Effect of Supplementary Feeding on Milk Volume, Milk Composition, Blood Biochemical Index, and Fecal Microflora Diversity in Grazing Yili Mares
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Date and Location
2.2. Experimental Design
2.3. Feed Management
2.4. Sample Collection and Analysis
2.4.1. Milk
2.4.2. Blood
2.4.3. Feces
2.5. Sample Analysis
2.5.1. Daily Lactation Volume
2.5.2. Milk Composition
2.5.3. Plasma Biochemical Indices
2.5.4. Fecal Microflora Abundance and Diversity
2.6. Data Analyses
3. Results
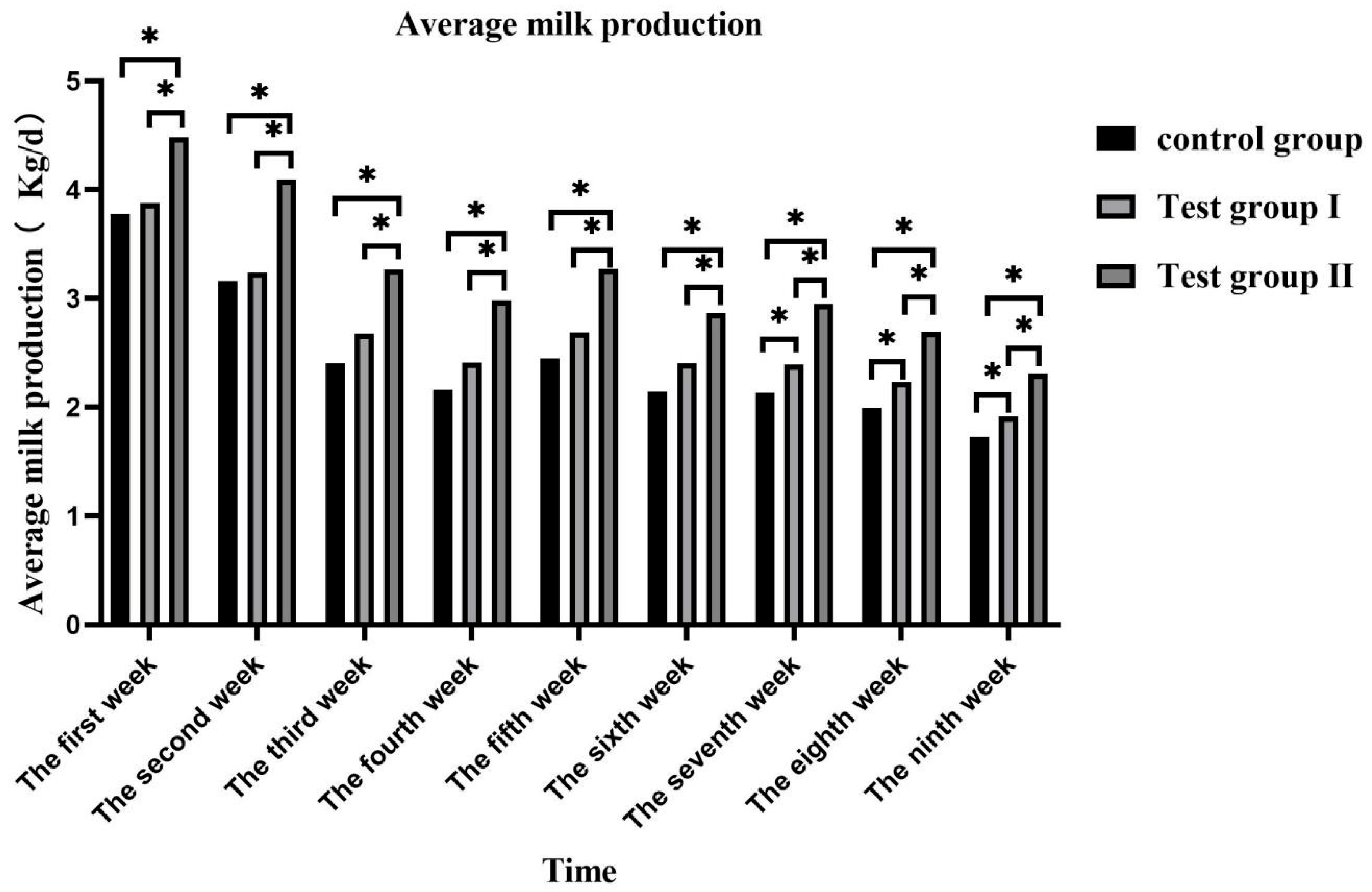
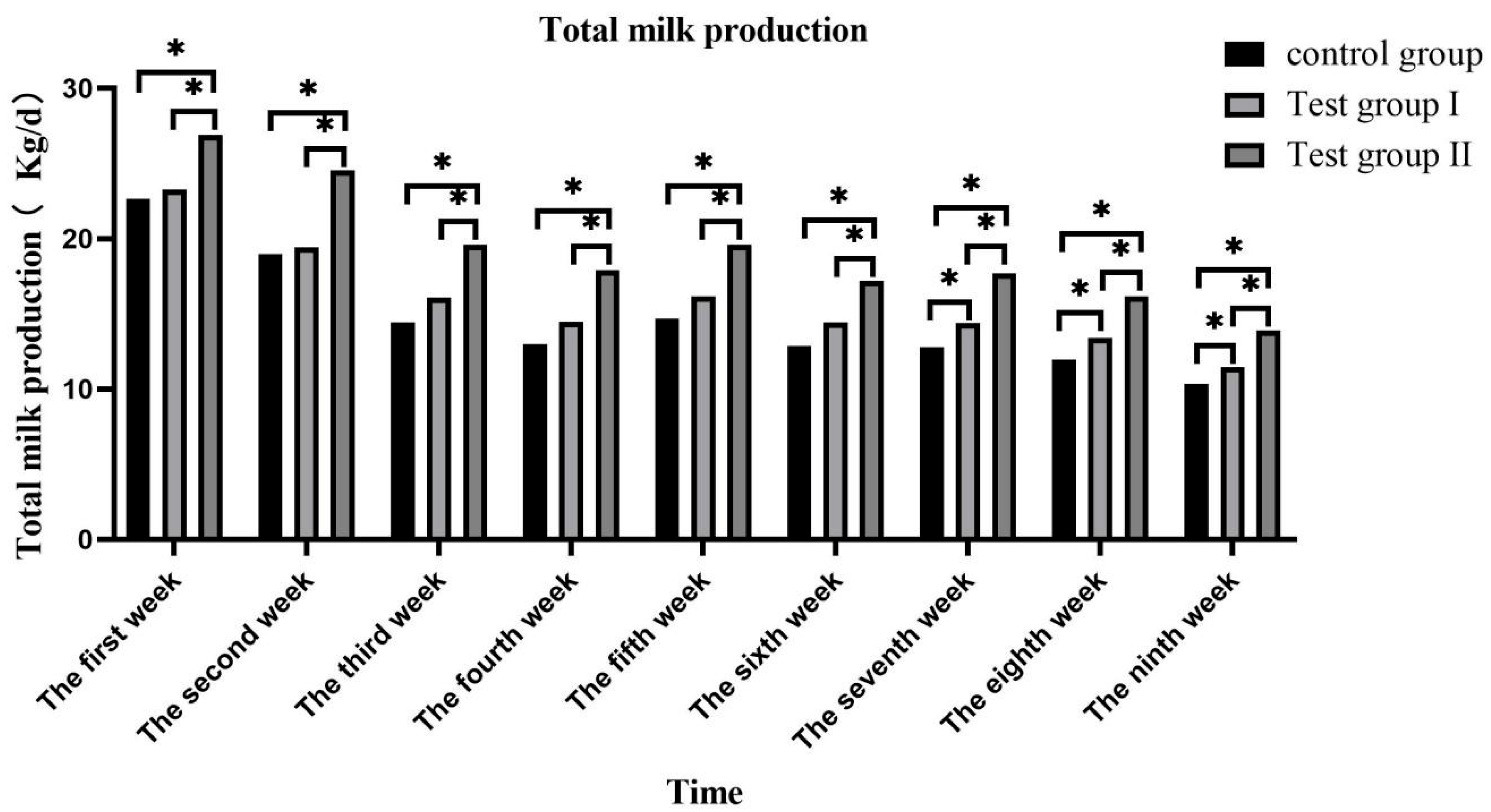
3.1. Effects of Concentrate and FA Supplementation on Milk Volume in Grazing Yili Horses
3.2. Effects of Feed Concentrate and FA Supplementation on the Composition of Grazing Yili Horse Milk
3.3. Effects of Feed Concentrate and FA Supplementation on FA Composition of Grazing Yili Horse Milk
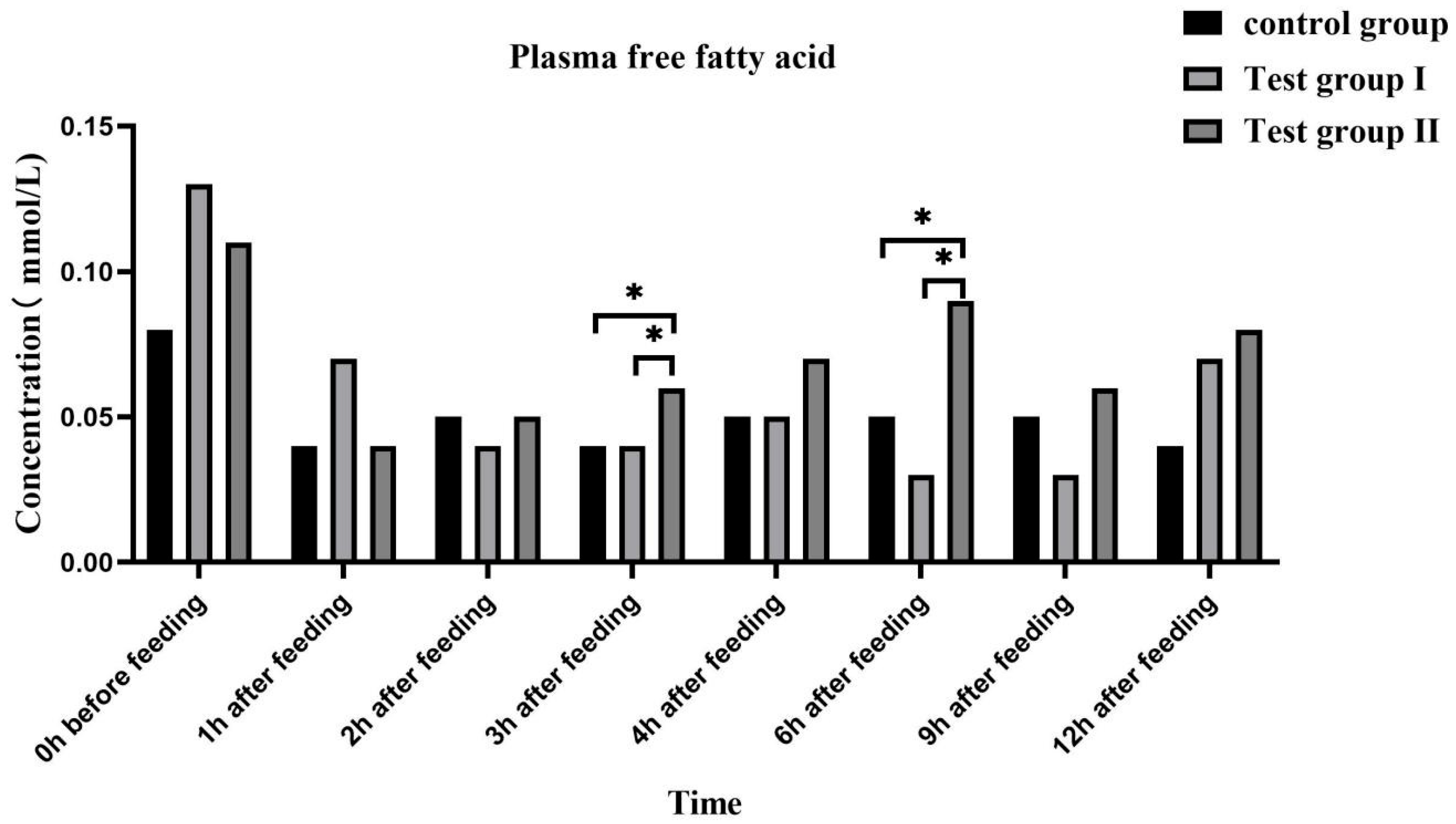
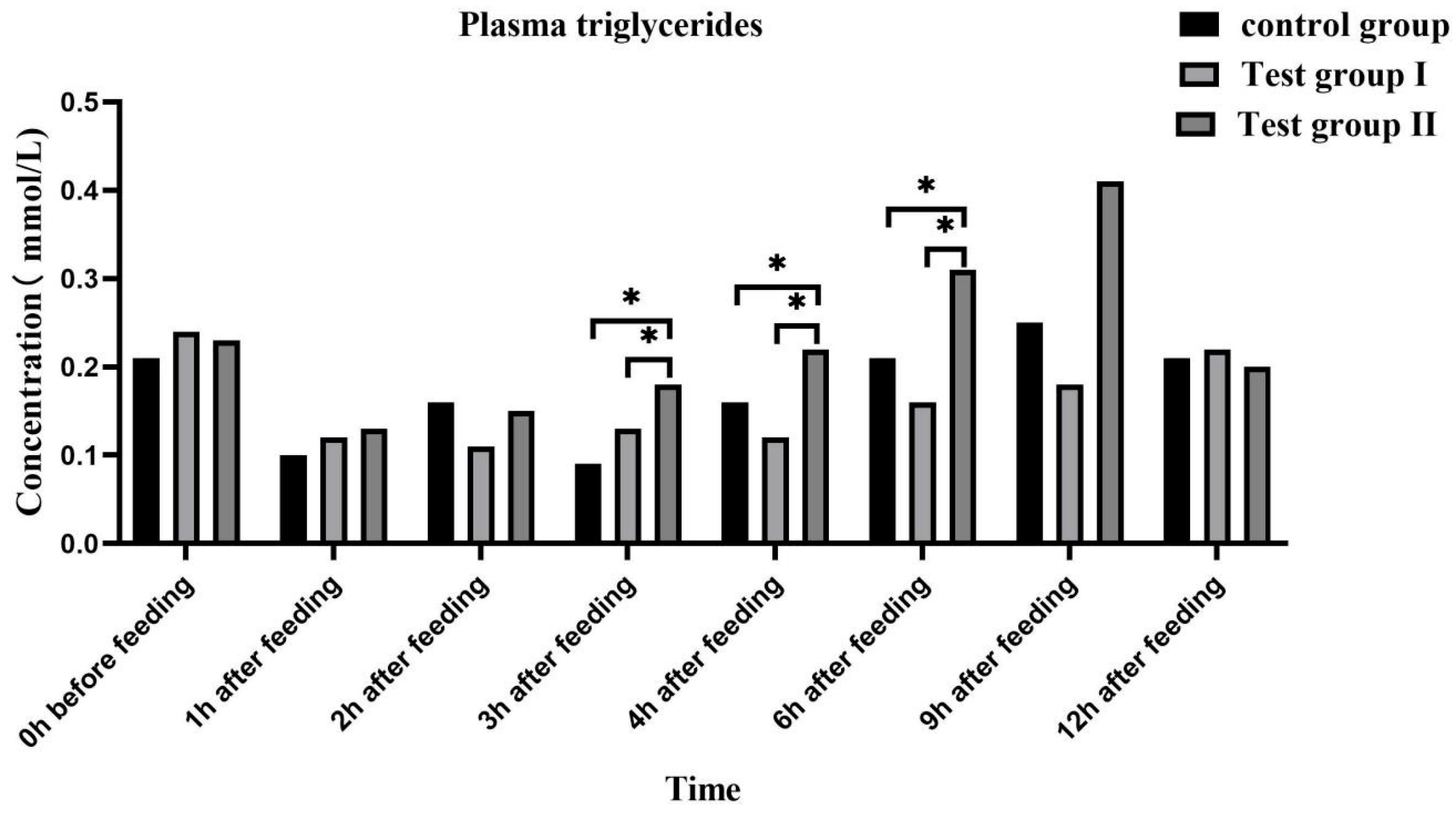
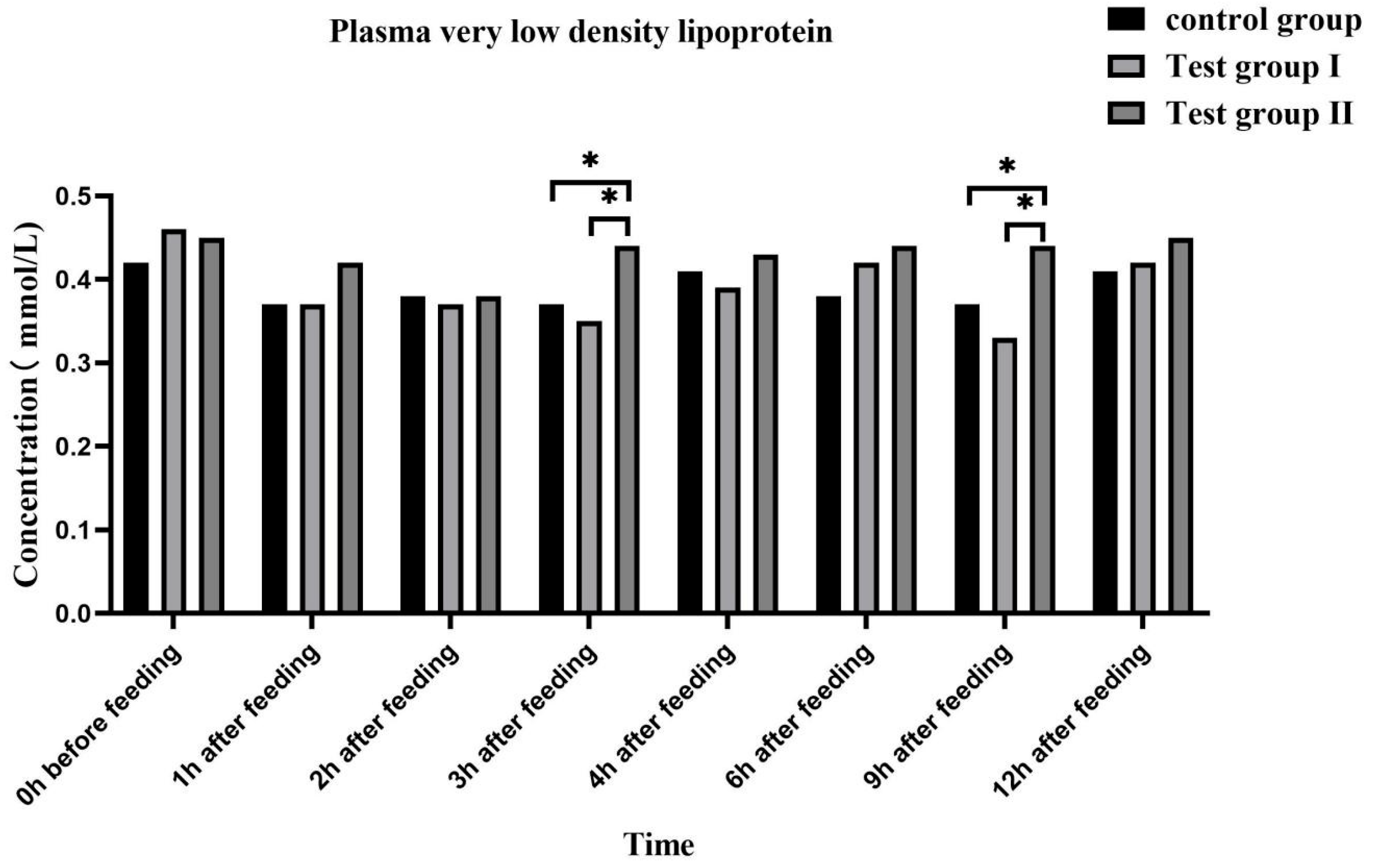
3.4. Effects of Feed Concentrate and FA Supplementation on the Plasma Biochemical Indices of Grazing Yili Horses
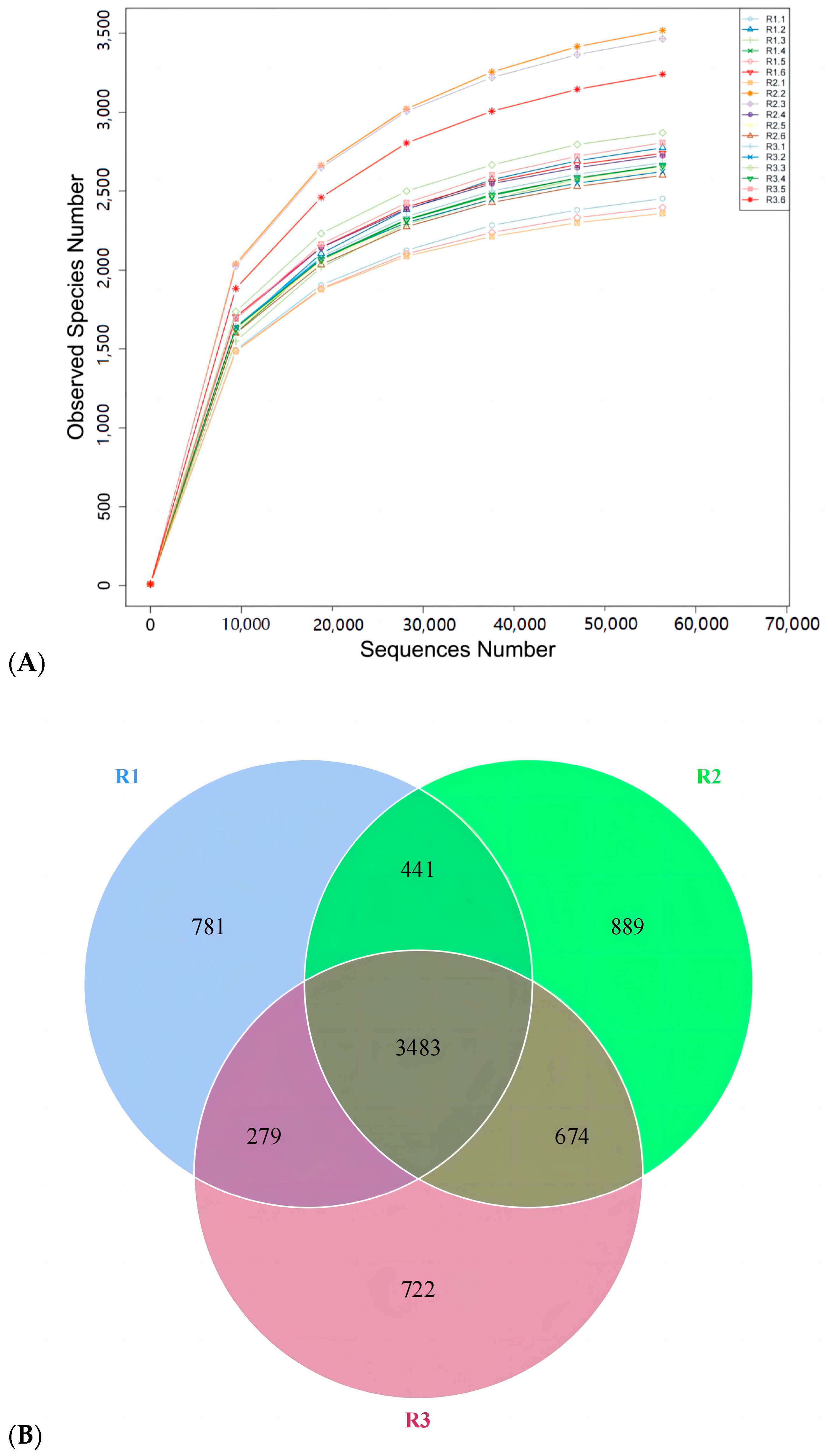
3.5. Effects of Feed Concentrate and FA Supplementation on Fecal Microflora Diversity in Grazing Yili Horses
3.6. Effects of Feed Concentrate and FA Supplementation on Alpha Diversity of Fecal Bacterial Phyla in Grazing Yili Horses
3.7. Effects of Feed Concentrate and FAs on Fecal Bacterial Phylum Abundance in Grazing Yili Horses
3.8. Effects of Feed Concentrate and FA Supplementation on Fecal Bacterial Family Abundance in Grazing Yili Horses
3.9. Effects of Concentrate and Fatty Acid Supplementation on Fecal Bacterial Genus Abundance in Grazing Yili Horses
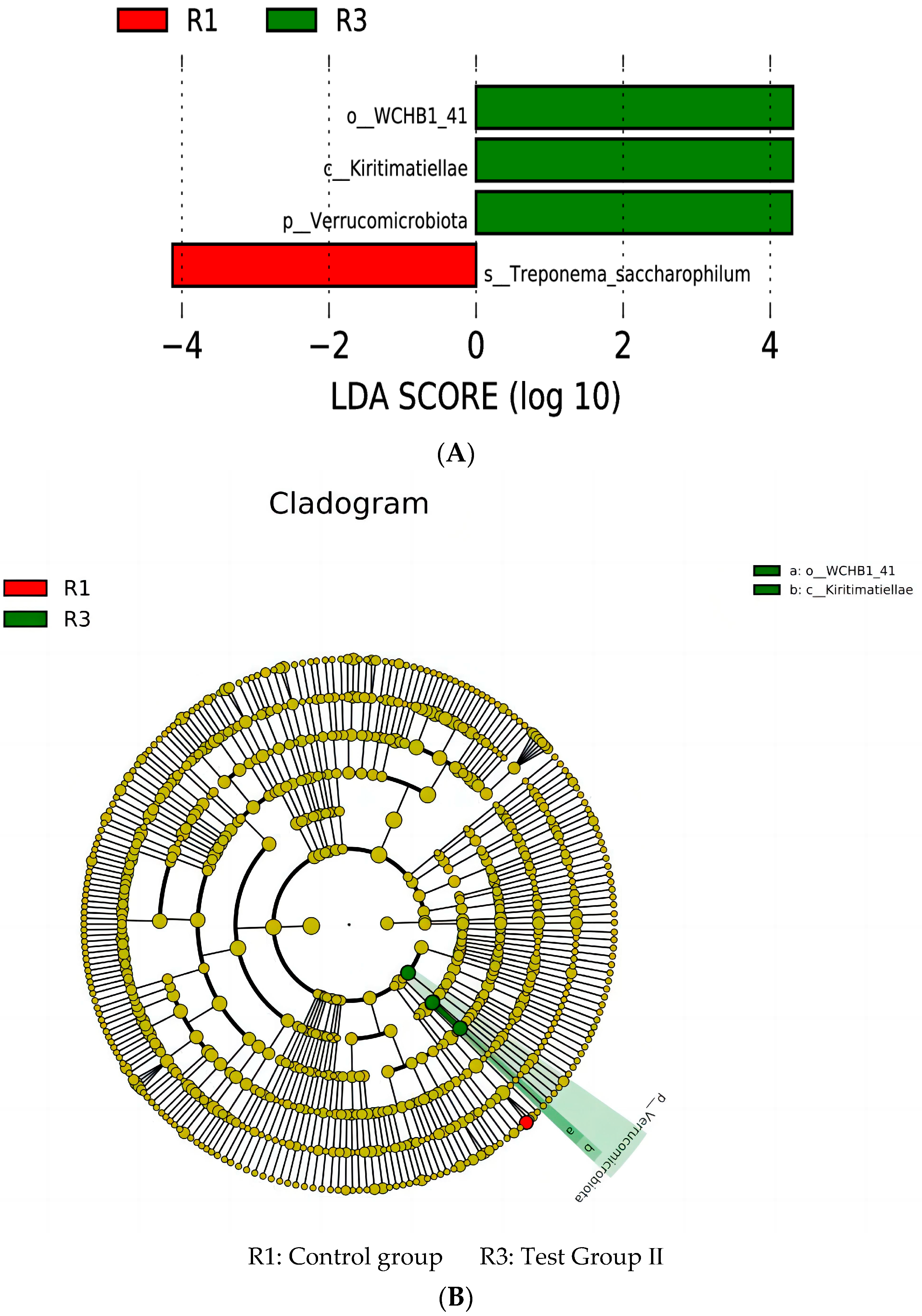
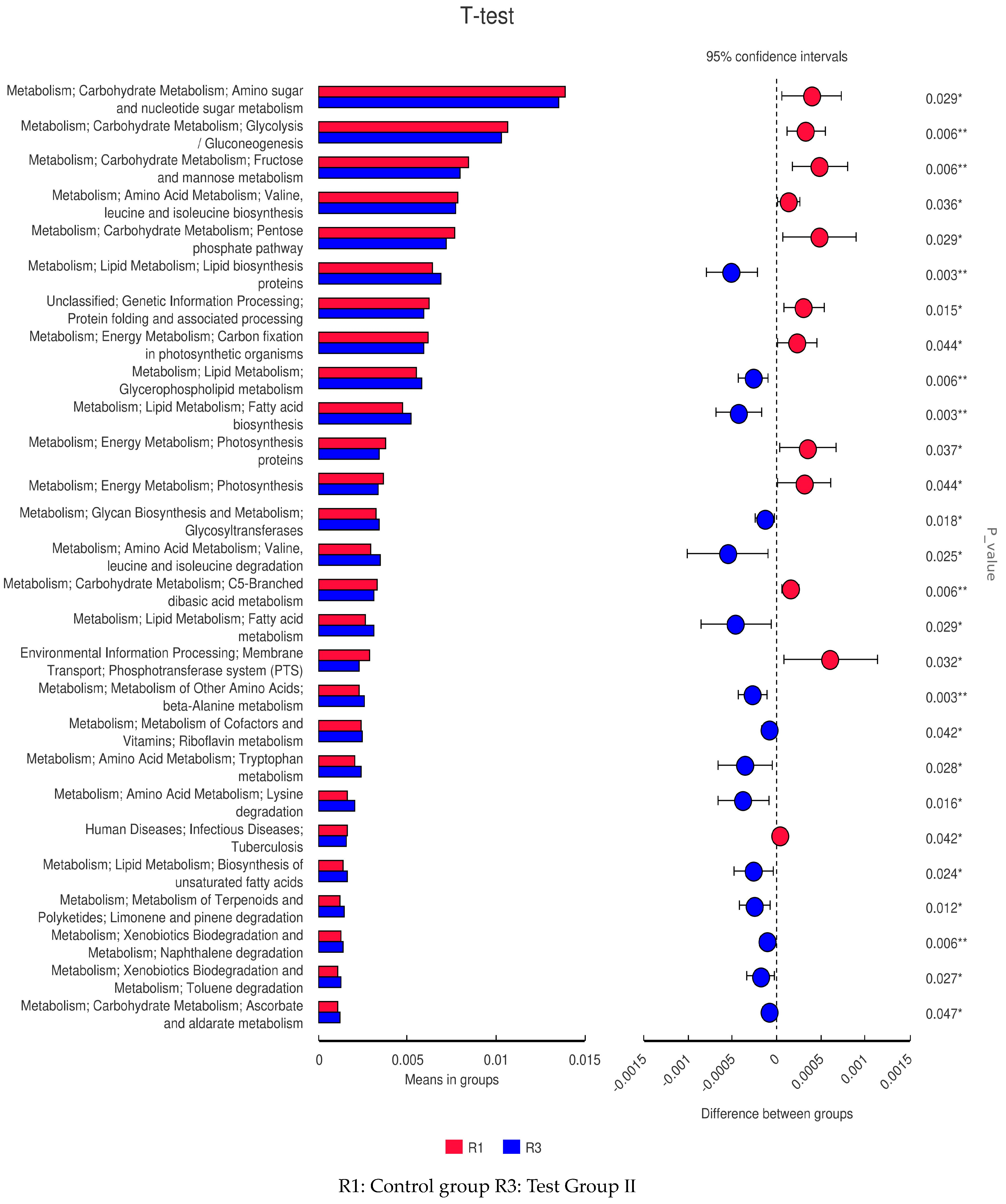
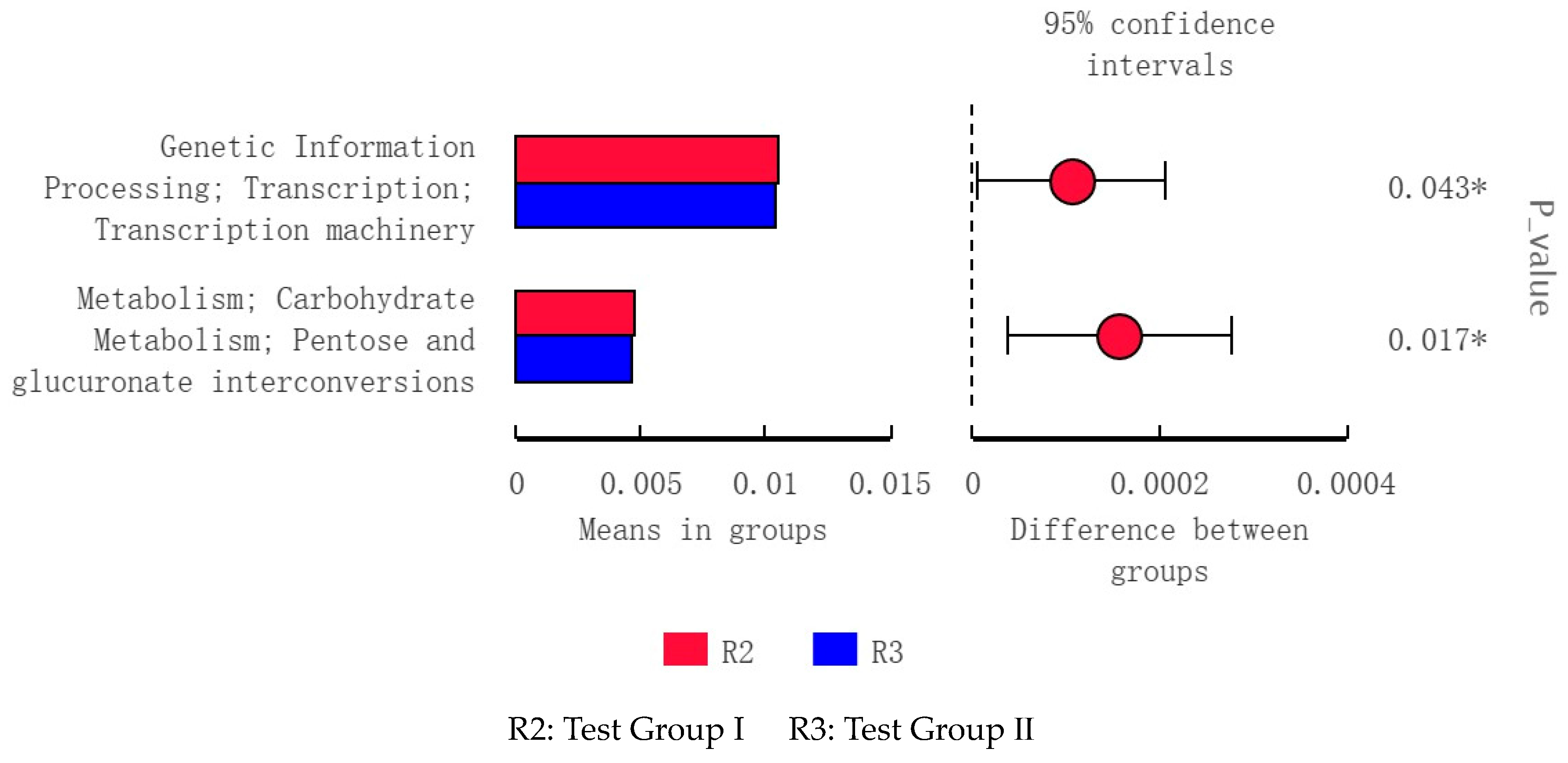
3.10. LEfSe Analysis and Tax4Fun Function Prediction of Fecal Bacterial Taxa in Grazing Yili Horses Supplemented with Feed Concentrate and FAs
3.11. PICRUSt Functional Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korosue, K.; Murase, H.; Sato, F.; Ishimaru, M.; Harada, T.; Watanabe, G.; Taya, K.; Nambo, Y. Successful induction of lactation in a barren Thoroughbred mare: Growth of a foal raised on induced lactation and the corresponding maternal hormone profiles. J. Vet. Med. Sci. 2012, 74, 995–1002. [Google Scholar] [CrossRef]
- Li, N.; Xie, Q.; Chen, Q.; Evivie, S.E.; Liu, D.; Dong, J.; Huo, G.; Li, B. Cow, Goat, and Mare Milk Diets Differentially Modulated the Immune System and Gut Microbiota of Mice Colonized by Healthy Infant Feces. J. Agric. Food Chem. 2020, 68, 15345–15357. [Google Scholar] [CrossRef]
- Yu, X.; Fang, C.; Liu, L.; Zhao, X.; Liu, W.; Cao, H.; Lv, S. Transcriptome study underling difference of milk yield during peak lactation of Kazakh horse. J. Equine Vet. Sci. 2021, 102, 103424. [Google Scholar] [CrossRef]
- Coenen, M.; Kienzle, E.; Vervuert, I.; Zeyner, A. Recent German Developments in the Formulation of Energy and Nutrient Requirements in Horses and the Resulting Feeding Recommendations. J. Equine Vet. Sci. 2011, 31, 219–229. [Google Scholar] [CrossRef]
- Mangwiro, Y.T.; Cuffe, J.S.; Vickers, M.H.; Reynolds, C.M.; Mahizir, D.; Anevska, K.; Gravina, S.; Romano, T.; Moritz, K.M.; Briffa, J.F.; et al. Maternal exercise alters rat fetoplacental stress response: Minimal effects of maternal growth restriction and high-fat feeding. Placenta 2021, 104, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Raspa, F.; Tarantola, M.; Muca, E.; Bergero, D.; Soglia, D.; Cavallini, D.; Vervuert, I.; Bordin, C.; De Palo, P.; Valle, E. Does Feeding Management Make a Difference to Behavioural Activities and Welfare of Horses Reared for Meat Production. Animals 2022, 12, 1740. [Google Scholar] [CrossRef]
- Raspa, F.; Vervuert, I.; Capucchio, M.T.; Colombino, E.; Bergero, D.; Forte, C.; Greppi, M.; Cavallarin, L.; Giribaldi, M.; Antoniazzi, S.; et al. A high-starch vs. high-fibre diet: Effects on the gut environment of the different intestinal compartments of the horse digestive tract. BMC Vet. Res. 2022, 18, 187. [Google Scholar] [CrossRef] [PubMed]
- Spadari, A.; Gialletti, R.; Gandini, M.; Valle, E.; Cerullo, A.; Cavallini, D.; Bertoletti, A.; Rinnovati, R.; Forni, G.; Scilimati, N.; et al. Short-Term Survival and Postoperative Complications Rates in Horses Undergoing Colic Surgery: A Multicentre Study. Animals 2023, 13, 1107. [Google Scholar] [CrossRef]
- Cavallini, D.; Penazzi, L.; Valle, E.; Raspa, F.; Bergero, D.; Formigoni, A.; Fusaro, I. When Changing the Hay Makes a Difference: A Series of Case Reports. J. Equine Vet. Sci. 2022, 113, 103940. [Google Scholar] [CrossRef] [PubMed]
- Vinassa, M.; Cavallini, D.; Galaverna, D.; Baragli, P.; Raspa, F.; Nery, J.; Valle, E. Palatability assessment in horses in relation to lateralization and temperament. Appl. Anim. Behav. Sci. 2020, 232, 105110. [Google Scholar] [CrossRef]
- Spers, R.C.; Spers, A.; Fernandes, W.R.; Visintin, J.A.; de Arruda, R.P.; Garcia, C.A. Effect of dietary supplementation with coco-nut babaçu oil on performance of lactating mares. Braz. J. Vet. Res. Anim. Sci. 2006, 43, 120–128. [Google Scholar] [CrossRef][Green Version]
- Doreau, M.; Boulot, S.; Bauchart, D.; Barlet, J.-P.; Martin-Rosset, W. Voluntary intake, milk production and plasma metabolites in nursing mares fed two different diets. J. Nutr. 1992, 122, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Rouel, J.; Leroux, C.; Ferlay, A.; Faulconnier, Y.; Legrand, P.; Chilliard, Y. Mammary lipid metabolism and milk fatty acid secretion in Alpine goats fed vegetable lipids. J. Dairy Sci. 2005, 88, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Davison, K.; Potter, G.; Greene, L.; Evans, J.; McMullan, W. Lactation and reproductive performance of mares fed added dietary fat during late gestation and early lactation. J. Equine Vet. Sci. 1991, 11, 111–115. [Google Scholar] [CrossRef]
- Ericsson, A.C.; Johnson, P.J.; Lopes, M.A.; Perry, S.C.; Lanter, H.R. A Microbiological Map of the Healthy Equine Gastrointestinal Tract. PLoS ONE 2016, 11, e0166523. [Google Scholar] [CrossRef] [PubMed]
- Vermorel, M.; Martin-rosset, W.; Vernet, J. Energy utilization of twelve forages or mixed diets for maintenance by sport horses. Livest. Prod. Sci. 1997, 47, 157–167. [Google Scholar] [CrossRef]
- Moneeb, A.H.M.; Hammam, A.R.A.; Ahmed, A.K.A.; Ahmed, M.E.; Alsaleem, K.A. Effect of fat extraction methods on the fatty acids composition of bovine milk using gas chromatography. Food Sci. Nutr. 2021, 9, 2936–2942. [Google Scholar] [CrossRef]
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. Wavelength-ratiometric and colorimetric probes for glucose determination. Dye. Pigment. 2006, 68, 159–163. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Weizhong, L.; Adam, G. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 2016, e2584. [Google Scholar] [CrossRef]
- Frederic, D.; Jean-francois, L.; MR, W.; CN, F. Allometric scaling of the elevation of maternal energy intake during lactation. Front. Zool. 2016, 13, 32. [Google Scholar]
- Stein, H.H. Nutrient requirementsof swine. Feedstuffs 2018, 90 (Suppl. S1), 15–21. [Google Scholar]
- Miraglia, N.; Burger, D.; Kapron, M.; Flanagan, J.; Langlois, B.; Martin-Rosset, W. Local animal resources and products in sustainable development: Role and potential of equids. Publ. Eur. Assoc. Anim. Prod. 2006, 118, 217. [Google Scholar]
- Hristov, A.N.; Degaetano, A.T.; Rotz, C.A.; Hollinger, D.Y. Climate change effects on livestock in the Northeast US and strategies for adaptation. Clim. Chang. 2018, 146, 33–45. [Google Scholar] [CrossRef]
- Laws, J.; Juniper, D.T.; Lean, I.J.; Amusquivar, E.; Herrera, E.; Dodds, P.F.; Clarke, L. Supplementing sow diets with palm oil during late gestation and lactation: Effects on milk production, sow hormonal profiles and growth and development of her offspring. Animal 2018, 12, 2578–2586. [Google Scholar] [CrossRef]
- Doreau, M.; Martin-Rosset, W. Animals that Produce Dairy Foods|Horse. In Encyclopedia of Dairy Sciences, 2nd ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 40, pp. 358–364. [Google Scholar]
- Harper, M.T.; Oh, J.; Melgar, A.; Nedelkov, K.; Risnen, S. Production effects of feeding extruded soybean meal to early-lactation dairy cows-ScienceDirect. J. Dairy Sci. 2019, 102, 8999–9016. [Google Scholar] [CrossRef]
- Kadegowda, A.K.G.; Piperova, L.S.; Delmonte, P.; Erdman, R.A. Abomasal Infusion of Butterfat Increases Milk Fat in Lactating Dairy Cows. J. Dairy Sci. 2008, 91, 2370–2379. [Google Scholar] [CrossRef]
- Dabadie, H.; Peuchant, E.; Bernard, M.; LeRuyet, P.; Mendy, F. Moderate intake of myristic acid in sn-2 position has beneficial lipidic effects and enhances DHA of cholesteryl esters in an interventional study. J. Nutr. Biochem. 2005, 16, 375–382. [Google Scholar] [CrossRef]
- Glasser, F.; Ferlay, A.; Chilliard, Y. Oilseed Lipid Supplements and Fatty Acid Composition of Cow Milk: A Meta-Analysis. J. Dairy Sci. 2008, 91, 4687–4703. [Google Scholar] [CrossRef]
- Massimo, M.; Martuzzi, F.; Summer, A. Mariani Primo. Protein and fat composition of mare’s milk: Some nutritional remarks with reference to human and cow’s milk. Int. Dairy J. 2002, 12, 869–877. [Google Scholar]
- Doreau, M.; Martuzzi, F. Milk yield of nursing and dairy mares. In Nutrition and Feeding of the Broodmare; Miraglia, N., Martin-Rosset, W., Eds.; No 120; French National Institute for Agriculture: Paris, France, 2006; pp. 57–64. [Google Scholar]
- Salimei, E. Animals that Produce Dairy Foods|Donkey. In Encyclopedia of Dairy Sciences, 2nd ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 365–373. [Google Scholar]
- Summer, A.; Sabbioni, A.; Formaggioni, P.; Mariani, P. Trend in ash and mineral element content of milk from Haflinger nursing mares throughout six lactation months. Livest. Prod. Sci. 2004, 88, 55–62. [Google Scholar] [CrossRef]
- Hosoi, S.; Honma, K.; Daimatsu, T.; Kiyokawa, M.; Aikawa, T.; Watanabe, S. Lower energy content of human milk than calculated using conversion factors. Pediatr. Int. 2005, 47, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, N.; Yamada, M.; Kan-No, T.; Kojima, T.; Kaneko, T.; Yonekubo, A. Macronutrient, mineral and trace element composition of breast milk from Japanese women. J. Trace Elem. Med. Biol. 2005, 19, 171–181. [Google Scholar] [CrossRef]
- Uniacke-Lowe, T. Encyclopedia of dairy sciences milk equid milk. In Encyclopedia of Dairy Sciences, 2nd ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 9, pp. 518–529. [Google Scholar]
- Aroua, M.; Jemmali, B.; Ben Said, S.; Kbaier, H.B.H.; Mahouachi, M. Physicochemical properties of North African donkey milk. Agric. Res. Technol. Open Access J. 2019, 21, 556155. [Google Scholar] [CrossRef]
- Malacarne, M.; Criscione, A.; Franceschi, P.; Bordonaro, S.; Formaggioni, P.; Marletta, D.; Summer, A. New insights into chemical and mineral composition of donkey milk throughout nine months of lactation. Animals 2019, 9, 1161–1174. [Google Scholar] [CrossRef]
- Fantuz, F.; Ferraro, S.; Todini, L.; Piloni, R.; Mariani, P.; Salimei, E. Donkey milk concentration of calcium, phosphorus, potassium, sodium and magnesium. Int. Dairy J. 2012, 24, 143–145. [Google Scholar] [CrossRef]
- Zeyner, A.; Bessert, J.; Gropp, J.M. Effect of feeding exercised horses on high-starch or high-fat diets for 390 days. Equine Vet. J. 2010, 34, 50–57. [Google Scholar] [CrossRef]
- Krasnow, S.M.; Steiner, R.A. Physiological Mechanisms Integrating Metabolism and Reproduction-ScienceDirect. In Knobil and Neill’s Physiology of Reproduction, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 2553–2625. [Google Scholar]
- Han, X.F.; Feng, F.J.; Yu, J.P.; Tang, S.X.; Wang, M. Effects of conjugated linoleic acid supplementation on growth, carcass characteristics and fatty acid profiles of muscle and fat in growing-finishing pigs. J. Anim. Feed. Sci. 2011, 20, 171–185. [Google Scholar] [CrossRef][Green Version]
- Li, J.Z.; Lei, Y.; Wang, Y.; Zhang, Y.X.; Ye, J.; Xia, X.Y.; Pan, X.M.; Li, P. Control of cholesterol biosynthesis, uptake and storage in hepatocytes by Cideb. BBA-Mol. Cell Biol. Lipids 2010, 1801, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Structure, J.H.B. Function and diversity of the healthy human microbiome. Nature 2012, 486, 53–82. [Google Scholar]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of Mammals and Their Gut Microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.J.; Brown, J.M.; Murray, R.D.; Getty, B.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Characterization of novel bovine gastrointestinal tract Treponema isolates and comparison with bovine digital dermatitis treponemes. Appl. Env. Microbiol. 2011, 77, 138–147. [Google Scholar] [CrossRef]
- Jiang, F.; Song, P.; Wang, H.; Zhang, J.; Liu, D.; Cai, Z.; Gao, H.; Chi, X.; Zhang, T. Comparative analysis of gut microbial composition and potential functions in captive forest and alpine musk deer. Appl. Microbiol. Biotechnol. 2022, 106, 1325–1339. [Google Scholar] [CrossRef]
- Kendle, M.M.; Angelica, T.V.; Aylwin, N.; Jan, K.; Frederic, S.; Di, Y.; Heidi, C.S.; Michael, S.R.; Fabienne, M.; David, A.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar]
- Caro-Quintero, A.; Ritalahti, K.M.; Cusick, K.D.; Löffler, F.E.; Konstantinidis, K.T. The chimeric genome of Sphaerochaeta: Nonspiral spirochetes that break with the prevalent dogma in spirochete biology. mBio 2012, 3, 12–25. [Google Scholar] [CrossRef]
- Su, S.; Zhao, Y.; Liu, Z.; Liu, G.; Du, M.; Wu, J.; Bai, D.; Li, B.; Bou, G.; Zhang, X.; et al. Characterization and comparison of the bacterial microbiota in different gastrointestinal tract compartments of Mongolian horses. Microbiol. Open 2020, 9, 1085–1101. [Google Scholar] [CrossRef]
- Gong, L.; Cao, W.; Chi, H.; Wang, J.; Zhang, H.; Liu, J.; Sun, B. Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Res. Int. 2018, 103, 84–102. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nagai, F.; Morotomi, M. Characterization of Phascolarctobacterium succinatutens sp. nov, an Asaccharolytic, Succinate-Utilizing Bacterium Isolated from Human Feces. Appl. Environ. Microbiol. 2011, 78, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Chen, Z.D.; Zhou, S.; Song, X.Z.; Ouyang, K.H.; Pan, K.; Xu, L.J.; Liu, C.J.; Qu, M.R. Effects of daidzein on performance, serum metabolites, nutrient digestibility, and fecal bacterial community in bull calves. Anim. Feed. Sci. Technol. 2017, 225, 87–96. [Google Scholar] [CrossRef]
- Michael, A.M.; Sara, K.L.; Philip, S.; Florin Timothy, H. Mucin dynamics and enteric pathogens. Nature reviews. Microbiology 2011, 9, 265–278. [Google Scholar]
- Caroline, A.M.; Daniela, B.; Ana, P.P.; Oliveira Bruno, C.M.; MaryRose, P.; Melissa, M.; Giovanni, W. Assessment of clinical and microbiota responses to fecal microbial transplantation in adult horses with diarrhea. PLoS ONE 2021, 16, e0244381. [Google Scholar]
- Edwards, J.E.; Shetty, S.A.; van den Berg, P.; Burden, F.; van Doorn, D.A.; Pellikaan, W.F.; Dijkstra, J.; Smidt, H. Multi-kingdom characterization of the core equine fecal microbiota based on multiple equine (sub) species. Anim. Microbiome 2020, 2, 231–268. [Google Scholar] [CrossRef] [PubMed]
- Caroline, A.M.; Oliveira, B.C.; Daniela, B.; Mary-Rose, P.; Melissa, M.; Sophie, S.; Alfredo, S.; Giovanni, W. The fecal microbiota of healthy donor horses and geriatric recipients undergoing fecal microbial transplantation for the treatment of diarrhea. PLoS ONE 2020, 15, e0230148. [Google Scholar]
- Byndloss, M.X.; Pernitzsch, S.R.; Bäumler, A.J. Healthy hosts rule within: Ecological forces shaping the gut microbiota. Mucosal Immunol. 2018, 11, 1299–1305. [Google Scholar] [CrossRef]
- Lindenberg, F.; Krych, L.; Fielden, J.; Kot, W.; Frøkiær, H.; van Galen, G.; Nielsen, D.S.; Hansen, A.K. Expression of immune regulatory genes correlate with the abundance of specific Clostridiales and Verrucomicrobia species in the equine ileum and cecum. Sci. Rep. 2019, 9, 12674. [Google Scholar] [CrossRef]
- Quercia, S.; Freccero, F.; Castagnetti, C.; Soverini, M.; Turroni, S.; Biagi, E.; Rampelli, S.; Lanci, A.; Mariella, J.; Chinellato, E.; et al. Early colonisation and temporal dynamics of the gut microbial ecosystem in Standardbred foals. Equine Vet. J. 2019, 51, 231–237. [Google Scholar] [CrossRef]



| Ingredient | Content | Nutrient (2) | Content |
|---|---|---|---|
| Barley | 55.54 | Dry matter | 89.25 |
| Corn | 36.00 | Crude protein | 14.56 |
| Soybean meal | 6.00 | Ether extract | 4.08 |
| CaHPO4 | 1.30 | Organic matter | 97.18 |
| Premix (1) | 1.16 | Neutral detergent fiber | 13.36 |
| Total | 100.00 | Acid detergent fiber | 5.18 |
| -- | -- | Ash | 2.82 |
| -- | -- | Calcium | 0.31 |
| -- | -- | Phosphorus | 0.46 |
| Items | Concentrate | Forage | Coated Fatty Acids |
|---|---|---|---|
| Butyric acid C4:0 | 0.43 | 0.82 | 0.24 |
| Hexanoic acid C6:0 | 0.06 | 0.43 | 0.49 |
| Octoic acid C8:0 | 0.25 | 1.17 | 0.37 |
| Decanoic acid C10:0 | 0.00 | 0.00 | 5.25 |
| Ricinoleic acid C11:0 | 0.00 | 0.00 | 0.02 |
| Lauric acid C12:0 | 0.00 | 4.06 | 44.83 |
| Tetradecanoic acid C14:0 | 0.12 | 0.86 | 18.42 |
| Pentadecanoic acid C15:0 | 0.03 | 0.00 | 0.02 |
| Palmitic acid C16:0 | 13.66 | 13.61 | 12.20 |
| Palmitoleic acid C16:1 | 0.12 | 0.33 | 0.02 |
| Margaric acid C17:0 | 0.05 | 0.32 | 0.01 |
| Heptadecanoic acid monoenoic acid C17:1 | 0.03 | 0.30 | 0.00 |
| Stearic acid C18:0 | 1.54 | 1.97 | 3.97 |
| Elaidic acid C18:1n9t | 0.03 | 0.00 | 0.00 |
| Oleic acid C18:1n9c | 25.17 | 3.02 | 3.91 |
| Linoleic acid C18:2n6c | 45.40 | 13.60 | 2.20 |
| γ- Linolenic acid C18:3n6 | 0.45 | 4.07 | 0.05 |
| α- Linolenic acid C18:3n3 | 2.44 | 28.67 | 0.56 |
| Arachidic acid C20:0 | 0.39 | 2.66 | 0.12 |
| Eicosaenoic acid C20:1 | 0.56 | 0.00 | 0.04 |
| Cis-11,14-eicosadienoic acid C20:2 | 0.06 | 0.00 | 0.00 |
| Behenic acid C22:0 | 0.20 | 1.11 | 0.00 |
| Total saturated fatty acids ∑SFA | 16.74 | 27 | 85.94 |
| Total unsaturated fat acids ∑UFA | 74.27 | 49.99 | 6.78 |
| Total monounsaturated fatty acids ∑MUFA | 25.92 | 3.65 | 3.97 |
| Total unsaturated fat acids ∑PUFA | 48.34 | 46.34 | 2.80 |
| Total saturated fatty acid/total unsaturated fat acid ∑SFA/∑UFA | 0.23 | 0.54 | 12.68 |
| Items | Control Group | Test Group I | Test Group Ⅱ | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|
| Grous | Time | G × T | |||||
| Butterfat content (%) | 1.47 | 1.45 | 1.49 | 0.07 | 0.944 | 0.104 | 0.897 |
| Milk fat production (g/d) | 3.59 B | 3.85 B | 4.77 A | 0.19 | <0.001 | 0.088 | 0.793 |
| Milk protein percentage (%) | 1.63 | 1.61 | 1.58 | 0.04 | 0.643 | 0.918 | 0.982 |
| Milk protein yield (g/d) | 3.97 B | 4.27 B | 5.06 A | 0.11 | <0.001 | 0.905 | 0.981 |
| Lactose percentage (%) | 6.68 | 6.76 | 6.76 | 0.04 | 0.307 | 0.563 | 0.555 |
| Lactose production (g/d) | 16.29 C | 17.90 B | 21.70 A | 0.11 | <0.001 | 0.625 | 0.608 |
| Total solids (%) | 9.86 | 9.90 | 9.88 | 0.10 | 0.963 | 0.67 | 0.469 |
| Somatic cell number (Thousand/mL) | 21.25 | 13.33 | 18.25 | 4.67 | 0.489 | 0.331 | 0.418 |
| Solid no fat (%) | 8.54 | 8.58 | 8.57 | 0.06 | 0.858 | 0.654 | 0.681 |
| Urea nitrogen (mg/dL) | 26.64 aA | 24.24 bB | 24.58 bAB | 0.61 | 0.02 | <0.001 | 0.967 |
| Items | Control Group | Test Group I | Test Group Ⅱ | SEM | p-Value |
|---|---|---|---|---|---|
| Butyric acid C4:0 | 0.34 | 0.42 | 0.37 | 0.02 | 0.390 |
| Hexanoic acid C6:0 | 0.22 | 0.20 | 0.21 | 0.01 | 0.895 |
| Octoic acid C8:0 | 1.22 | 1.20 | 1.29 | 0.05 | 0.742 |
| Decanoic acid C10:0 | 2.83 | 2.94 | 3.26 | 0.13 | 0.380 |
| Ricinoleic acid C11:0 | 0.35 | 0.37 | 0.38 | 0.02 | 0.805 |
| Lauric acid C12:0 | 3.93 b | 4.32 b | 8.78 a | 0.55 | <0.001 |
| Tetradecanoic acid C14:0 | 5.13 b | 5.64 b | 7.50 a | 0.27 | <0.001 |
| Myristoleic acid C14:1 | 0.77 | 0.77 | 0.91 | 0.03 | 0.091 |
| Pentadecanoic acid C15:0 | 0.32 a | 0.29 ab | 0.23 b | 0.01 | 0.013 |
| Palmitic acid C16:0 | 19.14 | 20.53 | 19.16 | 0.32 | 0.124 |
| Palmitoleic acid C16:1 | 7.98 | 7.77 | 6.93 | 0.23 | 0.139 |
| Margaric acid C17:0 | 0.18 | 0.17 | 0.16 | 0.01 | 0.738 |
| Heptadecanoic acid monoenoic acid C17:1 | 0.61 a | 0.59 a | 0.46 b | 0.02 | 0.005 |
| Stearic acid C18:0 | 0.67 | 0.73 | 0.78 | 0.03 | 0.265 |
| Elaidic acid C18:1n9t | 0.19 | 0.21 | 0.17 | 0.01 | 0.268 |
| Oleic acid C18:1n9c | 12.66 | 12.63 | 13.31 | 0.20 | 0.304 |
| Linoleic acid C18:2n6c | 7.90 a | 7.73 ab | 7.24 b | 0.12 | 0.056 |
| γ- Linolenic acid C18:3n6 | 8.31 a | 7.90 a | 6.32 b | 0.26 | <0.001 |
| α- Linolenic acid C18:3n3 | 20.55 a | 18.74 a | 15.32 b | 0.62 | <0.001 |
| Cis-11,14-eicosadienoic acid C20:2 | 0.15 b | 0.13 b | 0.18 a | 0.01 | 0.002 |
| Cis-8,11,14-eicosotrienic acid C20:3n6 | 0.16 | 0.15 | 0.17 | 0.01 | 0.704 |
| Cis-11,14,17-eicosotrienic acid C20:3n3 | 0.41 | 0.40 | 0.39 | 0.01 | 0.928 |
| Total saturated fatty acids ∑SFA | 34.35 b | 36.81 b | 42.12 a | 0.94 | <0.001 |
| Total unsaturated fat acids ∑UFA | 59.70 a | 57.03 a | 51.38 b | 1.04 | <0.001 |
| Total monounsaturated fatty acids ∑MUFA | 22.22 | 21.97 | 21.77 | 0.28 | 0.824 |
| Total unsaturated fat acids ∑PUFA | 37.48 a | 35.06 a | 29.61 b | 0.93 | <0.001 |
| Total saturated fatty acid/total unsaturated fat acid ∑SFA/∑UFA | 0.58 b | 0.65 b | 0.82 a | 0.03 | <0.001 |
| Items | Control Group | Test Group I | Test Group Ⅱ | SEM | p-Value |
|---|---|---|---|---|---|
| Observed species | 2163.67 | 2884.33 | 2814.00 | 76.73 | 0.348 |
| Shannon index | 9.30 | 9.59 | 9.53 | 0.06 | 0.108 |
| Simpson index | 0.99 | 1.00 | 1.00 | 0.0006 | 0.426 |
| Chao1 index | 2811.71 | 3084.11 | 3026.10 | 81.91 | 0.382 |
| ACE index | 2829.28 | 3107.67 | 3045.80 | 83.87 | 0.386 |
| Goods coverage (%) | 0.99 | 0.99 | 0.99 | 0.0003 | 0.883 |
| Items | Control Group | Test Group I | Test Group Ⅱ | SEM | p-Value |
|---|---|---|---|---|---|
| Bacteroidetes | 44.20 | 42.75 | 43.51 | 1.30 | 0.911 |
| Firmicutes | 32.59 | 33.60 | 31.52 | 1.02 | 0.736 |
| Spirochaetes | 6.41 | 3.39 | 2.98 | 0.87 | 0.221 |
| Verrucomicrobia | 3.92 c | 6.81 b | 7.92 a | 0.63 | 0.017 |
| Unidentified_Bacterri | 2.27 | 2.61 | 3.01 | 0.21 | 0.372 |
| Proteobacteria | 1.20 | 1.66 | 2.17 | 0.25 | 0.309 |
| Euryarchaeota | 0.66 | 0.43 | 0.52 | 0.14 | 0.817 |
| Halobacterota | 0.72 | 0.43 | 0.52 | 0.14 | 0.248 |
| Fibrobacterota | 1.25 | 0.87 | 1.00 | 0.09 | 0.186 |
| Acidobacteriota | 0.18 | 0.32 | 0.23 | 0.08 | 0.804 |
| Others | 6.60 | 7.14 | 7.05 | 0.35 | 0.817 |
| Items | Control Group | Test Group I | Test Group Ⅱ | SEM | p-Value |
|---|---|---|---|---|---|
| Rikenellaceae | 11.95 | 12.84 | 13.77 | 0.98 | 0.773 |
| Lachnospiraceae | 11.18 | 10.39 | 9.96 | 0.61 | 0.733 |
| Spirochaetaceae | 6.26 | 3.25 | 2.82 | 0.86 | 0.213 |
| Prevotellaceae | 8.39 | 6.42 | 5.88 | 0.54 | 0.138 |
| p-251-o5 | 8.72 | 6.34 | 6.44 | 0.67 | 0.279 |
| F082 | 6.04 | 7.80 | 7.62 | 0.39 | 0.123 |
| Bacteroidales_RF16_group | 2.37 | 2.30 | 2.95 | 0.29 | 0.632 |
| Oscillospiraceae | 3.55 | 4.36 | 4.25 | 0.17 | 0.107 |
| Clostridiaceae | 0.96 | 0.48 | 0.52 | 0.22 | 0.644 |
| Ruminococcaceae | 2.42 | 2.43 | 2.46 | 0.13 | 0.992 |
| Others | 38.16 | 43.38 | 43.32 | 1.16 | 0.104 |
| Items | Control Group | Test Group I | Test Group Ⅱ | SEM | p-Value |
|---|---|---|---|---|---|
| Treponema | 6.09 | 3.13 | 2.74 | 0.85 | 0.219 |
| Rikenellaceae_RC9_gut_group | 9.33 | 9.60 | 10.33 | 0.69 | 0.849 |
| Clostridium_sensu_stricto_1 | 0.90 | 0.34 | 0.34 | 0.22 | 0.518 |
| Prevotellaceae_UCG-001 | 1.78 | 1.11 | 1.02 | 0.18 | 0.171 |
| UCG-004 | 0.89 | 0.63 | 0.65 | 0.15 | 0.750 |
| Prevotellaceae_UCG-004 | 1.24 | 1.61 | 1.49 | 0.11 | 0.405 |
| Ruminococcus | 2.00 | 1.87 | 1.80 | 0.12 | 0.810 |
| Prevotellaceae_UCG-003 | 1.85 | 1.21 | 1.21 | 0.13 | 0.065 |
| Lachnospiraceae_UCG-009 | 1.21 | 0.89 | 0.71 | 0.12 | 0.230 |
| Faecalibaculum | 0.53 | 0.44 | 0.43 | 0.17 | 0.227 |
| Others | 73.98 b | 79.17 a | 79.72 a | 1.00 | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Zhang, W.; Sun, S.; Mei, Y.; Zhao, G.; Yang, K. Effect of Supplementary Feeding on Milk Volume, Milk Composition, Blood Biochemical Index, and Fecal Microflora Diversity in Grazing Yili Mares. Animals 2023, 13, 2415. https://doi.org/10.3390/ani13152415
Lu H, Zhang W, Sun S, Mei Y, Zhao G, Yang K. Effect of Supplementary Feeding on Milk Volume, Milk Composition, Blood Biochemical Index, and Fecal Microflora Diversity in Grazing Yili Mares. Animals. 2023; 13(15):2415. https://doi.org/10.3390/ani13152415
Chicago/Turabian StyleLu, Hao, Wenjie Zhang, Shuo Sun, Yingying Mei, Guodong Zhao, and Kailun Yang. 2023. "Effect of Supplementary Feeding on Milk Volume, Milk Composition, Blood Biochemical Index, and Fecal Microflora Diversity in Grazing Yili Mares" Animals 13, no. 15: 2415. https://doi.org/10.3390/ani13152415
APA StyleLu, H., Zhang, W., Sun, S., Mei, Y., Zhao, G., & Yang, K. (2023). Effect of Supplementary Feeding on Milk Volume, Milk Composition, Blood Biochemical Index, and Fecal Microflora Diversity in Grazing Yili Mares. Animals, 13(15), 2415. https://doi.org/10.3390/ani13152415





