Return of Sound Production as a Biomarker of Bottlenose Dolphin Emergence from Anesthesia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Anesthetic Procedures
2.2. Emergence from Anesthesia
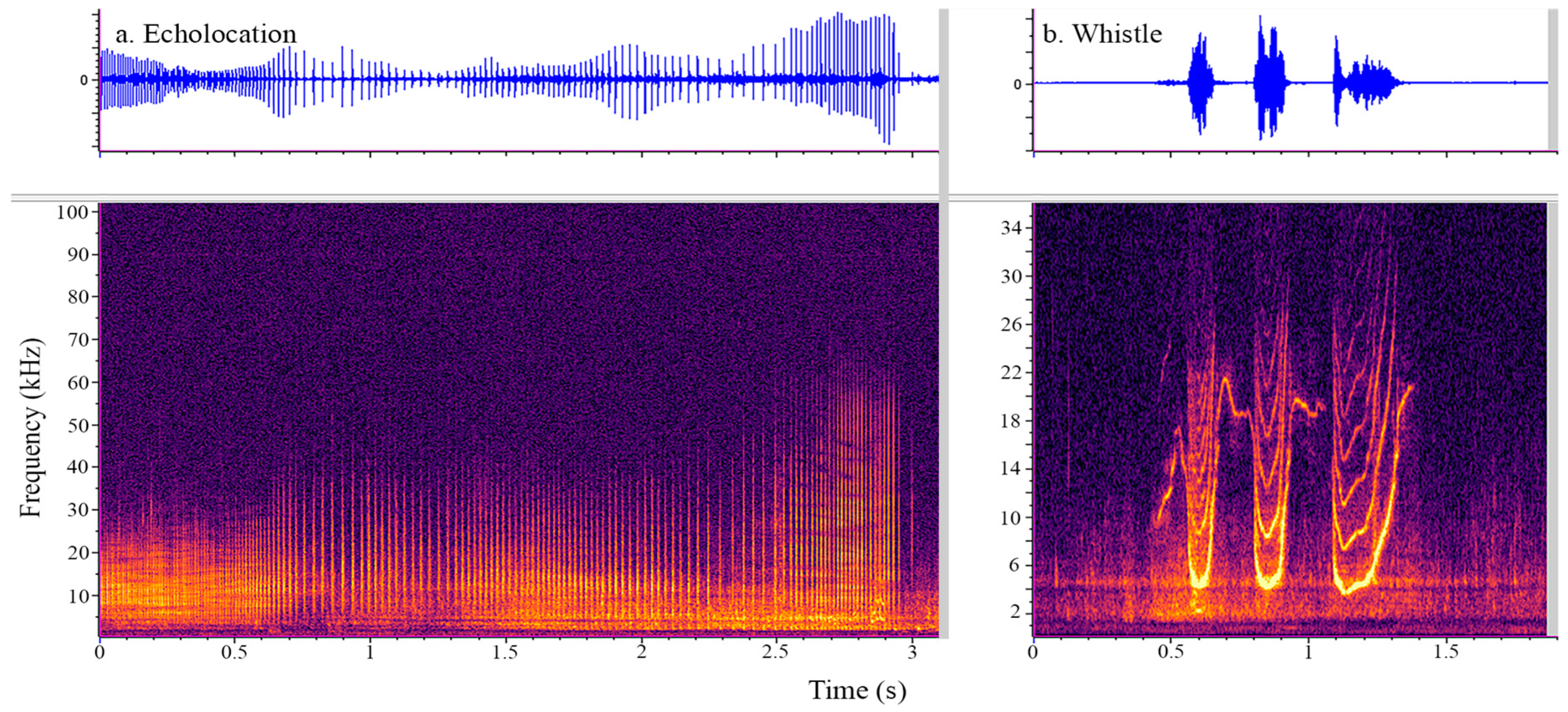
2.3. Acoustic Data Acquisition and Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hight, D.F.; Dadok, V.M.; Szeri, A.J.; Garcãa, P.S.; Voss, L.; Sleigh, J.W. Emergence from general anesthesia and the sleep-manifold. Front. Syst. Neurosci. 2014, 8, 146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cascella, M.; Bimonte, S.; Di Napoli, R. Delayed emergence from Anesthesia: What we know and how we act. Local Reg. Anesthesia 2020, 13, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Friedman, E.B.; Sun, Y.; Moore, J.T.; Hung, H.-T.; Meng, Q.C.; Perera, P.; Joiner, W.J.; Thomas, S.A.; Eckenhoff, R.G.; Sehgal, A.; et al. A Conserved behavioral state barrier impedes transitions between Anesthetic-induced unconsciousness and wakefulness: Evidence for neural inertia. PLoS ONE 2010, 5, e11903. [Google Scholar] [CrossRef] [PubMed]
- Kelz, M.B.; García, P.S.; Mashour, G.A.; Solt, K. Escape from oblivion: Neural mechanisms of emergence from general Anesthesia. Obstet. Anesthesia Dig. 2019, 128, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Jensen, E.D.; Meegan, J.; Ivančić, M.; Bailey, J.; Hendrickson, D.; Weiss, J.; Grindley, J.; Costidis, A.M.; Wisbach, G. Surgical management of a chronic neck abscess in a U.S. navy bottlenose dolphin. Mil. Med. 2019, 184, e360–e364. [Google Scholar] [CrossRef]
- McCormick, J.G. Relationship of sleep, respiration, and anesthesia in the porpoise: A preliminary report. Proc. Natl. Acad. Sci. USA 1969, 62, 697–703. [Google Scholar] [CrossRef]
- McCormick, J.G.; Ridgway, S.H. History of the development of Anesthesia for the dolphin: A quest to study a brain as large as man’s. Anesthesiology 2018, 129, 11–21. [Google Scholar] [CrossRef]
- Medway, W.; McCormick, J.G.; Ridgway, S.; Crump, J.F. Effects of prolonged halothane anesthesia on some cetaceans. J. Am. Vet. Med. Assoc. 1970, 157, 576–582. [Google Scholar]
- Ridgway, S.H.; McCormick, J.G. Anesthetization of porpoises for major surgery. Science 1967, 158, 510–512. [Google Scholar] [CrossRef]
- Ridgway, S.H.; McCormick, J.G. Anesthesia of the porpoise. In Textbook of Veterinary Anesthesia; Soma, L.R., Ed.; Williams & Wilkins: Baltimore, MA, USA, 1971; pp. 394–403. [Google Scholar]
- Ridgway, S.H.; McCormick, J.G.; Wever, E.G. Surgical approach to the dolphin’s ear. J. Exp. Zool. 1974, 188, 265–276. [Google Scholar] [CrossRef]
- Russell, J.; Jeffery, N.; Bailey, J.; Osborn, S.; Le-Bert, C.; Whitehead, H.; Nollens, H. Cerebrospinal fluid sampling in a bottlenose dolphin (Tursiops truncatus) under general Anesthesia. J. Zoo Wildl. Med. 2021, 51, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Dold, C.; Ridgway, S. Cetaceans. In Zoo Animal and Wildlife Immobilization and Anesthesia, 2nd ed.; West, G., Heard, D., Caulkett, N., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 679–691. [Google Scholar] [CrossRef]
- Haulena, M.; Schmitt, T. Anesthesia. In Handbook of Marine Mammal Medicine, 3rd ed.; Gulland, F.M.D., Dierauf, L.A., Whitman, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 567–606. [Google Scholar]
- Ridgway, S.H.; Carder, D.A.; Green, R.F.; Gaunt, A.S.; Gaunt, S.L.L.; Evans, W.E. Electromyographic and pressure events in the nasolaryngeal system of dolphins during sound production. In Animal Sonar Systems; Busnel, R.G., Fish, J.F., Eds.; Springer: Boston, MA, USA, 1980; NATO Advanced Study Institutes Series; Volume 28, pp. 239–249. [Google Scholar] [CrossRef]
- Ridgway, S.H. Neural time and movement time in choice of whistle or pulse burst responses to different auditory stimuli by dolphins. J. Acoust. Soc. Am. 2011, 129, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Reidenberg, J.S.; Laitman, J.T. Existence of vocal folds in the larynx of odontoceti (toothed whales). Anat. Rec. 1988, 221, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Madsen, P.T.; Jensen, F.H.; Carder, D.; Ridgway, S. Dolphin whistles: A functional misnomer revealed by heliox breathing. Biol. Lett. 2011, 8, 211–213. [Google Scholar] [CrossRef]
- Jones, B.; Zapetis, M.; Samuelson, M.M.; Ridgway, S. Sounds produced by bottlenose dolphins (Tursiops): A review of the defining characteristics and acoustic criteria of the dolphin vocal repertoire. Bioacoustics 2019, 29, 399–440. [Google Scholar] [CrossRef]
- Luís, A.R.; Couchinho, M.N.; dos Santos, M.E. A Quantitative analysis of pulsed signals emitted by wild bottlenose dolphins. PLoS ONE 2016, 11, e0157781. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, C.; Amundin, M. High-frequency burst-pulse sounds in agonistic/aggressive interactions in bottlenose dolphins, Tursiops truncatus. In Echolocation in Bats and Dolphins, 1st ed.; Thomas, J.A., Moss, C.F., Vater, M., Eds.; University of Chicago Press: Chicago, IL, USA, 2004; pp. 425–431. [Google Scholar]
- Caldwell, M.C.; Caldwell, D.K. Individualized whistle contours in bottle-nosed dolphins (Tursiops truncatus). Nature 1965, 207, 434–435. [Google Scholar] [CrossRef]
- Lilly, J.C.; Miller, A.M. Sounds emitted by the bottlenose dolphin. Science 1961, 133, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, M.C.; Caldwell, D.K.; Tyack, P.L. Review of the signature-whistle hypothesis for the Atlantic bottlenose dolphin. In The Bottlenose Dolphin, 1st ed.; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 199–234. [Google Scholar] [CrossRef]
- Janik, V.M.; Sayigh, L.S. Communication in bottlenose dolphins: 50 years of signature whistle research. J. Comp. Physiol. A 2013, 199, 479–489. [Google Scholar] [CrossRef]
- Lilly, J.C. Distress call of the bottlenose dolphin: Stimuli and evoked behavioral responses. Science 1963, 139, 116–118. [Google Scholar] [CrossRef]
- Watwood, S.L.; Owen, E.C.; Tyack, P.L.; Wells, R.S. Signature whistle use by temporarily restrained and free-swimming bottlenose dolphins, Tursiops truncatus. Anim. Behav. 2005, 69, 1373–1386. [Google Scholar] [CrossRef]
- Esch, H.C.; Sayigh, L.S.; Wells, R.S. Quantifying parameters of bottlenose dolphin signature whistles. Mar. Mammal Sci. 2009, 25, 976–986. [Google Scholar] [CrossRef]
- Ridgway, S.H. Dolphin hearing and sound production in health and illness. In Hearing and Other Senses: Presentations in Honor of E.G. Weaver; Far, R.R., Gourevitch, G., Eds.; The Amphora Press: Groton, CT, USA, 1983; Volume XII, pp. 247–296. [Google Scholar]
- Kuczaj, S.A.; Frick, E.E.; Jones, B.L.; Lea, J.S.E.; Beecham, D.; Schnöller, F. Underwater observations of dolphin reactions to a distressed conspecific. Learn. Behav. 2015, 43, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Au, W.W.L. Echolocation in dolphins. In Hearing by Whales and Dolphins, 1st ed.; Au, W.W.L., Fay, R.R., Popper, A.N., Eds.; Springer Science & Business Media: New York, NY, USA, 2000; pp. 364–408. [Google Scholar] [CrossRef]
- Mayhew, D.; Mendonca, V.; Murthy, B.V.S. A review of ASA physical status—Historical perspectives and modern developments. Anaesthesia 2019, 74, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Portier, K.; Ida, K.K. The ASA physical status classification: What is the evidence for recommending its use in veterinary anesthesia?—A systematic review. Front. Vet. Sci. 2018, 5, 204. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, D.; Gordon, J.; McHugh, R.; McLaren, D.; Mellinger, D.K.; Redmond, P.; Thode, A.; Trinder, P.; Deng, X.Y. PAMGUARD: Semiautomated, open source software for real-time acoustic detection and localisation of cetaceans. In Proceedings of the Conference on Underwater Noise Measurement Impact and Mitigation, Red Hook, NY, USA, 14–15 October 2008; Volume 30, pp. 54–62. [Google Scholar]
- Jones, B.L.; Oswald, M.; Tufano, S.; Baird, M.; Mulsow, J.; Ridgway, S.H. A system for monitoring acoustics to supplement an animal welfare plan for bottlenose dolphins. J. Zool. Bot. Gard. 2021, 2, 222–233. [Google Scholar] [CrossRef]
- Moore, P.; Finneran, J.J. Auditory scene analysis in the echolocating dolphin. J. Acoust. Soc. Am. 2011, 129, 2469. [Google Scholar] [CrossRef]

| Dolphin | Sex | Age | Medical or Surgical Procedure | ASA Classification | Duration of Anesthesia (HH:MM) |
|---|---|---|---|---|---|
| 1 * | F | 37 | Dental extractions | II | 03:37 |
| 2 | M | 8 | Bronchoscopy | II | 03:41 |
| 3 | M | 29 | Dental extractions | II | 04:33 |
| 4 ^ | F | 45 | Corneal graft | II | 00:56 |
| 5 * | F | 38 | Dental extractions | II | 03:07 |
| 6 | M | 42 | Dental extractions | II | 03:34 |
| 7 | F | 16 | Dental extractions | II | 02:58 |
| 8 | F | 40 | Dental extractions | II | 03:16 |
| 9 | M | 41 | Corneal graft | II | 00:30 |
| 10 | M | 10 | Bronchoscopy | II | 04:03 |
| 11 | M | 37 | Dental extractions | II | 03:39 |
| 12 ^ | F | 46 | Ureteroscopy | IV | 03:08 |
| Time to Echolocation | Time to Return of Righting Reflex | Time to Whistle | |
|---|---|---|---|
| Dolphins | 11/12 | 11/12 | 9/12 |
| Minimum | 00:21:58 | 00:31:00 | 00:28:57 |
| Maximum | 01:32:09 | 02:16:00 | 22:08:08 |
| Mean | 00:43:41 | 01:13:44 | 04:57:47 |
| Standard Error | 00:06:28 | 00:09:02 | 02:22:31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, B.L.; McClain, A.M.; Sportelli, J.J.; Le-Bert, C.R. Return of Sound Production as a Biomarker of Bottlenose Dolphin Emergence from Anesthesia. Animals 2023, 13, 2531. https://doi.org/10.3390/ani13152531
Jones BL, McClain AM, Sportelli JJ, Le-Bert CR. Return of Sound Production as a Biomarker of Bottlenose Dolphin Emergence from Anesthesia. Animals. 2023; 13(15):2531. https://doi.org/10.3390/ani13152531
Chicago/Turabian StyleJones, Brittany L., Abby M. McClain, Jessica J. Sportelli, and Carolina Ruiz Le-Bert. 2023. "Return of Sound Production as a Biomarker of Bottlenose Dolphin Emergence from Anesthesia" Animals 13, no. 15: 2531. https://doi.org/10.3390/ani13152531
APA StyleJones, B. L., McClain, A. M., Sportelli, J. J., & Le-Bert, C. R. (2023). Return of Sound Production as a Biomarker of Bottlenose Dolphin Emergence from Anesthesia. Animals, 13(15), 2531. https://doi.org/10.3390/ani13152531






