsis, K.; Lazou, A.; Chatzifotis, S. Effects of Partial Dietary Supplementation of Fish Meal with Soymeal on the Stress and Apoptosis Response in the Digestive System of Common Dentex (Dentex dentex). J. Biol. Res. Thessalon. 2017, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Penninger, J.M. Mitogen-Activated Protein Kinases in Apoptosis Regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar] [CrossRef] [PubMed]
- Panteli, N.; Demertzioglou, M.; Feidantsis, K.; Karapanagiotis, S.; Tsele, N.; Tsakoniti, K.; Gkagkavouzis, K.; Mylonas, C.C.; Kormas, K.A.; Mente, E.; et al. Advances in Understanding the Mitogenic, Metabolic, and Cell Death Signaling in Teleost Development: The Case of Greater Amberjack (Seriola dumerili, Risso 1810). Fish Physiol. Biochem. 2022, 48, 1665–1684. [Google Scholar] [CrossRef]
- Watson, A.J.; Pritchard, D.M. Lessons from Genetically Engineered Animal Models. VII. Apoptosis in Intestinal Epithelium: Lessons from Transgenic and Knockout Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G1–G5. [Google Scholar] [CrossRef] [PubMed]
- Rumbos, C.I.; Adamaki-Sotiraki, C.; Gourgouta, M.; Karapanagiotidis, I.T.; Asimaki, A.; Mente, E.; Athanassiou, C.G. Strain Matters: Strain Effect on the Larval Growth and Performance of the Yellow Mealworm, Tenebrio molitor L. J. Insects Food Feed. 2021, 7, 1195–1205. [Google Scholar] [CrossRef]
- AOAC Official Method. Official Methods of Analysis of AOAC INTERNATIONAL, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Vasilopoulos, S.; Giannenas, I.; Savvidou, S.; Bonos, E.; Rumbos, C.I.; Papadopoulos, E.; Fortomaris, P.; Athanassiou, C.G. Growth Performance, Welfare Traits and Meat Characteristics of Broilers Fed Diets Partly Replaced with Whole Tenebrio molitor Larvae. Anim. Nutr. 2023, 13, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Witeska, M.; Kondera, E.; Ługowska, K.; Bojarski, B. Hematological Methods in Fish—Not Only for Beginners. Aquaculture 2022, 547, 737498. [Google Scholar] [CrossRef]
- López-Ruiz, A.; Esteban, M.A.; Meseguer, J. Blood Cells of the Gilthead Seabream (Sparus aurata L.): Light and Electron Microscopic Studies: Blood Cells of Gilthead Seabream. Gen. Histol. Cytol. 1992, 234, 161–171. [Google Scholar] [CrossRef]
- Clauss, T.M.; Dove, A.D.M.; Arnold, J.E. Hematologic Disorders of Fish. Vet. Clin. N. Am. Exot. Anim. Pract. 2008, 11, 445–462. [Google Scholar] [CrossRef]
- Ainsworth, A.J. Fish Granulocytes: Morphology, Distribution, and Function. Annu. Rev. Fish Dis. 1992, 2, 123–148. [Google Scholar] [CrossRef]
- Khandekar, G.; Kim, S.; Jagadeeswaran, P. Zebrafish Thrombocytes: Functions and Origins. Adv. Hematol. 2012, 2012, 857058. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.A. Stress in Fishes: A Diversity of Responses with Particular Reference to Changes in Circulating Corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.J.; Lawrence, M.J.; Raby, G.D.; Teffer, A.K.; Jeffries, K.M.; Danylchuk, A.J.; Clark, T.D. Comment: Practices for Drawing Blood Samples from Teleost Fish. N. Am. J. Aquac. 2019, 81, 424–426. [Google Scholar]
- Wendelaar Bonga, S.E. The Stress Response in Fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and Disorders of the Stress System. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.A.; Iwama, G.K. Physiological Changes in Fish from Stress in Aquaculture with Emphasis on the Response and Effects of Corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Anastasiou, C.; Buchbauer, G. Essential Oils as Immunomodulators: Some Examples. Open Chem. 2017, 15, 352–370. [Google Scholar] [CrossRef]
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory Activities of Selected Essential Oils. Biomolecules 2020, 10, 1139. [Google Scholar] [CrossRef]
- Zuo, X.; Gu, Y.; Wang, C.; Zhang, J.; Zhang, J.; Wang, G.; Wang, F. A Systematic Review of the Anti-Inflammatory and Immunomodulatory Properties of 16 Essential Oils of Herbs. Evid. Based Complement. Altern. Med. 2020, 2020, 8878927. [Google Scholar] [CrossRef]
- Lang, M.; Ferron, P.-J.; Bursztyka, J.; Montjarret, A.; Duteil, E.; Bazire, A.; Bedoux, G. Evaluation of Immunomodulatory Activities of Essential Oils by High Content Analysis. J. Biotechnol. 2019, 303, 65–71. [Google Scholar] [CrossRef]
- Shourbela, R.M.; El-Hawarry, W.N.; Elfadadny, M.R.; Dawood, M.A.O. Oregano Essential Oil Enhanced the Growth Performance, Immunity, and Antioxidative Status of Nile Tilapia (Oreochromis niloticus) Reared under Intensive Systems. Aquaculture 2021, 542, 736868. [Google Scholar] [CrossRef]
- Mssillou, I.; Bakour, M.; Slighoua, M.; Laaroussi, H.; Saghrouchni, H.; Amrati, F.E.Z.; Derwich, E. Investigation on wound healing effect of Mediterranean medicinal plants and some related phenolic compounds: A review. J. Ethnopharmacol. 2022, 298, 115663. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.B.; da Fraga, R.E.; Nishiyama, P.B.; Silva, I.S.S.; da Costa, N.L.B.; de Oliveira, L.A.A.; Rocha, M.A.; Juncá, F.A. Leukocyte Profiles in Odontophrynus carvalhoi (Amphibia: Odontophrynidae) Tadpoles Exposed to Organophosphate Chlorpyrifos Pesticides. Water Air Soil Pollut. 2020, 231, 372. [Google Scholar] [CrossRef]
- Weyts, F.A.A.; Verburg-van Kemenade, B.M.L.; Flik, G. Characterisation of Glucocorticoid Receptors in Peripheral Blood Leukocytes of Carp, Cyprinus carpio L. Gen. Comp. Endocrinol. 1998, 111, 1–8. [Google Scholar] [CrossRef]
- Ortuno, J.; Esteban, M.A.; Meseguer, J. Effects of Short-Term Crowding Stress on the Gilthead Seabream (Sparus aurata L.) Innate Immune Response. Fish Shellfish. Immunol. 2001, 11, 187–197. [Google Scholar] [CrossRef]
- Pickering, A.D. Cortisol-Induced Lymphocytopenia in Brown Trout, Salmo trutta L. Gen. Comp. Endocrinol. 1984, 53, 252–259. [Google Scholar] [CrossRef]
- Hua, K. A Meta-Analysis of the Effects of Replacing Fish Meals with Insect Meals on Growth Performance of Fish. Aquaculture 2021, 530, 735732. [Google Scholar] [CrossRef]
- Reyes, M.; Rodríguez, M.; Montes, J.; Barroso, F.G.; Fabrikov, D.; Morote, E.; Sánchez-Muros, M.J. Nutritional and Growth Effect of Insect Meal Inclusion on Seabass (Dicentrarchuss labrax) Feeds. Fishes 2020, 5, 16. [Google Scholar] [CrossRef]
- Su, J.; Liu, Y.; Xi, L.; Lu, Q.; Liu, H.; Jin, J.; Yang, Y.; Zhu, X.; Han, D.; Xie, S. The Effect of Dietary Tenebrio Molitor Meal Inclusion on Growth Performance and Liver Health of Largemouth Bass (Micropterus salmoides). J. Insects Food Feed. 2022, 8, 1297–1309. [Google Scholar] [CrossRef]
- Ma, H.; Lin, H.; Feng, H.; Putheti, R. Effects of Angelica Polysaccharide on Hepatocytes Apoptosis Induced by Exhaustive Exercise. Afr. J. Microbiol. Res. 2009, 3, 774–777. [Google Scholar]
- Zhou, B.; Wu, L.-J.; Li, L.-H.; Tashiro, S.; Onodera, S.; Uchiumi, F.; Ikejima, T. Silibinin Protects against Isoproterenol-Induced Rat Cardiac Myocyte Injury through Mitochondrial Pathway after up-Regulation of SIRT1. J. Pharmacol. Sci. 2006, 102, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Dokou, S.; Mellidou, I.; Savvidou, S.; Stylianaki, I.; Panteli, N.; Antonopoulou, E.; Wang, J.; Grigoriadou, K.; Tzora, A.; Jin, L. A Phytobiotic Extract, in an Aqueous or in a Cyclodextrin Encapsulated Form, Added in Diet Affects Meat Oxidation, Cellular Responses and Intestinal Morphometry and Microbiota of Broilers. Front. Anim. Sci. 2023, 4, 1050170. [Google Scholar] [CrossRef]
- Cardinaletti, G.; Randazzo, B.; Messina, M.; Zarantoniello, M.; Giorgini, E.; Zimbelli, A.; Bruni, L.; Parisi, G.; Olivotto, I.; Tulli, F. Effects of Graded Dietary Inclusion Level of Full-Fat Hermetia illucens Prepupae Meal in Practical Diets for Rainbow Trout (Oncorhynchus mykiss). Animals 2019, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted Black Soldier Fly (Hermetia Illucens) Larvae Meal in Diets for Juvenile Jian Carp (Cyprinus carpio Var. Jian): Growth Performance, Antioxidant Enzyme Activities, Digestive Enzyme Activities, Intestine and Hepatopancreas Histological Structure. Aquaculture 2017, 477, 62–70. [Google Scholar] [CrossRef]
- Roques, S.; Deborde, C.; Guimas, L.; Marchand, Y.; Richard, N.; Jacob, D.; Skiba-Cassy, S.; Moing, A.; Fauconneau, B. Integrative Metabolomics for Assessing the Effect of Insect (Hermetia illucens) Protein Extract on Rainbow Trout Metabolism. Metabolites 2020, 10, 83. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Saravana Bhavan, P.; Seenivasan, C.; Muralisankar, T.; Shanthi, R. Effects of Native Medicinal Herbs (Alternanthera sessilis, Eclipta alba and Cissus quadrangularis) on Growth Performance, Digestive Enzymes and Biochemical Constituents of the Monsoon River Prawn M Acrobrachium Malcolmsonii. Aquac. Nutr. 2015, 21, 496–506. [Google Scholar] [CrossRef]
- Xu, Z.; Li, X.Q.; Yang, H.; Poolsawat, L.; Wang, P.; Leng, X.J. Dietary Rutin Promoted the Growth, Serum Antioxidant Response and Flesh Collagen, Free Amino Acids Contents of Grass Carp (Ctenopharyngodon idella). Aquac. Nutr. 2021, 27, 544–555. [Google Scholar] [CrossRef]
- Lee, D.-H.; Ra, C.-S.; Song, Y.-H.; Sung, K.-I.; Kim, J.-D. Effects of Dietary Garlic Extract on Growth, Feed Utilization and Whole Body Composition of Juvenile Sterlet Sturgeon (Acipenser ruthenus). Asian-Australas. J. Anim. Sci. 2012, 25, 577. [Google Scholar] [CrossRef][Green Version]
- Feidantsis, K.; Poertner, H.O.; Markou, T.; Lazou, A.; Michaelidis, B. Involvement of P38 MAPK in the Induction of H Sp70 During Acute Thermal Stress in Red Blood Cells of the Gilthead Sea Bream, Sparus aurata. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2012, 317, 303–310. [Google Scholar] [CrossRef]
- Stenberg, O.K.; Holen, E.; Piemontese, L.; Liland, N.S.; Lock, E.-J.; Espe, M.; Belghit, I. Effect of Dietary Replacement of Fish Meal with Insect Meal on in Vitro Bacterial and Viral Induced Gene Response in Atlantic Salmon (Salmo salar) Head Kidney Leukocytes. Fish Shellfish. Immunol. 2019, 91, 223–232. [Google Scholar] [CrossRef]
- Seo, M.; Goo, T.-W.; Chung, M.Y.; Baek, M.; Hwang, J.-S.; Kim, M.-A.; Yun, E.-Y. Tenebrio molitor Larvae Inhibit Adipogenesis through AMPK and MAPKs Signaling in 3T3-L1 Adipocytes and Obesity in High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2017, 18, 518. [Google Scholar] [CrossRef]
- Antonopoulou, E.; Chatzigiannidou, I.; Feidantsis, K.; Kounna, C.; Chatzifotis, S. Effect of Water Temperature on Cellular Stress Responses in Meagre (Argyrosomus regius). Fish Physiol. Biochem. 2020, 46, 1075–1091. [Google Scholar] [CrossRef]
- Feidantsis, K.; Antonopoulou, E.; Lazou, A.; Pörtner, H.O.; Michaelidis, B. Seasonal Variations of Cellular Stress Response of the Gilthead Sea Bream (Sparus aurata). J. Comp. Physiol. B 2013, 183, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Somayeh, S.M.; Jiun, Z.; Loh, Y.; Mousavi, S.; Loh, J.-Y.; Zahedinezhad, S. A review on insect meals in aquaculture: The immunomodulatory and physiological effects. Int. Aquat. Res. 2020, 12, 100–115. [Google Scholar]
- Reverter, M.; Tapissier-Bontemps, N.; Sarter, S.; Sasal, P.; Caruso, D. Moving towards More Sustainable Aquaculture Practices: A Meta-Analysis on the Potential of Plant-Enriched Diets to Improve Fish Growth, Immunity and Disease Resistance. Rev. Aquac. 2021, 13, 537–555. [Google Scholar] [CrossRef]




| Ingredients (g/kg) | Control | TM-10 | MAP-TM-10 |
|---|---|---|---|
| Fish feed | 900 | 900 | 900 |
| Wheat (extruded) | 60 | 0 | 0 |
| Fish meal (71% CP) | 40 | 0 | 0 |
| “Conventional” insect meal (35% CP) | 0 | 100 | 0 |
| “Enriched” insect meal (35% CP) | 0 | 0 | 100 |
| Calculated analysis (as fed) | |||
| Digestible energy (Mj/kg) | 15.79 | 15.80 | 15.80 |
| Total phenolic content (TPC) of fish feed (mg GAE/L extract phenol) | 12.42 | 44.13 | 56.08 |
| Crude protein (g/kg) | 439.9 | 440.0 | 440.0 |
| Nitrogen-free extract (g/kg) | 163.8 | 126.0 | 126.0 |
| Parameters | Control (A) | TM-10 (B) | MAP-TM-10 (C) | Significance |
|---|---|---|---|---|
| Initial weight (g) | 208.84 ± 2.88 | 208.19 ± 7.04 | 205.88 ± 8.81 | Ns 1 |
| Final weight (g) | 334.92 ± 7.77 | 340.02 ± 3.72 | 343.90 ± 5.68 | Ns |
| Weight gain (g) | 129.08 ± 7.50 | 131.83 ± 4.09 | 138.02 ± 3.16 | Ns |
| 2 FR (%) | 1.5 | 1.5 | 1.5 | Ns |
| 3 SGR (%/day) | 0.58 ± 0.03 | 0.61 ± 0.03 | 0.63 ± 0.03 | Ns |
| 4 FCR (%) | 1.19 ± 0.01 | 1.14 ± 0.03 | 1.08 ± 0.02 | Ns |
| 5 PER | 2.04 ± 0.11 | 1.99 ± 0.06 | 2.10 ± 0.05 | Ns |
| Survival rate (%) | 98.08 | 99.23 | 98.46 | Ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonopoulou, E.; Kolygas, M.; Panteli, N.; Gouva, E.; Kontogeorgiou, P.; Feidantsis, K.; Chatzopoulos, A.; Bitchava, K.; Zacharis, C.; Bonos, E.; et al. Breeding Substrate Containing Distillation Residues of Mediterranean Medicinal Aromatic Plants Modulates the Effects of Tenebrio molitor as Fishmeal Substitute on Blood Signal Transduction and WBC Activation of Gilthead Seabream (Sparus aurata). Animals 2023, 13, 2537. https://doi.org/10.3390/ani13152537
Antonopoulou E, Kolygas M, Panteli N, Gouva E, Kontogeorgiou P, Feidantsis K, Chatzopoulos A, Bitchava K, Zacharis C, Bonos E, et al. Breeding Substrate Containing Distillation Residues of Mediterranean Medicinal Aromatic Plants Modulates the Effects of Tenebrio molitor as Fishmeal Substitute on Blood Signal Transduction and WBC Activation of Gilthead Seabream (Sparus aurata). Animals. 2023; 13(15):2537. https://doi.org/10.3390/ani13152537
Chicago/Turabian StyleAntonopoulou, Efthimia, Markos Kolygas, Nikolas Panteli, Evangelia Gouva, Panagiota Kontogeorgiou, Konstantinos Feidantsis, Achilleas Chatzopoulos, Konstantina Bitchava, Christos Zacharis, Eleftherios Bonos, and et al. 2023. "Breeding Substrate Containing Distillation Residues of Mediterranean Medicinal Aromatic Plants Modulates the Effects of Tenebrio molitor as Fishmeal Substitute on Blood Signal Transduction and WBC Activation of Gilthead Seabream (Sparus aurata)" Animals 13, no. 15: 2537. https://doi.org/10.3390/ani13152537
APA StyleAntonopoulou, E., Kolygas, M., Panteli, N., Gouva, E., Kontogeorgiou, P., Feidantsis, K., Chatzopoulos, A., Bitchava, K., Zacharis, C., Bonos, E., Giannenas, I., Skoufos, I., Andreadis, S. S., Skoulakis, G., Athanassiou, C. G., & Nathanailides, C. (2023). Breeding Substrate Containing Distillation Residues of Mediterranean Medicinal Aromatic Plants Modulates the Effects of Tenebrio molitor as Fishmeal Substitute on Blood Signal Transduction and WBC Activation of Gilthead Seabream (Sparus aurata). Animals, 13(15), 2537. https://doi.org/10.3390/ani13152537













