Simple Summary
Oral diseases, including dental problems, are of great significance in domestic animals. Disorders involving tissue localised in the teeth, periodontium, gums or tongue can be associated with pain and loss of appetite. It is particularly prominent in predatory animals such as cats. One of the most prominent dental diseases in that animal species is tooth resorption (also known as odontoclastic resorptive lesion). The disease is associated with damage of the tooth tissue that eventually leads to tooth loss. We have analysed dental charts of 174 cats diagnosed with tooth resorption. The changes were most often noted in premolar and molar teeth. We have not found correlations between the severity of the disease and clinical symptoms shown by the animals, but the disease progressed with animals’ age. Based on the obtained results, we indicated the need of a careful dental examination with intraoral radiography in cats and especially in animals showing any signs of oral disease, even in cases with preserved appetite.
Abstract
Feline tooth resorption (odontoclastic resorptive lesion) is a common and important issue in veterinary dentistry. This study aimed to analyse the disease’s severity and correlation with clinical information in the population of feline patients in Poland in the area of Lower Silesia. An analysis of the clinical charts of 174 cats with dental problems, which were diagnosed as tooth resorption, was conducted. The gender and breed had no influence on the disease severity, but the disease progressed with age. The lesions were mostly encountered within the third and fourth maxillary premolars (107, 108, 207, 208) and mandibular molars (309, 409). No direct correlation was found between the presence or severity of the disease and the clinical signs of affected cats. The study shows that feline tooth resorption is a common issue in feline dentistry and should be taken into account in all cases of animals with any signs of oral disease, including gingivitis and/or dental plaque with preserved appetite. A careful intraoral radiographic examination is essential to avoid false negative results in ambiguous cases.
1. Introduction
Feline oral diseases are considered to be a significant topic in veterinary medicine. The most frequent diseases in that group include: tooth resorption (TR; formerly feline odontoclastic resorptive lesion—FORL), lympho-plasmocytic (eosinophilic) stomatitis and periodontitis [1,2,3,4]. Tooth resorption was first described in cats as early as 1920 [5]. The first evidence of a TR-like disease comes from paleopathological descriptions of cats living 800 years ago [6]. However, paleopathological findings are rare, suggesting that TR is more of a modern problem. Similar changes to those occurring in the course of TR were described in 1930 by Hopewell-Smith and named odontolysis [7]. The report also describes histological changes present in the teeth. Moreover, Hopewell-Smith noted that the changes were associated with chronic marginal gingivitis. [7]. In the 1950s and 1960s, the disease was thought to be related to caries, and it was not until the 1970s that it was unequivocally determined that changes involved not caries, but resorption [8,9]. Tooth resorption has also been described in wild felids [10,11].
Due to the fact that TR can be accompanied by appetite loss and developing cachexia, a rapid and appropriate diagnosis as well as proper treatment are crucial for the animal’s wellbeing. The disease is characterised by a resorption in the cementum layer or enamel-covered surface of the tooth caused by polynuclear cells called odontoclasts. The lesions develop initially in the tooth ligament structures and cementum. As a result of the loss of root substance, the tooth destruction disturbs the stability of the crown causing its fracture [2,4,12]. Based on clinical examination and radiography, five stages of the disease have been distinguished [4]:
- The resorption has minimal character. It involves the surface of the enamel or cementum with no loss of the dentine; the changes can be difficult to notice due to the low extent.
- In this stage, the dentine loss is present, although the lesion does not involve dental pulp. The lesion has a mild character.
- In the third stage of the disease, the resorption involves cementum, enamel, dentine and pulp cavity, but the tooth maintains its stability within the alveolar sac.
- In the next stage, the resorption progresses involving all tooth structures and causes the loss of teeth stability.
- The fifth stage can be subdivided into two types: 5a in which the tooth loses its crown while the root(s) remain unchanged apart from being significantly resorbed, and 5b in which a vast resorption of the root(s) is accompanied by the presence of an almost intact crown.
The division of either the fourth or fifth stage of the disease into further types differs between the authors. The American Veterinary Dental College suggests dividing the fourth stage into 4a, 4b and 4c types (https://avdc.org/; online access: 1 June 2023).
The disease aetiology is not understood completely, although many theories have been formed to explain this phenomenon. Factors that are being considered involve: the domestication of the cat (influencing the nutrition, vaccinations and neutering programs), as well as infectious agents and chronic inflammation associated with plaque accumulation. The contribution of anatomical factors related to the specific structure of feline teeth cannot be ruled out.
It is conceivable that the feline diet plays a role in the aetiology of TR [13,14]. The process of overfeeding with raw liver can contribute to the disease because both retinol and tretinoin present in the liver can directly stimulate the activity of clastic cells [4]. Moreover, cats with TR show significantly higher urine specific gravity and higher concentration of serum 25-hydroxyvitamin D3 (25(OH)D3) than cats without the disease, which confirms that the activity of odontoclasts is a result of not only local changes but also systemic disturbances [6]. Since food is the only source of vitamin D in cats, the dietary factor is decisive for the level of 25(OH)D3 in the blood serum. Taking the above-mentioned hypotheses into account, one cannot rule out that the domestication process and the resulting changes in feline diet play a significant role in the occurrence of TR. Nonetheless, finding the disease in wild cats casts doubt on this theory [14,15].
The role of viral infections in the course of the disease is poorly known. Some researchers describe the influence of calicivirus (FCV) on the development of TR [16]. Feline immunodeficiency virus (FIV) was also found in affected cats [17]. Significant metabolic changes were also localised in gingiva-derived stromal cells obtained from cats with TR and concomitant caliciviral infections [18]. However, the association of feline leukaemia virus (FeLV) with feline oral disease has not been demonstrated [19].
Chronic periodontitis has also been believed to have an influence on the development of odontoclastic lesions. The accumulation of dental plaque can cause inflammation of the periodontium t hat results in a local immune response and the release of inflammatory mediators (e.g., cytokines) and bacterial toxins (e.g., lipopolysaccharides), which play a significant role both in the stimulation of the inflammatory process and in the differentiation and infiltration of the clastic cells. Among the bacteria that may contribute to TR, gram negative anaerobic Fusobacterium and Bacteroides spp. [20] have been mentioned. In humans, antibodies against Actinobacterium sp. have also been found in the course of the resorptive disease. Although the majority of TR cases are combined with the presence of inflammation in the surrounding tissue, early lesions combined with a seemingly normal appearance of the tissue may cause confusion. Therefore, the inflammation is rather secondary than primary to the disease [6].
The specific structure of cats’ teeth may also contribute to the occurrence of TR. A lack of coverage of the dentin by cement in the area of the cementoenamel junction forming an area of increased root risk can be defined as a significant factor. The lack of cement and the presence of mineralized dentine could attract the odontoclast. A thin layer of cement in the area of furcation between the roots is another risk point [4].
It is also believed that mechanical forces and occlusal stress can contribute to the progression of TR. The repeating compressive and tensile forces caused by micro-movements during chewing and malocclusion can disrupt the chemical bonds between enamel rods, causing enamel abfraction and dentin exposure. Nonetheless, the disease can also affect teeth which are not subject to the abovementioned forces [4,6].
The disease incidence grows with age. Patients with tooth loss found during clinical examination are more susceptible to development of the disease [6]. The data regarding the incidence of TR are various, depending on diagnostic criteria. Some of the research is based on the results of clinical examination, while others also include the results of radiographic examination [3,21,22]. The early stages of the disease are very often not manifested by the animals and are difficult to discover during clinical examination. However, they can be recognised during radiography [21]. The affected teeth show gingivitis and often gingival hypertrophy. Cavities in dental crowns can be filled by gingival granulation tissue [23].
Veterinary medicine is gradually changing: on the one hand, diagnostic tools are constantly improving and becoming more available in routine practice; on the other hand, the awareness of owners has grown, leading to more careful care and providing more information to the veterinarian. Therefore, studies including animals’ history, clinical examination and specific procedures can improve our knowledge on changes in the disease prevalence and clinical picture. This study aimed at analysing: (1) the severity of the disease in affected animals based on radiographic examination and (2) the relationship between the disease severity and clinical signs noted by owners and during clinical examination.
2. Materials and Methods
The research included a retrospective analysis of data sheets of 174 cats presented for the first time to the Dental Clinic and diagnosed with tooth resorption. The group comprised 58% of males and 42% of females aged from 18 months to 18 years (median 7.5 years) representing various breeds with the majority (79%) representing domestic shorthair cats (Table 1).

Table 1.
The breed distribution in the examined group.
All cats were diagnosed on the basis of the history, clinical examination and intraoral radiographic examination.
The medical history, including symptoms of oral disease, i.e., diminished or lost appetite, signs of pain and fetor ex ore was collected. All the animals underwent clinical examination. The presence of gingivitis, dental plaque and gingival bleeding (resulting from examination) were evaluated. The oral examination was performed initially on unanaesthetised animals and repeated after sedation for confirmation. The data obtained from examination without sedation were taken into account in the analysis. The clinical examination was complemented with an intraoral radiographic examination using iM3 Revolution 4DC device (iM3, Ireland) and CR 7 Vet Image Plate X-ray Scanner (iM3, Ireland). Radiological diagnostics were performed under general anaesthesia appropriate for each patients’ condition.
The number of absent teeth and affected teeth as well as the advancement of lesions were assessed on the obtained images. The severity of the disease was evaluated using staging available on the American Veterinary Dental College website (https://avdc.org/; online access: 1 June 2023).
The data underwent statistical analysis using StatisticaPL 13.3 (StatSoft, Kraków, Poland) software. The data normality were tested using Shapiro–Wilk analysis. The correlation of the results was tested using Spearman’s rang correlation analysis. The difference among groups was tested using either Mann–Whitney U analysis or Kruskal–Wallis analysis. The significance level was set at p < 0.05.
3. Results
The radiographic examination of affected cats revealed lesions in 1613 teeth, inclusively. The pathological process was present in all types of teeth (from incisors to molars) and in all degrees of disease advancement (Figure 1, Figure 2 and Figure 3).
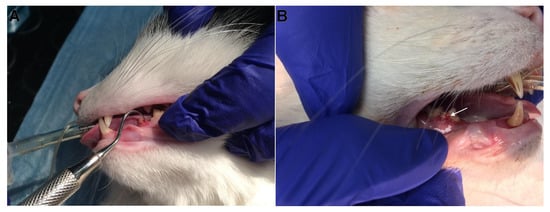
Figure 1.
Clinical presentation of examined cats. Deep odontoclastic lesions penetrating to pulp cavity in examined teeth. (A) The lesion is located on the caudal aspect of 307 tooth; (B) the lesion is located on the buccal surface of 408 tooth (arrow), the 407 tooth is missing.
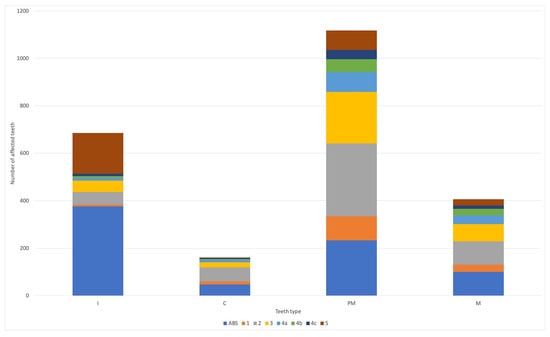
Figure 2.
The degree of radiological lesions among individual teeth types in studied animals. ABS—absent; I—incisors; C—canine; PM—premolars; M—molars; 1–5—the degree of dental lesions according to American Veterinary Dental College (https://avdc.org/; online access: 1 June 2023).
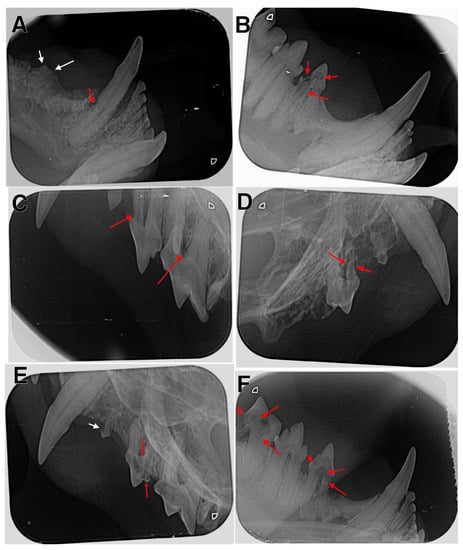
Figure 3.
Radiographic examination of affected cats. (A) The right mandible with type 2 lesion in tooth 404 (red arrow) and type 5a lesion in tooth 407 (white arrows); (B) the right mandible with type 4 lesion in tooth 407; (C) the left maxilla with type 1 lesions in teeth 207 and 208 (red arrows); (D) the right maxilla with type 4 lesions in tooth107 (red arrows); (E) the left maxilla with type 1 lesion in tooth 206 (white arrow) and type 4 lesions in tooth 207 (red arrows); (F) the right mandible with type 4 lesions in teeth 407 and 409 (red arrows).
The examined cats showed resorptive lesions of 1–28 teeth (median 9 teeth). The lesions were noted in one (n = 9; 5.2%), two (n = 24; 13.8%), three (n = 36; 20.7%) or even four (n = 105; 60.3%) dental arches (Table 2). Among the 24 animals that showed lesions in two dental arches, the disease involved mainly only mandibles (n = 10) or maxilla (n = 9), followed by cats with the disease present only on the left side (n = 2) or present diagonally (n = 3). The lesions were mostly encountered within the third and fourth maxillary premolars (107, 108, 207, 208) and mandibular molars (309, 409) (Table 3; Figure 4). The affected teeth showed lesions scored from the first stage to the fifth stage with a median of the fifth stage.

Table 2.
The number and arrangement of involved dental arches in examined animals.

Table 3.
The table presents the number of animals showing tooth resorption of various degrees in each of the examined teeth, including information about missing teeth; ABS—absent.
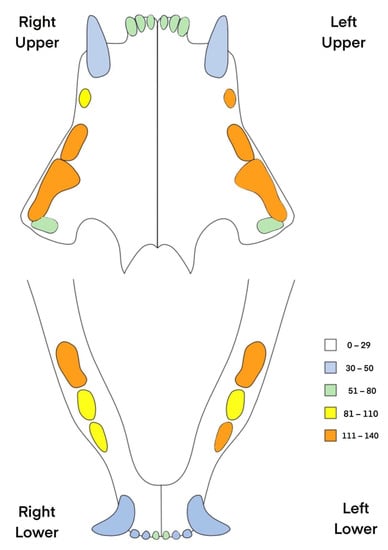
Figure 4.
The incidence of resorptive lesion in each feline tooth in the examined animals. The most frequently affected teeth were maxillary premolars (107, 108, 206, 207, 208) and mandibular premolar and molars (307, 309, 409).
Moreover, a total number of 756 teeth were missing (Table 3) in the examined animals with a medium value of 3 per animal and a maximum value of 20 in one animal. The most commonly missing teeth included: 106 (n = 62), 206 (n = 53), 301 (n = 46) and 401 (n = 46).
There was a correlation between the animals’ age and number of missing teeth (p < 0.05; r = 0.37; Spearman correlation analysis), number of affected teeth (p < 0.05; r = 0.19; Spearman correlation analysis) and the highest degree of lesions (p < 0.05; r = 0.29; Spearman correlation analysis). Moreover, we have noted a positive correlation (Spearman correlation analysis) between the degree of lesions and the animal’s age in teeth number: 107 (p < 0.05; r = 0.23), 108 (p < 0.05; r = 0.22), 208 (p < 0.05; r = 0.29), 307 (p < 0.05; r = 0.38), 404 (p < 0.05; r = 0.53) and 409 (p < 0.05; r = 0.30). We have also noted multiple correlations in the degree of observed lesions between corresponding groups of teeth (Table 4 and Table 5).

Table 4.
Correlations in the severity of the disease (degree of tooth resorption) between teeth within the same type; Spearman correlation analysis.

Table 5.
Correlations in the severity of the disease (degree of tooth resorption) between shearing teeth within right side, left side, maxilla or mandible; Spearman correlation analysis.
There was no significant difference between males and females regarding the number of affected teeth, number of missing teeth and the highest degree of lesions (p > 0.05; Mann–Whitney U analysis); also, there was no difference in the above mentioned parameters in relation to animals’ breed (p > 0.05; Kruskal–Wallis analysis).
A diminished appetite was noted in 37 (21.3%) cats, fetor ex ore in 14 (8%) cats, the presence of gingivitis in 89 (51.1%), dental plaque in 67 (38.5%) animals and gingival bleeding during examination in 24 (13.8%) animals. Apart from gingivitis, no other signs of inflammation were noted (including faucitis). There were no differences in the number of affected teeth or number of affected dental arches depending on the presence of clinical symptoms (p > 0.05).
4. Discussion
Diseases involving the lysis of dental hard tissues (including TR) resulting from the activity of blast cells were described in various animal species as well as in humans [24,25,26]. In horses, a disease resembling TR was reported for the first time as late as 2008 [27]. Tooth resorption is less common in dogs than in cats. In the case of dogs, we more often deal with external replacement resorption and external inflammatory resorption [28]. As in the case of cats, the cause of the disease cannot be clearly identified [29]. The cause of the disease differs among species. In humans and cats, the lysis predominates being accompanied partly by inflammation and very limited hypercementosis, while in horses, a very intense periodontitis combined with secondary hypercementosis dominates [25,30,31,32]. In horses, the disease affects mostly incisors and canine teeth, whereas in cats the lesions are most frequently present in premolar and molar teeth [3,25,33]. Ingham et al. in a study of 228 cats found that teeth 307 and 407 were most commonly affected, with the lesions being symmetrical in most cases [3]. Also in our study, the cheek teeth were predominantly affected but teeth number 107, 108, 207, 208, 309 and 409 were more frequently affected than teeth number 307 and 407. Although Ingham et al. [3] showed that the disease was most frequently affecting the mandibular teeth, the majority of cats presented in our study showed changes in three or all four dental arches. Moreover, we have shown that teeth of the same type (canine or shearing teeth) or of the ipsilateral (right, left, mandibular, maxillar) tend to have a similar severity of changes. It points to the need for a complete oral examination and a full oral radiography in a case of noticing changes in at least one tooth.
The data regarding the incidence of TR in cats are diverse, which is likely caused by the differences in diagnostic processes [3,21]. The number of publications on this disease in cats is not wide. Our study consisted of clinical history, clinical examination and intraoral radiographic examination. The number of patients is large and allows conclusions to be drawn. The study conducted by Ingham et al. [3] on 228 domestic shorthair cats and 9 British shorthair cats showed TR in 29% of animals, with females being affected more often than males. Lund et al. [34] noted TR in 48% of cats older than one year. In our study, we have recognised TR with no influence of sex or breed on the presence of the disease, but with more advanced disease in older animals (higher number of affected and/or missing teeth and higher degree of changes).
It seems that since TR’s first descriptions in the 1920’s, the disease’s incidence has increased. In addition to the improvement in diagnostic possibilities, it may be related to other factors, e.g., animal nutrition [4]. What is riveting is a low percentage of animals showing loss of appetite despite the severity of the lesions. This may be related to the fact that cats are strict carnivores which is reflected in the way they take and handle food. They show an increase in their teeth’s ability to cut and a concomitant loss of their ability to crush food, which also applies to the molars. Thus, the food taken in is subjected to little processing, apart from grinding it into smaller particles [35]. Hence, the involvement of the chewing apparatus when eating dry commercial feed is low [36]. Cats tend to swallow such food. On the other hand, due to disorders affecting part of the periodontal mechanoreceptors, they may disrupt the chewing process [37]. The most common clinical signs were gingivitis and presence of dental plaque, pointing to a conclusion that a thorough dental examination including intraoral radiography should be performed in all cats showing any signs of gingivitis and/or dental plaque, regardless of their appetite and dietary habits.
Unfortunately, due to a retrospective character of our study, the data regarding animals’ diet, vaccination history or viral infection status were not available, therefore could not serve for extended analysis.
The obtained results show explicitly that TR is a very common feline disease that requires intraoral radiographic examination as an addition to clinical examination of cats, especially when the results of clinical examination are ambiguous. It is consistent with the results presented by other authors [3,21]. Our results highlight the need of a through oral examination (including intraoral radiography) in any case of suspicion of oral disease or oral pain and moreover, in all elderly cats. Although previously third mandibular premolar teeth were considered the most frequently affected [3], we have shown that the disease can be present more often in other teeth (including maxillary teeth).
5. Conclusions
TR should be taken into account in the differential diagnosis in all cases of cats with appetite disorder, signs of oral pain, gingivitis, dental plaque and fetor ex ore, regardless of the animal’s age. The most affected teeth are premolar and molar teeth with a tendency to involve multiple teeth of the same group. An intraoral radiographic examination is essential to confirm the disease in ambiguous cases with nonspecific clinical symptoms.
Author Contributions
Conceptualization, M.J.; Methodology, P.P., I.J. and M.J.; Software, I.J.; Validation, I.J. and M.J.; Investigation, P.P., I.J. and M.J.; Resources, I.J. and M.J.; Writing—original draft, P.P. and I.J.; Writing—review & editing, M.J. and M.D.; Supervision, P.P. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The study included a review of archived patient records. All patients underwent diagnostic and treatment procedures in a veterinary clinic with oral consent obtained from the owners. All patient records were blinded in terms of personal data. According to national law, no additional approval is required for studies including solely patients’ records.
Informed Consent Statement
The data analysed in the study were obtained during standard clinical procedures. All animals’ owners gave their consent to clinical examination and data collection.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Conflicts of Interest
The authors report no conflict of interest.
References
- Gorell, C. Tooth resorption in cats. Pathopysiology and treatment options. J. Feline Med. Surg. 2015, 17, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Gorell, C.; Larsson, A. Feline odontoclastic resorptive lesions: Unveiling the early lesion. J. Small Anim. Pract. 2002, 43, 482–488. [Google Scholar] [CrossRef]
- Ingham, K.E.; Gorrel, C.; Blackburn, J.; Farnsworth, W. Prevalence of odontoclastic resorptive lesions in a population of clinically healthy cats. J. Small Anim. Pract. 2001, 42, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Reiter, A.M.; Mendoza, K.A. Feline odontoclastic resorptive lesions. An unsolved enigma in veterinary dentistry. Vet. Clin. N. Am. Small Anim. Pract. 2002, 32, 791–837. [Google Scholar] [CrossRef]
- Reiter, A.M. Feline “odontolysis” in the 1920s: The forgotten histopathological study of feline odontoclastic resorptive lesions (FORL). J. Vet. Dent. 1998, 15, 35–41. [Google Scholar] [CrossRef]
- Reiter, A.M.; Lewis, J.R.; Okuda, A. Update on the etiology of tooth resorption in domestic cats. Vet. Clin. N. Am Small Anim. Pract. 2005, 35, 913–942. [Google Scholar] [CrossRef]
- Hopewell-Smith, A. The process of osteolysis and odontolysis, or so-called “absorption” of calcified tissues: A new and original investigation. A. The loosening of the deciduous and permanent dentitions. B. The bone of attachment. C. The alveolar processes of the jaws. Dent. Cosmos. 1930, 72, 323–345. [Google Scholar]
- Kerebel, B.; Daculsi, G. Histologie et histopathologie dentaires du chat. Sci. Rech. Odontostomatol. 1971, 7, 29–32. [Google Scholar]
- Schneck, G.W.; Osborn, J.W. Neck lesions in the teeth of cats. Vet. Rec. 1976, 99, 100. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Schawalder, P.; Stich, H.; Lussi, A. Neck Lesion bei Grosskatzen; Untersuchungen beim Leoparden (Panthera pardus). Kleintierpraxis 1995, 40, 537–549. [Google Scholar]
- Berger, M.; Schawalder, P.; Stich, H.; Lussi, A. Feline dental resorptive lesions in captive and wild leopards and lions. J. Vet. Dent. 1996, 13, 13–21. [Google Scholar] [CrossRef]
- Lommer, M.J.; Verstraete, F.J.M. Prevalence of odontoclastic resorption lesions and periapical radiographic lucencies in cats: 265 cases. JAVMA 2000, 217, 1866–1869. [Google Scholar] [CrossRef]
- von Schlup, D. Epidemiologische und morphologische Untersuchungen am Katzengebiss I. Mitteilung: Epidemiologische Untersuchungen. Kleintierpraxis 1982, 27, 87–94. [Google Scholar]
- Zetner, K. The influence of dry food and the development of feline neck lesions. J. Vet. Dent. 1992, 9, 2–4. [Google Scholar] [CrossRef]
- Okuda, A.; Harvey, C.E. Etiopathogenesis of feline dental resorptive lesions. Vet. Clin. N. Am. Small Anim. Pract. 1992, 22, 1385–1404. [Google Scholar] [CrossRef]
- Thomas, S.; Lappin, D.F.; Spears, J.; Bennett, D.; Nile, C.; Riggio, M.P. Prevalence of feline calicivirus in cats with odontoclastic resorptive lesions and chronic gingivostomatitis. Res. Vet. Sci. 2017, 111, 124–126. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Berger, M.; Sigrist, B.; Schawalder, P.; Lutz, H. Feline immunodeficiency virus (FIV) infection leads toincreased incidence of feline odontoclastic resorptive lesions (FORL). Vet. Immunol. Immunopathol. 1998, 65, 299–308. [Google Scholar] [CrossRef]
- Sotero-Rivera, M.; Groborz, S.; Janeczek, M.; Kornicka, J.; Wierzgoń, M.; Boaz, A.; Marycz, K. Gingiva-derived Stromal Cells Isolated from Cats Affected with Tooth Resorption Exhibit Increased Apoptosis, Inflammation and Oxidative Stress while Experiencing Deteriorated Expansion and Anti-Oxidative Defence. Stem Cell Rev. Rep. 2023, 16, 1343–1355. [Google Scholar]
- Diehl, K.; Rosychuk, R.A.W. Feline gingivitis–stomatitis–pharyngitis. Vet. Clin. N. Am. Small Anim. Pract. 1993, 23, 139–153. [Google Scholar] [CrossRef]
- Dobrescu, M.V. Nekrobazillose der Zähne bei der Katze. Tierärztl. Umschav 1994, 49, 498–501. [Google Scholar]
- Heaton, M.; Wilkinson, J.; Gorell, C.; Butterwick, R. A rapid screening technique for feline odontoclastic resorptive lesions. J. Small Anim. Pract. 2004, 45, 598–601. [Google Scholar] [CrossRef]
- DuPont, G.A. Radiographic Evaluation and Treatment of Feline Dental Resorptive Lesions. Vet. Clin. N. Am. Small Anim. Pract. 2005, 35, 943–962. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.E. Feline oral pathology, diagnosis and management. In Manual of Small Animal Dentistry; BSAVA: Gloucester, UK, 1995; pp. 129–138. [Google Scholar]
- Kim, P.H.; Heffez, L.B. Multiple idiopathic resorption in the primary dentition: Review of the literature and case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 501–505. [Google Scholar] [CrossRef]
- Smedley, R.C.; Earley, E.T.; Galloway, S.S.; Baratt, R.M.; Rawlinson, J.E. Equine odontoclastic tooth resorption and hypercementosis: Histopathologic features. Vet. Pathol. 2015, 52, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Watanabe, K.; Ozawa, T. Odontoclastic resorptive lesions in dogs. J. Vet. Med. Sci. 2008, 70, 103–105. [Google Scholar] [CrossRef]
- Staszyk, C.; Bienert, A.; Kreutzer, R.; Wohlsein, P.; Simhofer, H. Equine odontoclastic tooth resorption and hypercementosis. Vet. J. 2008, 178, 372–379. [Google Scholar] [CrossRef]
- Peralta, S.; Verstraete, F.J.M.; Kass, P.H. Radiographic evaluation of the types of tooth resorption in dogs. Am. J. Vet. Res. 2010, 71, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Arnbjerg, J. Idiopathic Dental Root Replacement Resorption in Old Dogs. J. Vet. Dent. 1996, 13, 97–99. [Google Scholar] [CrossRef]
- DeLaurier, A.; Boyde, A.; Horton, M.A.; Price, J.S. A scanning electron microscopy study of idiopathic external tooth resorption in the cat. J. Periodontol. 2005, 76, 1106–1112. [Google Scholar] [CrossRef]
- Lewis, J.R.; Okuda, A.; Shofer, F.S.; Pachtinger, G.; Harvey, C.E.; Reiter, A.M. Significant association between tooth extrusion and tooth resorption in domestic cats. J. Vet. Dent. 2008, 25, 86–95. [Google Scholar] [CrossRef]
- Schätzle, M.; Tanner, S.D.; Bosshardt, D.D. Progressive, generalized, apical idiopathic root resorption and hypercementosis. J. Periodontol. 2005, 76, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- van Wessum, R.; Harvey, C.E.; Hennet, P. Feline dental resorptive lesions. Prevalence patterns. Vet. Clin. N. Am. Small Anim. Pract. 1992, 22, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.M.; Bohacek, L.K.; Dahlke, J.L.; King, V.L.; Kramek, B.A.; Logan, E.I. Prevalence and risk factors for odontoclastic resorptive lesions in cats. J. Am. Vet. Med. Assoc. 1998, 212, 392–395. [Google Scholar] [PubMed]
- Wittenberg, P.A. Phylogenetic, Behavioral and Dietary Constrains on Felid Masticatory Morphology; Graduate School of Duke University: Durham, NC, USA, 1995. [Google Scholar]
- Gorniak, G.C.; Gans, C. Quantitative Assay Electromyograms During Mastication in Domestic Cats (Felis catus). J. Morph. 1985, 163, 253–281. [Google Scholar] [CrossRef]
- Kim, S.E.; Boaz, A.; Garcia, T.C.; Verstraete, F.J.M. Bite Forces and Their Measurement in Dogs and Cats. Front. Vet. Sci. 2018, 5, 76. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).