1. Introduction
Maintaining a good gut functionality generally depends on diet composition and keeping a well-balanced gut microbiota [
1]. In this context, many commercial foods already include prebiotics, which are ingredients that aim to improve intestinal functionality by modulating the gut microbiota [
2,
3]
According to the International Scientific Association of Probiotics and Prebiotics (ISAPP), a prebiotic is defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” [
4]. In other words, prebiotics are selectively fermented by the enzymes of unicellular organisms, leading to changes in the population and/or activity of the gut microbiota and providing an enabling environment for the growth of bacteria considered beneficial [
1,
3,
5,
6]. Prebiotics can be extracted from natural sources, such as plants or yeast, or can be produced through acid or enzymatic hydrolysis of polysaccharides [
5]. One of the prebiotics widely used in the nutrition of dogs and cats is the mannan oligosaccharide (MOS), a short-chain carbohydrate composed of 3 to 10 mannose residues, capable of modulating the intestinal microbiota. MOS is usually obtained through enzymatic, alkaline, or acidic hydrolysis of the cell wall mannans derived from yeast (
Saccharomyces cerevisiae) cell walls or from plant galactomannans [
7].
β-glucans can also be obtained from
S. cerevisiae cell walls. These are a group of glucose polymers that escape digestion and alter the composition of the gut microbiota, promoting mainly immunomodulatory effects in dogs [
8]. β-glucans are classified as natural immunostimulants or biological response modifiers, since they interfere with various cell types and biological processes in dogs, such as effects on immune cells and the regulation of stress or cholesterol levels [
9].
In addition to prebiotics, other natural additives, such as essential oils (EOs), can contribute to gut functionality and to diet digestibility and palatability in dogs. EOs are natural bioactive compounds extracted from plant parts, such as leaves, flowers, seeds, and roots. They are volatile at room temperature, aromatic, and liquid, and, in addition to promoting gut benefits, some EOs have antimicrobial, anti-inflammatory, and antioxidant potential, especially observed in pigs and broilers [
10]. An example is the oregano EO, obtained by drying the leaves and flowers of
Origanum vulgare. This EO presents the phenolic isomers carvacrol and thymol as the main components, which constitute about 78–85% of the oil [
11]. These bioactive compounds are strongly related to the important antimicrobial (especially against Gram-positive bacteria) and antioxidant properties of oregano EO [
12].
In addition to intestinal functionality, digestibility and palatability of the diet can be influenced by the inclusion of feed additives [
1,
13,
14]. Even though yeast products are known as palatability enhancers in dogs [
15], research about the effects of EOs on diet palatability and digestibility are limited. As such, it is possible that the combination of yeast cell wall products and oregano EO has additional effects on intestinal functionality and on diet digestibility and palatability in dogs.
To the author’s knowledge, no studies on the combination of prebiotics and EOs in canine nutrition has been published. Therefore, the aim of this study was to evaluate the combined effects of yeast cell wall and oregano EO on nutrient digestibility, diet palatability, intestinal fermentation products, and fecal microbiota in dogs.
4. Discussion
The use of functional ingredients by the pet food industry follows the growing concern of pet owners for the health and welfare of their pets. Due to the diverse properties of yeast cell wall components and EO, these become important additives to be studied and used in canine nutrition. In the present study, it potential beneficial effects of adding the YCO blend to the diet on the intestinal functionality of dogs were observed, given the modulation of the intestinal microbiota and reduction in ammonia, phenols, and histamine concentrations in feces.
The difference observed in the ATTD of DM with the 3.0YCO diet compared to the control diet contradicts previous studies that used prebiotics or EOs in dogs’ and pigs’ nutrition [
1,
27,
28,
29]. Possibly, the reduction observed in the ATTD of DM does not have a major nutritional impact, since the ATTD of other nutrients or ME were not different and a trend was only found in the digestibility of GE.
Ammonia is one of the main metabolites originating from protein fermentation by the gut microbiota [
30]. When protein is not digested by host enzymes in the small intestine, gut microorganisms can hydrolyze it using extracellular proteases and peptidases, which generate free amino acids and peptides that can be absorbed by microorganisms. After the deamination process, which is the catabolic step responsible for removing the amine group from amino acids, ammonia is produced [
31,
32]. Therefore, the luminal ammonia concentration in the intestine can vary depending on the combined effects of microbial deamination and microbial protein synthesis [
3]. A reduction in the fecal ammonia concentrations in dogs fed 3.0YCO was found in comparison to the control group. This was possibly due to the effects of YCO in controlling the growth of some proteolytic bacteria in the gut, such as
Streptococcus.
The reduction in fecal ammonia concentrations corroborates with the result found related to fecal odor in dogs fed 3.0YCO. Ammonia, as well as biogenic amines, phenols, and BCFA, are some of the putrefactive compounds responsible for foul fecal odor [
33,
34]. These compounds are produced during colonic fermentation of endogenous and undigested amino acids and some of them are toxic to gut mucosal cells [
35,
36]. In this study, most of the evaluators judged the odor of fresh feces from dogs fed YCO as less fetid compared to the control group, in agreement with previous studies performed in dogs and cats [
37,
38]. Possibly, this improvement in the fecal odor occurred due to the decreased production of one or more volatile compounds from protein metabolism [
37,
38].
The higher fecal concentrations of some biogenic amines seem contradictory to the reduction in ammonia concentration and fecal odor in dogs fed 3.0YCO. However, it is known that some polyamines, such as putrescine and cadaverine, are extremely important for the regulation of intestinal cell physiology, such as membrane stability, correct cell proliferation, and differentiation [
32,
39,
40,
41,
42,
43,
44]. Indeed, some studies have shown that dogs and humans with inflammatory bowel disease have reduced putrescine and spermidine concentrations [
45,
46].
Faecalibacterium, one of the bacterial genera that presented higher abundance in dogs supplemented with YCO, is able to catalyze the transfer of the propylamine group from the amine donor S-adenosylmethioninamine to putrescine, producing spermidine and increasing putrescine concentrations [
46]. In addition, a relevant result was the nearly five-fold reduction in histamine concentration in the YCO-fed dogs. Histamine is an important signaling agent for toxic substances in the gut, and higher concentrations are related to intestinal inflammatory processes, such as inflammatory bowel disease, irritable bowel syndrome, and food allergy [
47,
48,
49,
50]. Thus, its reduction may be indicative of a protective effect of YCO in the gut, which may help regulate inflammatory processes.
The other fecal characteristics, such as pH, DMf, fecal score, and fecal output did not differ among treatments, contrary to Middelbos et al. [
51], who identified increased fecal pH and reduced fecal output in dogs fed 0.05 to 0.65% dietary yeast cell wall. On the other hand, the study of Swanson et al. [
34] demonstrated that there was no difference related to fecal characteristics in dogs fed 1 g of MOS, although a trend towards increased fecal pH was identified in animals receiving the prebiotic when compared to the control group. It is important to highlight that both studies mentioned utilized beet pulp as a fiber source, unlike the fiber composition of the present study. A possible explanation for the fact that fecal pH did not differ among treatments in the present study is the amount of YCO added to the diet. The amount added may have been insufficient to generate changes in intestinal fermentation that could be measured by fecal pH [
52]. Furthermore, there is a complex interaction among fermentative metabolites produced in the gut that might or might not alter the fecal pH.
Although there was a lower mean concentration of phenols in the feces of dogs that consumed YCO diets compared to the control diet, there was no difference among the diets regarding indole production. Contrary to this result, Swanson et al. [
53] identified a tendency for reduced fecal indole concentration in dogs supplemented with a mixture of oligosaccharides (MOS + FOS) and observed no difference in phenol concentration in any of the treatments.
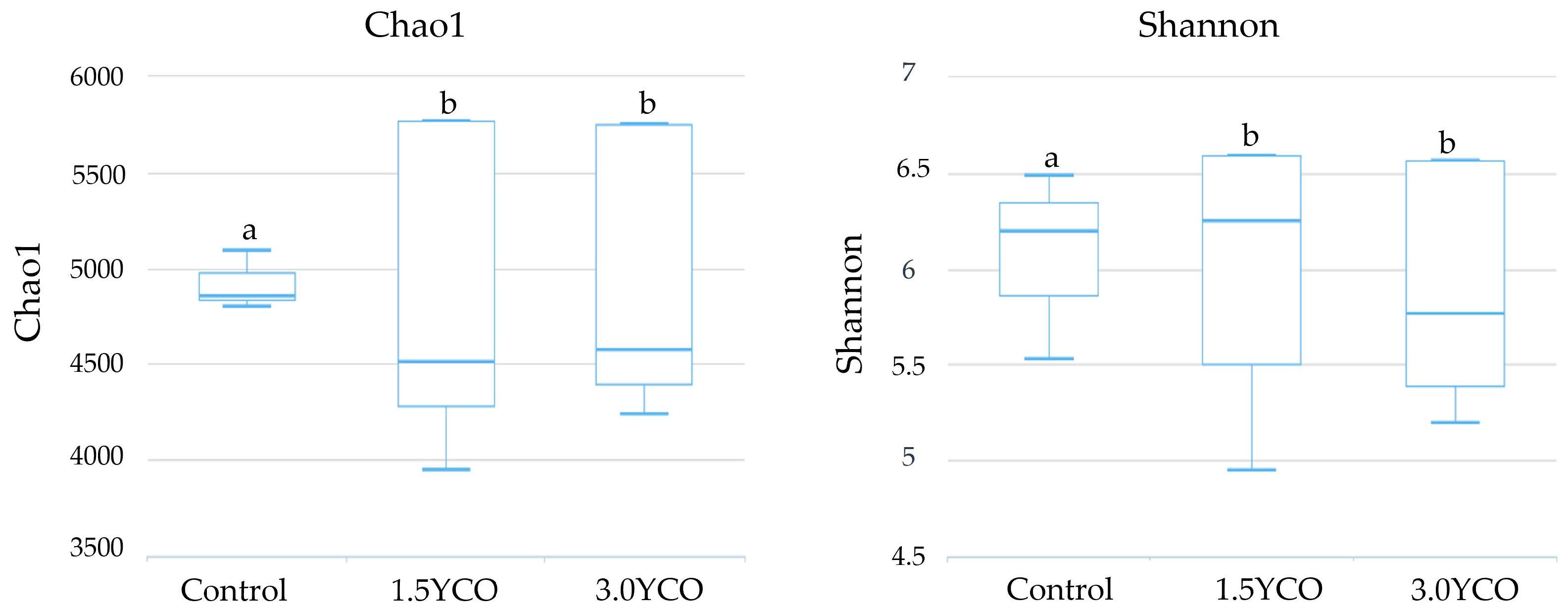
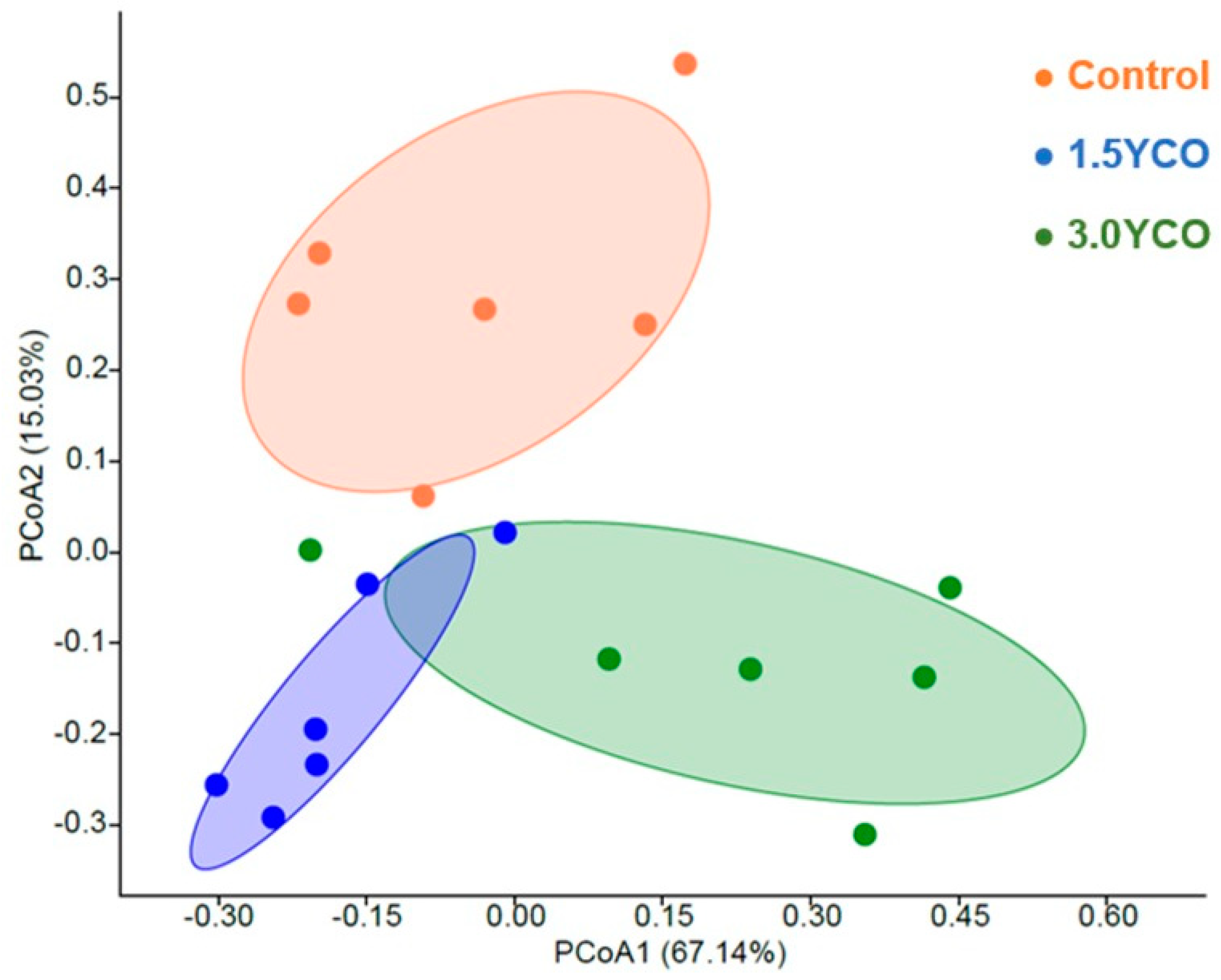
The differences observed in the fecal concentrations of ammonia, biogenic amines, phenols, and indoles possibly occurred in response to changes in the gut microbiota. Animals fed 1.5YCO and 3.0YCO showed an increase in alpha diversity when compared to the control group. The type of diet ingested and feed additives [
54,
55,
56], the segment of the gastrointestinal tract, and the particularities of each animal [
57] are some of the factors that exert influence on the diversity of gut microbiota. MOS can modulate the gut microbiota mainly through its ability to adhere to type I fimbriae from some bacteria [
58,
59]. This type of fimbriae is present in most Gram-negative bacteria, such as
Escherichia coli,
Klebsiella sp., and some
Salmonella sp., such as
S. typhimurium and
S. enteritidis [
60]. Type I fimbriae allow attachment of the bacteria to the enterocyte and exert an agglutinating effect on these cells [
61]. However, agglutination is blocked by D-mannose or α-methylmannosidium and by concanavalin A. Therefore, by binding with this type of fimbriae, MOS can limit intestinal colonization by potentially pathogenic microorganisms [
62]. Another mechanism presented by MOS to modulate gut microbiota is through selective fermentation, which benefits the growth of certain bacterial groups, such as
Lactobacillus and
Bifidobacterium, and the production of SCFAs, such as acetate, propionate, and butyrate [
63]. Bacteria of the genus
Lactobacillus help maintain a proper enteric environment, as they suppress the growth of potentially pathogenic bacteria through the production of SCFAs [
64]. It is important to note that this selective fermentation mechanism of the gut microbiota is secondary, as MOS is moderately fermentable [
28]. Furthermore, due to the partial solubility of SCFAs in the membrane, these gut fermentation products can alter the integrity and fluidity of the membrane of pathogens, providing another mechanism for inhibiting the growth of microorganisms considered pathogenic [
7].
Although there was a difference in gut bacterial diversity and richness in dogs fed YCO, fecal SCFA concentrations did not differ among treatments. Possibly, this happened because (1) SCFAs were rapidly absorbed in the gut and metabolized by the intestinal epithelium, liver, and muscle [
65,
66] or (2) the inclusion levels of YCO were not high enough to detect differences. Similarly, no difference in fecal SCFAs was found with the inclusion of 15 g/kg of MOS in the diet for dogs [
1]. However, the inclusion of 5.23% of a prebiotic and soluble fiber blend (combination of beet pulp, FOS, inulin, MOS, and kelp) promoted increases in fecal SCFA concentrations of dogs [
67].
Dogs fed 1.5YCO and 3.0YCO showed a reduction in the Bacteroidetes phylum and an increase in Firmicutes, compared to the control group, possibly as a result of the modulation of microorganisms related to intestinal eubiosis. This result contradicts the findings of Van den Abbeele et al. [
68], since the authors identified a significant increase in the Bacteroidetes and Actinobacteria phyla when evaluating the effects of a
Saccharomyces cerevisiae-based product, containing 27.5% β-glucans and 22.5% MOS, in an in vitro simulation of the canine gastrointestinal tract. On the other hand, research performed in yellow-feathered chickens showed an increase in ileal Firmicutes and a reduction in the relative abundance of ileal Proteobacteria and Actinobacteria in chickens supplemented with 150 or 300 mg/kg of oregano EO [
69].
Another component of the yeast cell wall that could have had an association with the modulation of gut microbiota and intestinal functionality is the β-glucans. It is known that β-glucans exert biological effects on the organism, such as immunomodulation [
70,
71]. When in the host, β-glucans bind to the Dectin-1 receptor, stimulating the production of many cytokines or other mechanisms of immune and non-immune reactions [
72]. Research performed in rats revealed that β-glucans can produce effects on the systemic immune system and interact with the gut-associated lymphoid tissue, modulating the expression of pattern recognition receptors in this tissue [
73]. Furthermore, another positive effect of β-glucans on gastrointestinal functionality reported in mice is the regulation of gut microbiota through the production of SCFAs [
74].
Beyond yeast cell wall components, EOs may be associated with the modulation of gut microbiota and intestinal functionality. The antimicrobial action of EOs and the modulation of the gut microbiota occur due to the action of bioactive compounds on several targets in the bacterial cell, such as damage to the cell membrane, cytoplasmic membrane, and protein membrane, extravasation of cell contents, coagulation of the cytoplasm, and the depletion of the proton motor force [
75]. In general, EOs have a greater spectrum of action on Gram-positive bacteria than Gram-negative bacteria, since Gram-negative bacteria are more resistant to EOs [
76]. However, carvacrol and thymol, recognized for having intense antimicrobial activity, also have action on Gram-negative bacteria. These phenolic compounds can disintegrate the outer membrane of Gram-negative bacteria, releasing lipopolysaccharides and increasing the permeability of the cytoplasmic membrane [
77]. It is also possible that the variation in composition among EOs is sufficient to vary the degree of susceptibility of Gram-negative and Gram-positive bacteria. Due to the hydrophobicity of EO and their compounds, the membrane polysaccharides, fatty acids, and phospholipid layer of the bacterial cell wall and mitochondria are injured, generating changes in structural conformation, and making the membrane permeable [
78,
79].
In a study by Zeng et al. [
80], when evaluating the use of an EO blend consisting of 4.5% cinnamaldehyde and 13.5% thymol in weaned pigs, animals fed EOs showed a significant reduction in
E. coli and total anaerobic bacteria in the rectum and a quantitative increase in
Lactobacillus in the colon and rectum, when compared to pigs that did not receive such supplementation. Extrapolating to other animal species, some studies encompass the use of phytogenic compounds in canine nutrition. In one of these [
81], it was shown that dogs fed a blend composed of 21.55 mg/g carvacrol, 18.76 mg/g, thymol, and 27.62 mg/g cinnamaldehyde showed a reduction in the total bacterial count, total coliforms,
Salmonella spp., and
E. coli, revealing the important effect of these compounds in improving the host interaction with the gastrointestinal microbiota, which is one of the key components of intestinal functionality [
82].
From the bacterial genera that increased in the feces of dogs fed the YCO,
Faecalibacterium and
Blautia are known as butyrate producers and are associated with a lower incidence and severity of inflammatory processes in the gut. These bacteria are considered biomarkers of gut functionality in terms of normal and stable microbiota, effective immune status, and gut mucosa [
66,
82,
83]. Also,
Faecalibacterium prausnitzii, the only known species of this genus, secretes metabolites that block the activation of NF-kB factor transcription, consequently resulting in the inhibition of the production of pro-inflammatory interleukins, such as interleukin 8 [
84].
It is important to highlight the greater relative abundance of
Clostridium in the feces of dogs fed 1.5YCO when compared to the control group. Although the
Clostridium genus is recognized for having species with potential pathogenicity to animals, such as
C. difficile and
C. perfringens [
85,
86], studies reveal the beneficial effects of
C. hiranonis in dogs by converting primary bile acids into secondary ones [
66]. Secondary bile acids control the growth of
C. difficile spores and, in previous studies in dogs, have been shown to stimulate the growth of
Faecalibacterium and inhibit
E. coli [
87], being a mechanism for controlling the growth of potentially pathogenic microorganisms. Considering this, it is important that future studies evaluate the effects of YCO and other additives on gut bacterial species to better understand the relationships among microbial species and to aid in the development of beneficial feed additives.
The greater bacterial diversity in the feces of dogs fed YCO is one of the main findings related to improved intestinal functionality. Dogs with gastroenteritis, such as inflammatory bowel disease and acute and chronic diarrhea, have a lower diversity of the gut microbiota, characterizing dysbiosis [
88]. Unlike the healthy dogs enrolled in this study, which have a higher abundance of
Faecalibacterium and
Blautia and a lower concentration of
Streptococcus, several studies show that dogs with gastroenteritis have reduced concentrations of key microorganisms, such as
Faecalibacterium,
Blautia, and
Turicibacter, and increased
Streptococcus [
88,
89,
90,
91,
92].
Regarding palatability, to the author’s knowledge, no studies that evaluate the palatability of diets containing oregano EO in dogs have been published. However, one study evaluating a blend of EOs (copaiba, cashew nutshell, and peppers) described a possible negative effect of the EO blend evaluated on diet palatability in dogs [
13].
In this study, the inclusion of YCO resulted in lower feed consumption compared to the control diet, even though some yeast-derived products, like sugarcane yeast, are usually known to make diets more palatable [
15]. Such result may have occurred due to (1) the organoleptic characteristics of oregano EO, which presents intense odor and flavor, and (2) the inclusion of the YCO blend by coating, which may have accentuated the flavor and odor. Possibly, the inclusion of the YCO blend in the dough before extrusion would have less influence on palatability and feed consumption. However, further studies are needed to confirm this hypothesis.