Effect of Honey, Coenzyme Q10, and β-Carotene/α-Tocopherol as Novel Additives in Rabbit-Sperm Cryopreservation Extender
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals and Housing Conditions
2.3. Experimental Design
2.4. Semen Samples Collection
2.5. Hormonal Seminal Plasma Analyses
2.6. Sperm Cryopreservation
2.7. Post-Thawing Sperm Analyses
2.7.1. Subjective Motility
2.7.2. Concentration
2.7.3. Viability and Acrosomal Integrity
2.7.4. Mitochondrial Status
2.7.5. ProAKAP4 Analysis
2.8. Statistical Analyses
3. Results
3.1. Fresh Sperm-Quality Analyses
3.2. SP Analyses
3.2.1. Validation of the EIA: Cortisol, Testosterone, and AMH
3.2.2. Cortisol, Testosterone, and AMH Level Analyses of SP
3.3. Honey Addition of 2.5% Improves the Post-Thawing Motility but Not the Viability (Experiment 1)
3.4. CoQ10 Addition Is Deleterious for the Sperm Cryopreservation, Whereas No Effect Has Been Detected in the β-Carotene/α-Tocopherol Treatment Groups (Experiment 2)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvariño, J.M.R. Reproductive Performance of Male Rabbits. In Proceedings of the 7th World Rabbit Congress, Valencia, Spain, 4–7 July 2000. [Google Scholar]
- Carluccio, A.; Robbe, D.; Amicis, I.D.; Contri, A.; Tosi, U.; Russo, F.; Paoletti, M. Technical Note: Artificial Insemination in Rabbits: Laboratory and Field Trial with Three Different Semen Extenders. World Rabbit. Sci. 2004, 12, 65–79. [Google Scholar] [CrossRef]
- Mocé, E.; Vicente, J.S. Rabbit Sperm Cryopreservation: A Review. Anim. Reprod. Sci. 2009, 110, 1–24. [Google Scholar] [CrossRef]
- Rosato, M.P.; Iaffaldano, N. Cryopreservation of Rabbit Semen: Comparing the Effects of Different Cryoprotectants, Cryoprotectant-Free Vitrification, and the Use of Albumin plus Osmoprotectants on Sperm Survival and Fertility after Standard Vapor Freezing and Vitrification. Theriogenology 2013, 79, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F. The Causes of Reduced Fertility with Cryopreserved Semen. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Barbas, J.P.; Mascarenhas, R.D. Cryopreservation of Domestic Animal Sperm Cells. Cell Tissue Bank. 2009, 10, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Amidi, F.; Pazhohan, A.; Shabani Nashtaei, M.; Khodarahmian, M.; Nekoonam, S. The Role of Antioxidants in Sperm Freezing: A Review. Cell Tissue Bank. 2016, 17, 745–756. [Google Scholar] [CrossRef]
- Darin-Bennett, A.; White, I.G. Influence of the Cholesterol Content of Mammalian Spermatozoa on Susceptibility to Cold-Shock. Cryobiology 1977, 14, 466–470. [Google Scholar] [CrossRef]
- Isachenko, E.; Isachenko, V.; Katkov, I.I.; Dessole, S.; Nawroth, F. Vitrification of Mammalian Spermatozoa in the Absence of Cryoprotectants: From Past Practical Difficulties to Present Success. Reprod. Biomed. Online 2003, 6, 191–200. [Google Scholar] [CrossRef]
- Mocé, E.; Vicente, J.S.; Lavara, R. Effect of Freezing–Thawing Protocols on the Performance of Semen from Three Rabbit Lines after Artificial Insemination. Theriogenology 2003, 60, 115–123. [Google Scholar] [CrossRef]
- Majzoub, A.; Agarwal, A. Antioxidants in Sperm Cryopreservation. In Male Infertility; Springer International Publishing: Cham, Switzerland, 2020; pp. 671–678. [Google Scholar]
- Marcantonini, G.; Bartolini, D.; Zatini, L.; Costa, S.; Passerini, M.; Rende, M.; Luca, G.; Basta, G.; Murdolo, G.; Calafiore, R.; et al. Natural Cryoprotective and Cytoprotective Agents in Cryopreservation: A Focus on Melatonin. Molecules 2022, 27, 3254. [Google Scholar] [CrossRef]
- Ball, D.W. The Chemical Composition of Honey. J. Chem. Educ. 2007, 84, 1643. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; Zaat, S.A.J. Antibacterial Components of Honey. IUBMB Life 2012, 64, 48–55. [Google Scholar] [CrossRef]
- Nasreen, S.; Awan, M.A.; Ul-Husna, A.; Rakha, B.A.; Ansari, M.S.; Holt, W.; Akhter, S. Honey as an Alternative to Antibiotics for Cryopreservation of Nili-Ravi Buffalo Bull Spermatozoa. Biopreserv. Biobank. 2020, 18, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Jerez-Ebensperger, R.A.; Luño, V.; Olaciregui, M.; González, N.; de Blas, I.; Gil, L. Effect of Pasteurized Egg Yolk and Rosemary Honey Supplementation on Quality of Cryopreserved Ram Semen. Small Rumin. Res. 2015, 130, 153–156. [Google Scholar] [CrossRef]
- Banday, M.N.; Lone, F.A.; Rasool, F.; Rather, H.A.; Rather, M.A. Does Natural Honey Act as an Alternative to Antibiotics in the Semen Extender for Cryopreservation of Crossbred Ram Semen? Iran. J. Vet. Res. 2017, 18, 258–263. [Google Scholar]
- Fakhrildin, M.-B.M.-R.; Alsaadi, R.A.-R. Honey Supplementation to Semen-Freezing Medium Improves Human Sperm Parameters Post-Thawing. J. Fam. Reprod. Health 2014, 8, 27–31. [Google Scholar]
- El-Sheshtawy, R.I.; El-Badry, D.A.; El-Sisy, G.A.; El-Nattat, W.S.; Abo Almaaty, A.M. Natural Honey as a Cryoprotectant to Improve Arab Stallion Post-Thawing Sperm Parameters. Asian Pac. J. Reprod. 2016, 5, 331–334. [Google Scholar] [CrossRef]
- El-Sheshtawy, R.; El-Nattat, W.; Sabra, H.; Ali, A. Effect of Honey Solution on Semen Preservability of Local Breeds of Cattle Bulls. World Appl. Sci. J. 2014, 32, 2076–2078. [Google Scholar]
- Yimer, N.; Muhammad, N.; Sarsaifi, K.; Rosnina, Y.; Wahid, H.; Khumran, A.; Kaka, A. Effect of Honey Supplementation into Tris Extender on Cryopreservation of Bull Sper-Matozoa. Mysore J. Agric. Sci. 2015, 18, 47–54. [Google Scholar]
- Lan, Q.; Xie, Y.; Pan, J.; Chen, Q.; Xiao, T.; Fang, S. The Antibacterial and Antioxidant Roles of Buckwheat Honey (BH) in Liquid Preservation of Boar Semen. BioMed Res. Int. 2021, 2021, 5573237. [Google Scholar] [CrossRef]
- Catalán, J.; Yánez-Ortiz, I.; Tvarijonaviciute, A.; González-Arostegui, L.G.; Rubio, C.P.; Yeste, M.; Miró, J.; Barranco, I. Impact of Seminal Plasma Antioxidants on Donkey Sperm Cryotolerance. Antioxidants 2022, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Riesco, M.F.; Alvarez, M.; Anel-Lopez, L.; Neila-Montero, M.; Palacin-Martinez, C.; Montes-Garrido, R.; Boixo, J.C.; de Paz, P.; Anel, L. Multiparametric Study of Antioxidant Effect on Ram Sperm Cryopreservation—From Field Trials to Research Bench. Animals 2021, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Baishya, S.K.; Govindasamy, K.; Deka, B.C.; Biswas, R.K.; Sinha, S.; Singh, M. Effect of Reduced Glutathione, Water Soluble Vitamin E Analogue and Butylated Hydroxytoluene on the Post Thaw Characteristics of Boar Spermatozoa. Cryo Lett. 2018, 39, 227–234. [Google Scholar]
- Pranay Kumar, K.; Swathi, B.; Shanmugam, M. Effect of Supplementing Vitamin e Analogues on Post-Thaw Semen Parameters and Fertility in Chicken. Br. Poult. Sci. 2019, 60, 340–345. [Google Scholar] [CrossRef]
- Domínguez-Rebolledo, Á.E.; Fernández-Santos, M.R.; Bisbal, A.; Ros-Santaella, J.L.; Ramón, M.; Carmona, M.; Martínez-Pastor, F.; Garde, J.J. Improving the Effect of Incubation and Oxidative Stress on Thawed Spermatozoa from Red Deer by Using Different Antioxidant Treatments. Reprod. Fertil. Dev. 2010, 22, 856–870. [Google Scholar] [CrossRef]
- Mata-Campuzano, M.; Álvarez-Rodríguez, M.; Álvarez, M.; Tamayo-Canul, J.; Anel, L.; de Paz, P.; Martínez-Pastor, F. Post-Thawing Quality and Incubation Resilience of Cryopreserved Ram Spermatozoa Are Affected by Antioxidant Supplementation and Choice of Extender. Theriogenology 2015, 83, 520–528. [Google Scholar] [CrossRef]
- Mehdipour, M.; Kia, H.D.; Najafi, A.; Mohammadi, H.; Álvarez-Rodriguez, M. Effect of Crocin and Naringenin Supplementation in Cryopreservation Medium on Post-Thaw Rooster Sperm Quality and Expression of Apoptosis Associated Genes. PLoS ONE 2020, 15, e0241105. [Google Scholar] [CrossRef]
- Eriani, K.; Azhar, A.; Ihdina, M.; Rosadi, B.; Rizal, M.; Boediono, A. Quality Enhancement of Aceh Swamp Buffalo (Bubalus Bubalis) Frozen Semen by Supplementing β-Carotene. Trop. Anim. Sci. J. 2018, 41, 1–7. [Google Scholar] [CrossRef]
- Alcázar-Fabra, M.; Navas, P.; Brea-Calvo, G. Coenzyme Q Biosynthesis and Its Role in the Respiratory Chain Structure. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016, 1857, 1073–1078. [Google Scholar] [CrossRef]
- Talevi, R.; Barbato, V.; Fiorentino, I.; Braun, S.; Longobardi, S.; Gualtieri, R. Protective Effects of in Vitro Treatment with Zinc, d-Aspartate and Coenzyme Q10 on Human Sperm Motility, Lipid Peroxidation and DNA Fragmentation. Reprod. Biol. Endocrinol. 2013, 11, 81. [Google Scholar] [CrossRef]
- Safarinejad, M.R. Efficacy of Coenzyme Q10 on Semen Parameters, Sperm Function and Reproductive Hormones in Infertile Men. J. Urol. 2009, 182, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.A.M.; Canisso, I.F.; Bandeira, R.S.; Scheeren, V.F.C.; Freitas-Dell’Aqua, C.P.; Alvarenga, M.A.; Papa, F.O.; Dell’Aqua, J.A. Effects of Coenzyme Q10 on Semen Cryopreservation of Stallions Classified as Having Good or Bad Semen Freezing Ability. Anim. Reprod. Sci. 2018, 192, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Lançoni, R.; Celeghini, E.C.C.; Júnior, V.D.G.; de Carvalho, C.P.T.; Zoca, G.B.; Garcia-Oliveros, L.N.; Batissaco, L.; Oliveira, L.Z.; de Arruda, R.P. Coenzyme Q-10 Improves Preservation of Mitochondrial Functionality and Actin Structure of Cryopreserved Stallion Sperm. Anim. Reprod. 2021, 18, e20200218. [Google Scholar] [CrossRef] [PubMed]
- Appiah, M.O.; Asante-Badu, B.; Zhao, J.; Liu, H.; Wang, J.; Lu, W. Possible Protective Mechanisms of Coenzyme Q10 Action on Spermatozoa During Cryopreservation or Cooled-Stored Condition. Cryo Lett. 2020, 41, 246–256. [Google Scholar]
- de Albuquerque Lagares, M.; da Silva, G.C.; Cortes, S.F.; Luz, S.B.; de Resende, A.C.; Alves, N. de C.; Wenceslau, R.R.; Stahlberg, R. Does Coenzyme Q10 Exert Antioxidant Effect on Frozen Equine Sperm? J. Equine Vet. Sci. 2020, 88, 102964. [Google Scholar] [CrossRef]
- Rossi, M.; Falomo, M.E.; Mantovani, R. Role of Coenzyme Q and Vitamin E on Stallion Semen Motility Evaluated Both in Frozen and Cooled-Stored Semen. Ital. J. Anim. Sci. 2016, 15, 595–603. [Google Scholar] [CrossRef]
- Chavoshi Nezhad, N.; Vahabzadeh, Z.; Allahveisie, A.; Rahmani, K.; Raoofi, A.; Rezaie, M.J.; Rezaei, M.; Partovyan, M. The Effect of L-Carnitine and Coenzyme Q10 on the Sperm Motility, DNA Fragmentation, Chromatin Structure and Oxygen Free Radicals during, before and after Freezing in Oligospermia Men. Urol. J. 2021, 18, 330–336. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Martinez, E.A.; Calvete, J.J.; Peña Vega, F.J.; Roca, J. Seminal Plasma: Relevant for Fertility? Int. J. Mol. Sci. 2021, 22, 4368. [Google Scholar] [CrossRef]
- Hennessy, M.B. Hypothalamic-Pituitary-Adrenal Responses to Brief Social Separation. Neurosci. Biobehav. Rev. 1997, 21, 11–29. [Google Scholar] [CrossRef]
- Pani, L.; Porcella, A.; Gessa, G.L. The Role of Stress in the Pathophysiology of the Dopaminergic System. Mol. Psychiatry 2000, 5, 14–21. [Google Scholar] [CrossRef]
- Walker, W.H. Molecular Mechanisms of Testosterone Action in Spermatogenesis. Steroids 2009, 74, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Cai, J.; Zhang, C.; Jin, R.; Bai, S.; Yao, F.; Ding, H.; Zhao, B.; Chen, Y.; Wu, X.; et al. Semen Quality and Seminal Plasma Metabolites in Male Rabbits (Oryctolagus cuniculus) under Heat Stress. PeerJ 2023, 11, e15112. [Google Scholar] [CrossRef]
- Martínez-Fresneda, L.; O’Brien, E.; López Sebastián, A.; Velázquez, R.; Toledano-Díaz, A.; Tesfaye, D.; Schellander, K.; García-Vázquez, F.A.; Santiago-Moreno, J. In Vitro Supplementation of Testosterone or Prolactin Affects Spermatozoa Freezability in Small Ruminants. Domest. Anim. Endocrinol. 2020, 72, 106372. [Google Scholar] [CrossRef] [PubMed]
- La Marca, A.; Sighinolfi, G.; Radi, D.; Argento, C.; Baraldi, E.; Artenisio, A.C.; Stabile, G.; Volpe, A. Anti-Mullerian Hormone (AMH) as a Predictive Marker in Assisted Reproductive Technology (ART). Hum. Reprod. Update 2010, 16, 113–130. [Google Scholar] [CrossRef]
- Nery, S.F.; Vieira, M.a.F.; Dela Cruz, C.; Lobach, V.N.M.; Del Puerto, H.L.; Torres, P.B.; Rocha, A.L.L.; Reis, A.B.; Reis, F.M. Seminal Plasma Concentrations of Anti-Müllerian Hormone and Inhibin B Predict Motile Sperm Recovery from Cryopreserved Semen in Asthenozoospermic Men: A Prospective Cohort Study. Andrology 2014, 2, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.B.; Turner, R.M.O.; Burkert, K.L.; Butt, H.V.S.; Gerton, G.L. Conservation and Function of a Bovine Sperm A-Kinase Anchor Protein Homologous to Mouse AKAP82. Biol. Reprod. 1999, 61, 335–342. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, H.; Park, A.J.; O’Brien, D.A.; Shaw, G.; Renfree, M.B. A-Kinase Anchoring Protein 4 Has a Conserved Role in Mammalian Spermatogenesis. Reproduction 2009, 137, 645–653. [Google Scholar] [CrossRef]
- Nixon, B.; De Iuliis, G.N.; Hart, H.M.; Zhou, W.; Mathe, A.; Bernstein, I.R.; Anderson, A.L.; Stanger, S.J.; Skerrett-Byrne, D.A.; Fairuz Jamaluddin, M.B.; et al. Proteomic Profiling of Mouse Epididymosomes Reveals Their Contributions to Post-Testicular Sperm Maturation. Mol. Cell. Proteom. 2019, 18, S91–S108. [Google Scholar] [CrossRef]
- Luconi, M.; Carloni, V.; Marra, F.; Ferruzzi, P.; Forti, G.; Baldi, E. Increased Phosphorylation of AKAP by Inhibition of Phosphatidylinositol 3-Kinase Enhances Human Sperm Motility through Tail Recruitment of Protein Kinase A. J. Cell Sci. 2004, 117, 1235–1246. [Google Scholar] [CrossRef]
- Blommaert, D.; Sergeant, N.; Delehedde, M.; Jouy, N.; Mitchell, V.; Franck, T.; Donnay, I.; Lejeune, J.P.; Serteyn, D. Expression, Localization, and Concentration of A-Kinase Anchor Protein 4 (AKAP4) and Its Precursor (ProAKAP4) in Equine Semen: Promising Marker Correlated to the Total and Progressive Motility in Thawed Spermatozoa. Theriogenology 2019, 131, 52–60. [Google Scholar] [CrossRef]
- Brown, P.R.; Miki, K.; Harper, D.B.; Eddy, E.M. A-Kinase Anchoring Protein 4 Binding Proteins in the Fibrous Sheath of the Sperm Flagellum. Biol. Reprod. 2003, 68, 2241–2248. [Google Scholar] [CrossRef]
- Blommaert, D.; Sergeant, N.; Delehedde, M.; Donnay, I.; Lejeune, J.P.; Franck, T.; Serteyn, D. First Results about ProAKAP4 Concentration in Stallion Semen after Cryopreservation in Two Different Freezing Media. Cryobiology 2021, 102, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Dordas-Perpinyà, M.; Sergeant, N.; Ruelle, I.; Bruyas, J.-F.; Charreaux, F.; Michaud, S.; Carracedo, S.; Catalán, J.; Miró, J.; Delehedde, M.; et al. ProAKAP4 Semen Concentrations as a Valuable Marker Protein of Post-Thawed Semen Quality and Bull Fertility: A Retrospective Study. Vet. Sci. 2022, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Riesco, M.F.; Anel-Lopez, L.; Neila-Montero, M.; Palacin-Martinez, C.; Montes-Garrido, R.; Alvarez, M.; de Paz, P.; Anel, L. Proakap4 as Novel Molecular Marker of Sperm Quality in Ram: An Integrative Study in Fresh, Cooled and Cryopreserved Sperm. Biomolecules 2020, 10, 1046. [Google Scholar] [CrossRef] [PubMed]
- Kutluyer, F.; Kayim, M.; Öğretmen, F.; Büyükleblebici, S.; Tuncer, P.B. Cryopreservation of Rainbow Trout Oncorhynchus Mykiss Spermatozoa: Effects of Extender Supplemented with Different Antioxidants on Sperm Motility, Velocity and Fertility. Cryobiology 2014, 69, 462–466. [Google Scholar] [CrossRef]
- Motemani, M.; Chamani, M.; Sharafi, M.; Masoudi, R. Alpha-Tocopherol Improves Frozen-Thawed Sperm Quality by Reducing Hydrogen Peroxide during Cryopreservation of Bull Semen. Span. J. Agric. Res. 2017, 15, e0401. [Google Scholar] [CrossRef]
- Alvarez-Rodriguez, M.; Alvarez, M.; Gomes-Alves, S.; Borragan, S.; Martinez-Pastor, F.; de Paz, P.; Anel, L. Quality of Frozen-Thawed Semen in Brown Bear Is Not Affected by Timing of Glycerol Addition. Theriogenology 2011, 75, 1561–1565. [Google Scholar] [CrossRef]
- Sabés-Alsina, M.; Tallo-Parra, O.; Mogas, M.T.; Morrell, J.M.; Lopez-Bejar, M. Heat Stress Has an Effect on Motility and Metabolic Activity of Rabbit Spermatozoa. Anim. Reprod. Sci. 2016, 173, 18–23. [Google Scholar] [CrossRef]
- Maya-Soriano, M.J.; Taberner, E.; Sabés-Alsina, M.; Piles, M.; Lopez-Bejar, M. Absence of Beneficial Effects on Rabbit Sperm Cell Cryopreservation by Several Antioxidant Agents. Zygote 2015, 23, 1–10. [Google Scholar] [CrossRef]
- Domingo, P.; Gil-Sánchez, L. Preservación Seminal: Estado Actual En La Especie Cunícola. Boletín Cunicult. Lagomorpha 2016, 180, 40–45. [Google Scholar]
- Si, W.; Benson, J.D.; Men, H.; Critser, J.K. Osmotic Tolerance Limits and Effects of Cryoprotectants on the Motility, Plasma Membrane Integrity and Acrosomal Integrity of Rat Sperm. Cryobiology 2006, 53, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Domingo, P.; Olaciregui, M.; González, N.; De Blas, I.; Gil, L. Effects of Seminal Plasma and Different Cryoprotectants on Rabbit Sperm Preservation at 16 °C. Exp. Anim. 2018, 67, 413–420. [Google Scholar] [CrossRef]
- Vega, M.; Barrio-López, M.; Quintela-Arias, L.A.; Becerra-González, J.J.; Caínzos-Cagiao, J.M.; Prieto-Largo, A.; Rodríguez-Zamora, A.; García-Herradón, P.J. Evolución del Manejo Reproductivo En Cunicultura. ITEA 2012, 2, 172–190. [Google Scholar]
- Buhr, M.M.; Curtis, E.F.; Kakuda, N.S. Composition and Behavior of Head Membrane Lipids of Fresh and Cryopreserved Boar Sperm. Cryobiology 1994, 31, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, H.D.; Welch, G.R. Impact of Storage Prior to Cryopreservation on Plasma Membrane Function and Fertility of Boar Sperm. Theriogenology 2005, 63, 396–410. [Google Scholar] [CrossRef]
- Medeiros, C.M.O.; Forell, F.; Oliveira, A.T.D.; Rodrigues, J.L. Current Status of Sperm Cryopreservation: Why Isn’t It Better? Theriogenology 2002, 57, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Santos, M.R.; Martínez-Pastor, F.; García-Macías, V.; Esteso, M.C.; Soler, A.J.; de Paz, P.; Anel, L.; Garde, J.J. Extender Osmolality and Sugar Supplementation Exert a Complex Effect on the Cryopreservation of Iberian Red Deer (Cervus elaphus hispanicus) Epididymal Spermatozoa. Theriogenology 2007, 67, 738–753. [Google Scholar] [CrossRef]
- Chang, X.; Wang, J.; Yang, S.; Chen, S.; Song, Y. Antioxidative, Antibrowning and Antibacterial Activities of Sixteen Floral Honeys. Food Funct. 2011, 2, 541. [Google Scholar] [CrossRef]
- Len, J.S.; Koh, W.S.D.; Tan, S.-X. The Roles of Reactive Oxygen Species and Antioxidants in Cryopreservation. Biosci. Rep. 2019, 39, BSR20191601. [Google Scholar] [CrossRef]
- Saeed, A.; El-Nagar, H.; Wafa, W.; Hussein, Y. Effect of Coenzyme Q10 as an Antioxidant Added to Semen Extender during Cryopreservation of Buffalo and Cattle Semen. J. Anim. Poult. Prod. 2016, 7, 403–408. [Google Scholar] [CrossRef]
- Masoudi, R.; Sharafi, M.; Shahneh, A.Z. Effects of CoQ10 on the Quality of Ram Sperm during Cryopreservation in Plant and Animal Based Extenders. Anim. Reprod. Sci. 2019, 208, 106103. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, I.; Emamverdi, M.; Karamzadeh-Dehaghani, A.; Sabzian-Melei, R.; Zhandi, M.; Zare-Shahneh, A. Attenuation of Cryopreservation-Induced Oxidative Stress by Antioxidant: Impact of Coenzyme Q10 on the Quality of Post-Thawed Buck Spermatozoa. Cryobiology 2018, 81, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, R.; Sharafi, M.; Zare Shahneh, A.; Kohram, H.; Nejati-Amiri, E.; Karimi, H.; Khodaei-Motlagh, M.; Shahverdi, A. Supplementation of Extender with Coenzyme Q10 Improves the Function and Fertility Potential of Rooster Spermatozoa after Cryopreservation. Anim. Reprod. Sci. 2018, 198, 193–201. [Google Scholar] [CrossRef] [PubMed]
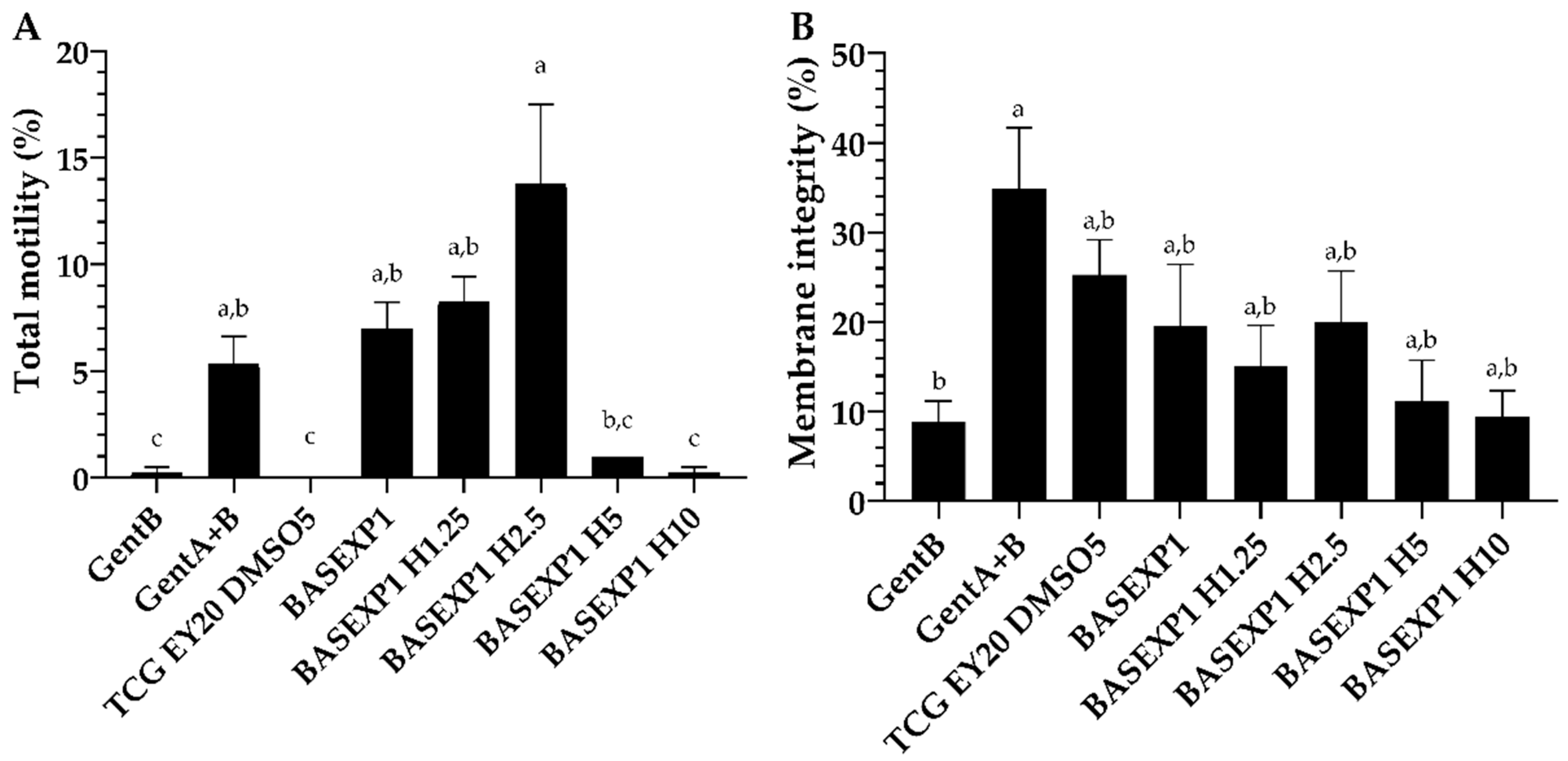
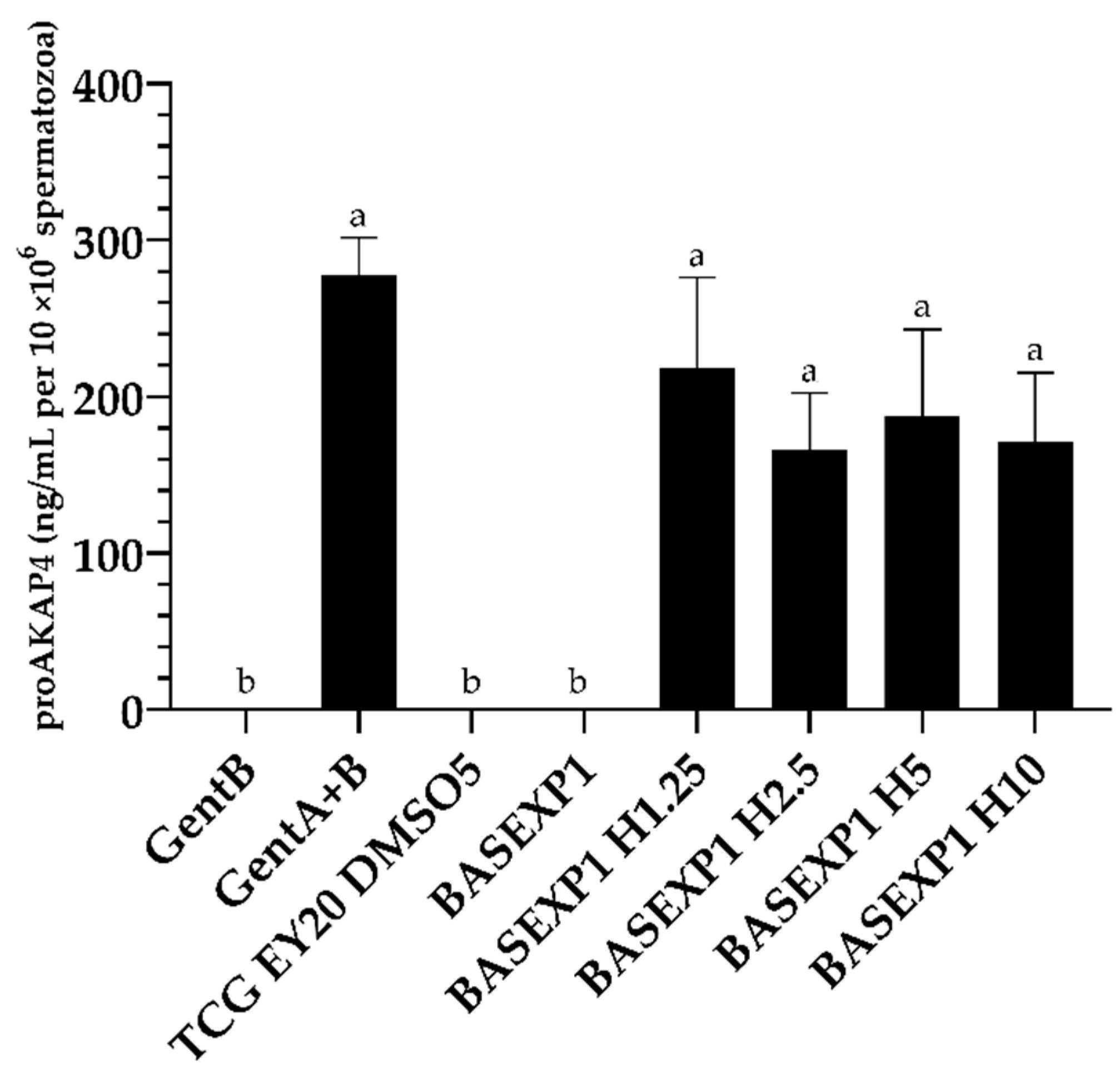
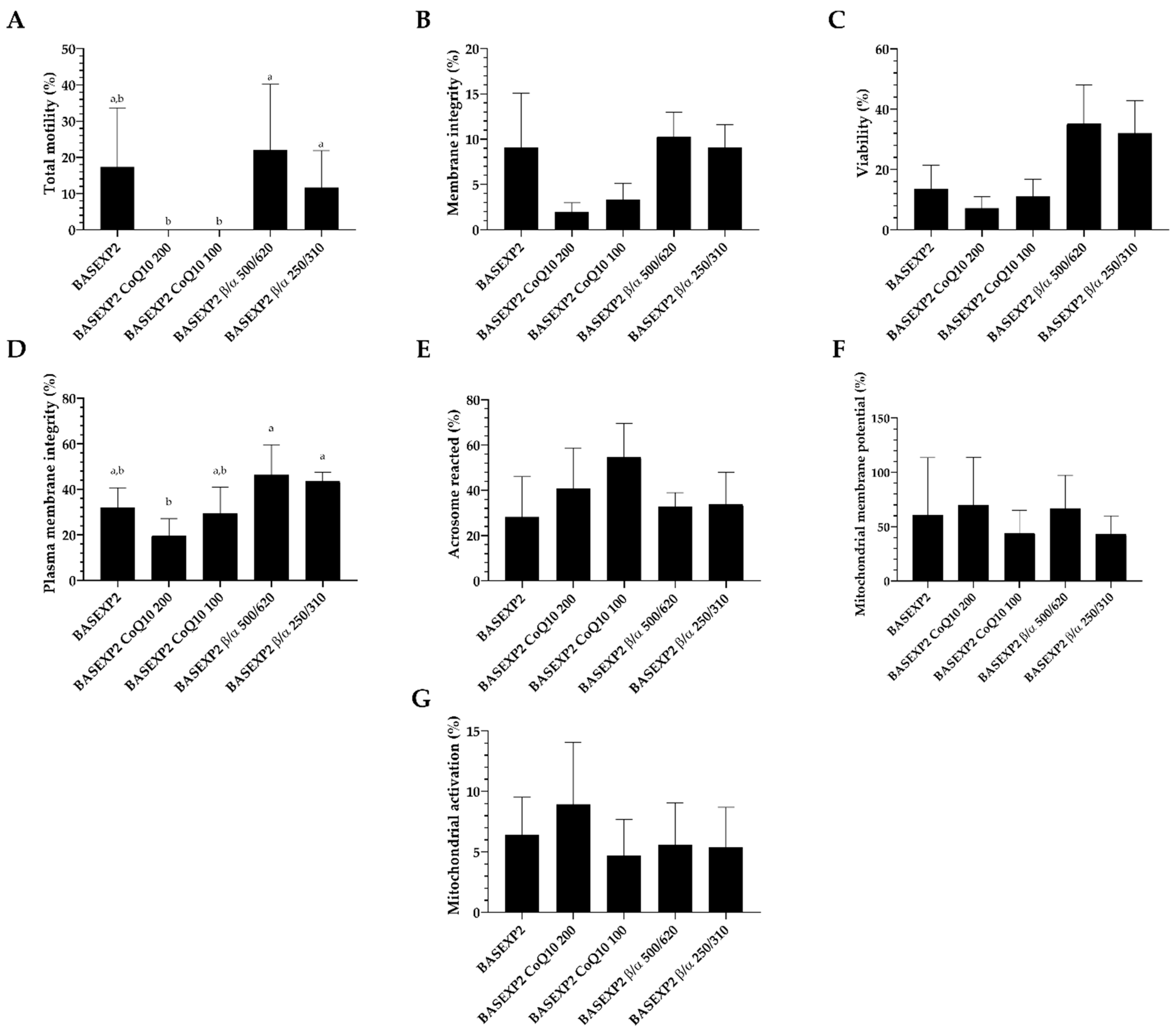
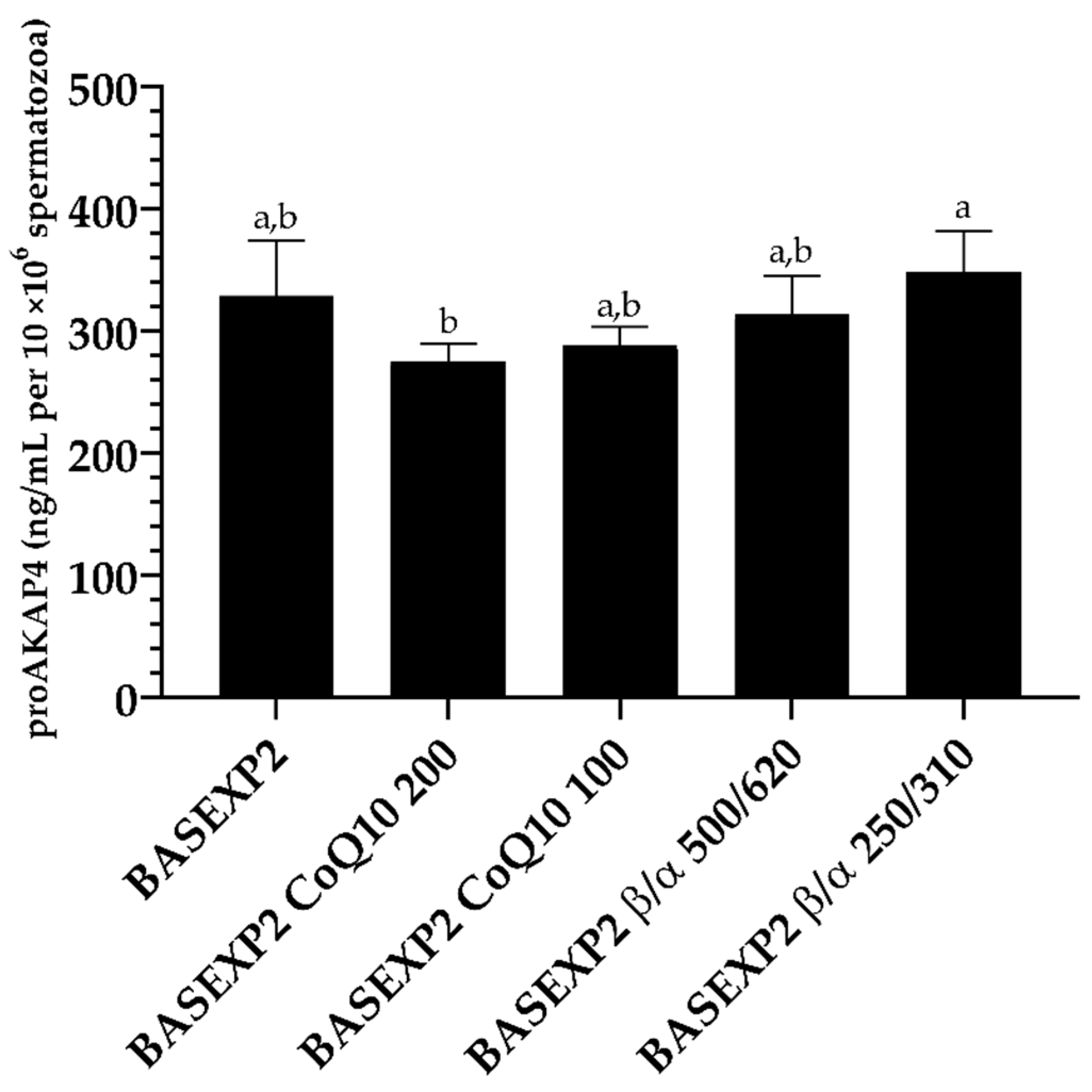
| Extender | Composition | Osmolality (mOsm/kg) | |
|---|---|---|---|
| Experiment 1 | Gent B | Egg-yolk-based commercial extender | Higher $ |
| Gent A + Gent B | Egg-yolk-based commercial extender | 749 | |
| TCG EY20 DMSO5 | Tris + citric acid + glucose + EY (20%) + DMSO (10%) * | 1110 | |
| BASEXP1 | GALAP + DMSO (10%) * | 1133 | |
| BASEXP1 H1.25 | GALAP + DMSO (10%) + H (1.25%) * | 1172 | |
| BASEXP1 H2.5 | GALAP + DMSO (10%) + H (2.5%) * | 1183 | |
| BASEXP1 H5 | GALAP + DMSO (10%) + H (5%) * | 1407 | |
| BASEXP1 H10 | GALAP + DMSO (10%) + H (10%) * | 1850 | |
| Experiment 2 | BASEXP2 | GALAP + EY (20%) + DMSO (10%) * | 1171 |
| BASEXP2 CoQ10 200 | GALAP + EY (20%) + DMSO (10%) + CoQ10 (200 μM) * | Higher $ | |
| BASEXP2 CoQ10 100 | GALAP + EY (20%) + DMSO (10%) + CoQ10 (100 μM) * | Higher $ | |
| BASEXP2 β/α 500/620 | GALAP + EY (20%) + DMSO (10%) + β/α (500 μM/620 μM) * | 1184 | |
| BASEXP2 β/α 250/310 | GALAP + EY (20%) + DMSO (10%) + β/α (250 μM/310 μM) * | 1160 |
| Experiment | Total Motility (%) | Membrane Integrity (%) | Viability (%) | Plasma Membrane Integrity (%) | Acrosome Reacted (%) | Mitochondrial Membrane Potential (%) | Mitochondrial Activation (%) |
|---|---|---|---|---|---|---|---|
| 1 | 60.0 ± 9.7 | 68.2 ± 10.2 | - | - | - | - | - |
| 2 | 53.3 ± 11.6 | 57.5 ± 13.5 | 72.1 ± 13.5 | 62.9 ± 12.2 | 19.9 ± 6.6 | 38.6 ± 13.3 | 10.9 ± 9.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardela, J.; Ruiz-Conca, M.; Palomares, A.; Olvera-Maneu, S.; García-Calvo, L.; López-Béjar, M.; Martínez-Pastor, F.; Álvarez-Rodríguez, M. Effect of Honey, Coenzyme Q10, and β-Carotene/α-Tocopherol as Novel Additives in Rabbit-Sperm Cryopreservation Extender. Animals 2023, 13, 2392. https://doi.org/10.3390/ani13142392
Gardela J, Ruiz-Conca M, Palomares A, Olvera-Maneu S, García-Calvo L, López-Béjar M, Martínez-Pastor F, Álvarez-Rodríguez M. Effect of Honey, Coenzyme Q10, and β-Carotene/α-Tocopherol as Novel Additives in Rabbit-Sperm Cryopreservation Extender. Animals. 2023; 13(14):2392. https://doi.org/10.3390/ani13142392
Chicago/Turabian StyleGardela, Jaume, Mateo Ruiz-Conca, Anna Palomares, Sergi Olvera-Maneu, Laura García-Calvo, Manel López-Béjar, Felipe Martínez-Pastor, and Manuel Álvarez-Rodríguez. 2023. "Effect of Honey, Coenzyme Q10, and β-Carotene/α-Tocopherol as Novel Additives in Rabbit-Sperm Cryopreservation Extender" Animals 13, no. 14: 2392. https://doi.org/10.3390/ani13142392
APA StyleGardela, J., Ruiz-Conca, M., Palomares, A., Olvera-Maneu, S., García-Calvo, L., López-Béjar, M., Martínez-Pastor, F., & Álvarez-Rodríguez, M. (2023). Effect of Honey, Coenzyme Q10, and β-Carotene/α-Tocopherol as Novel Additives in Rabbit-Sperm Cryopreservation Extender. Animals, 13(14), 2392. https://doi.org/10.3390/ani13142392








