Identification of Biomarkers Affecting Cryopreservation Recovery Ratio in Ram Spermatozoa Using Tandem Mass Tags (TMT)-Based Quantitative Proteomics Approach
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Animals and Ethics
2.3. Sample Collection and Preparation
2.4. Quantitative Mass Spectrometry Analysis
2.4.1. Protein Extraction and Digestion (PTM)
2.4.2. TMT Labeling
2.4.3. High-Performance Liquid Chromatography (HPLC) Fractionation and LC-MS/MS Analysis
2.4.4. Database Search and Bioinformatic Analysis
2.5. PRM Validation
2.6. Statistical Analysis
3. Results
3.1. Comparison of Sample Parameters between HCR and LCR
3.2. Proteomic Profile of Spermatozoa
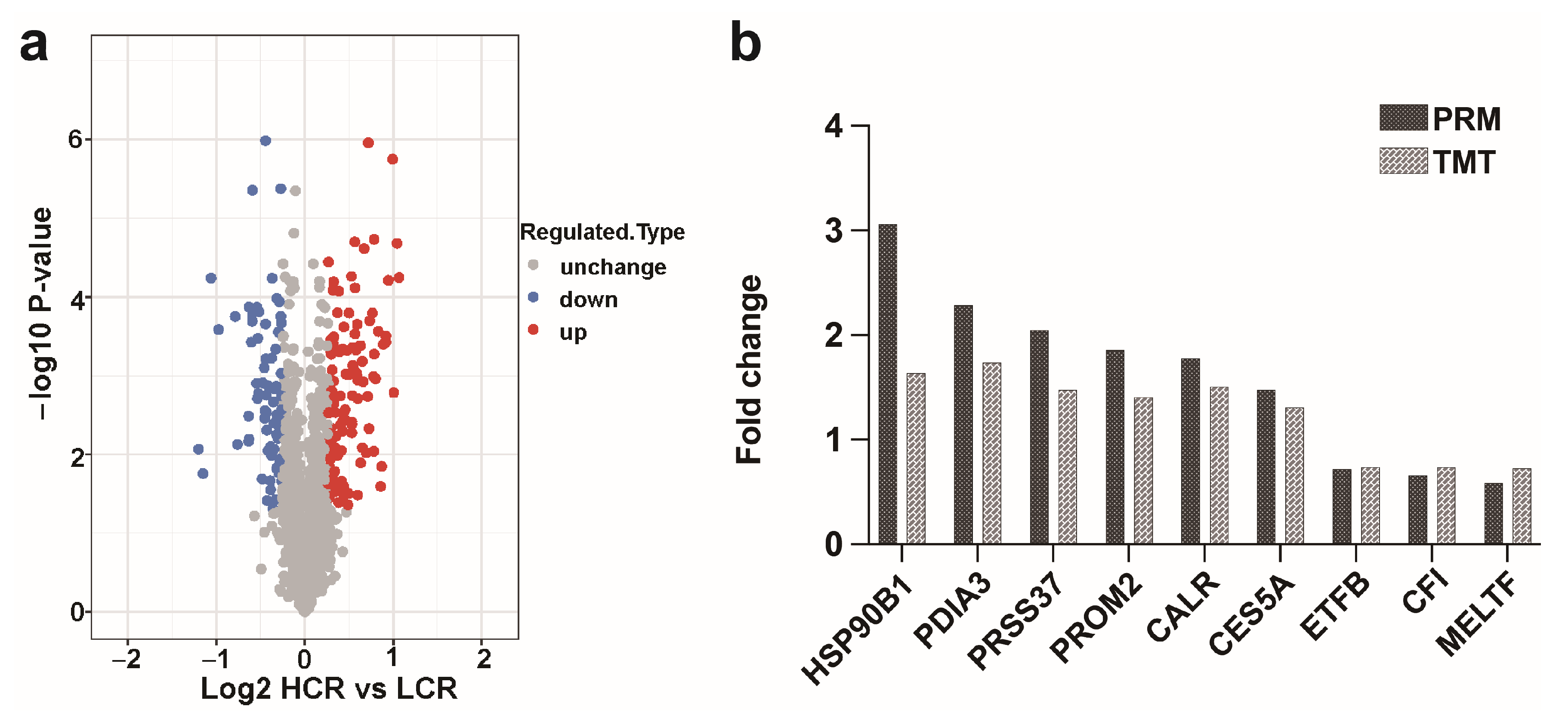
3.3. Validation of the Selected DEPs by PRM
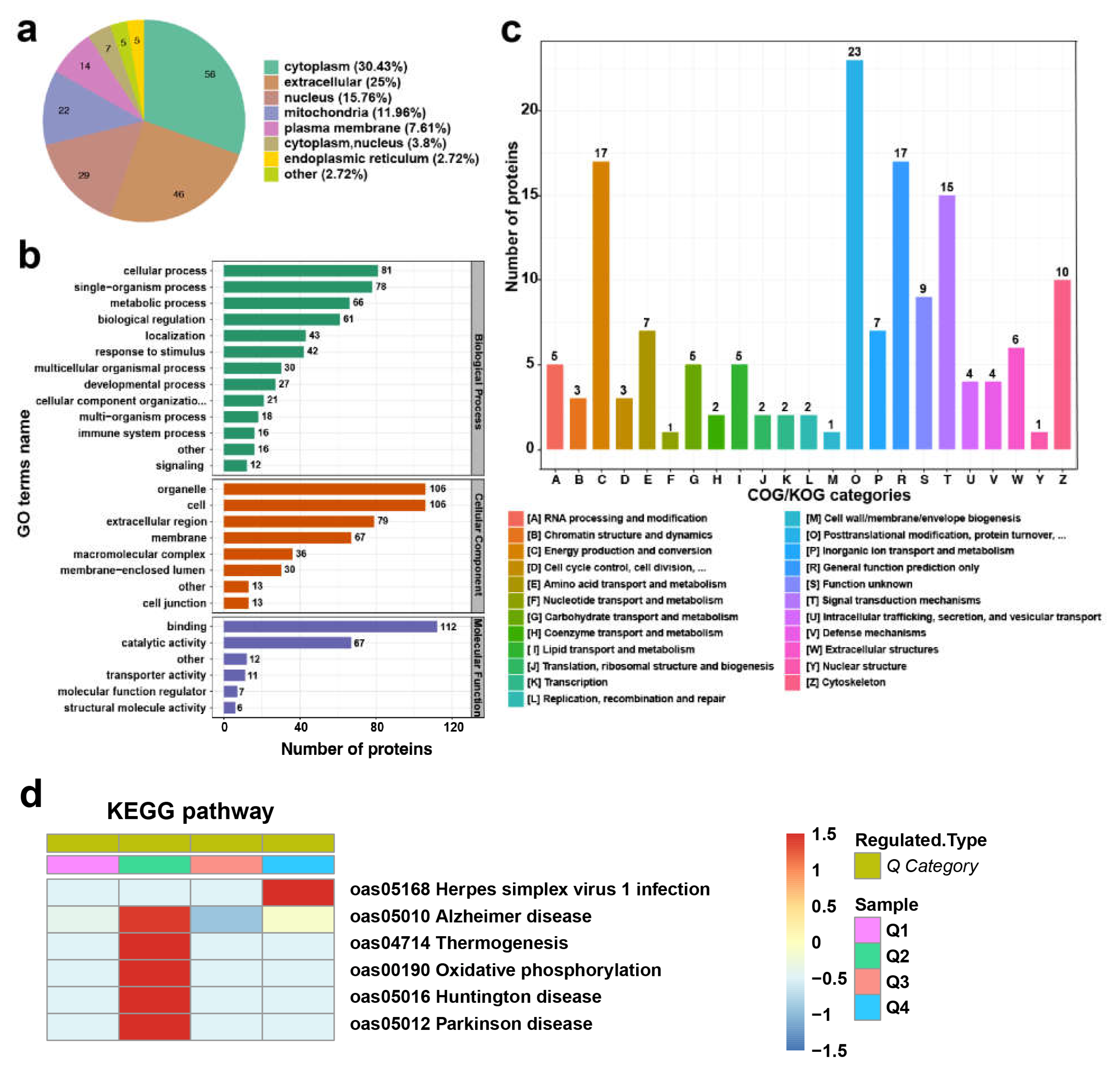
3.4. Bioinformatics Analysis of DEPs
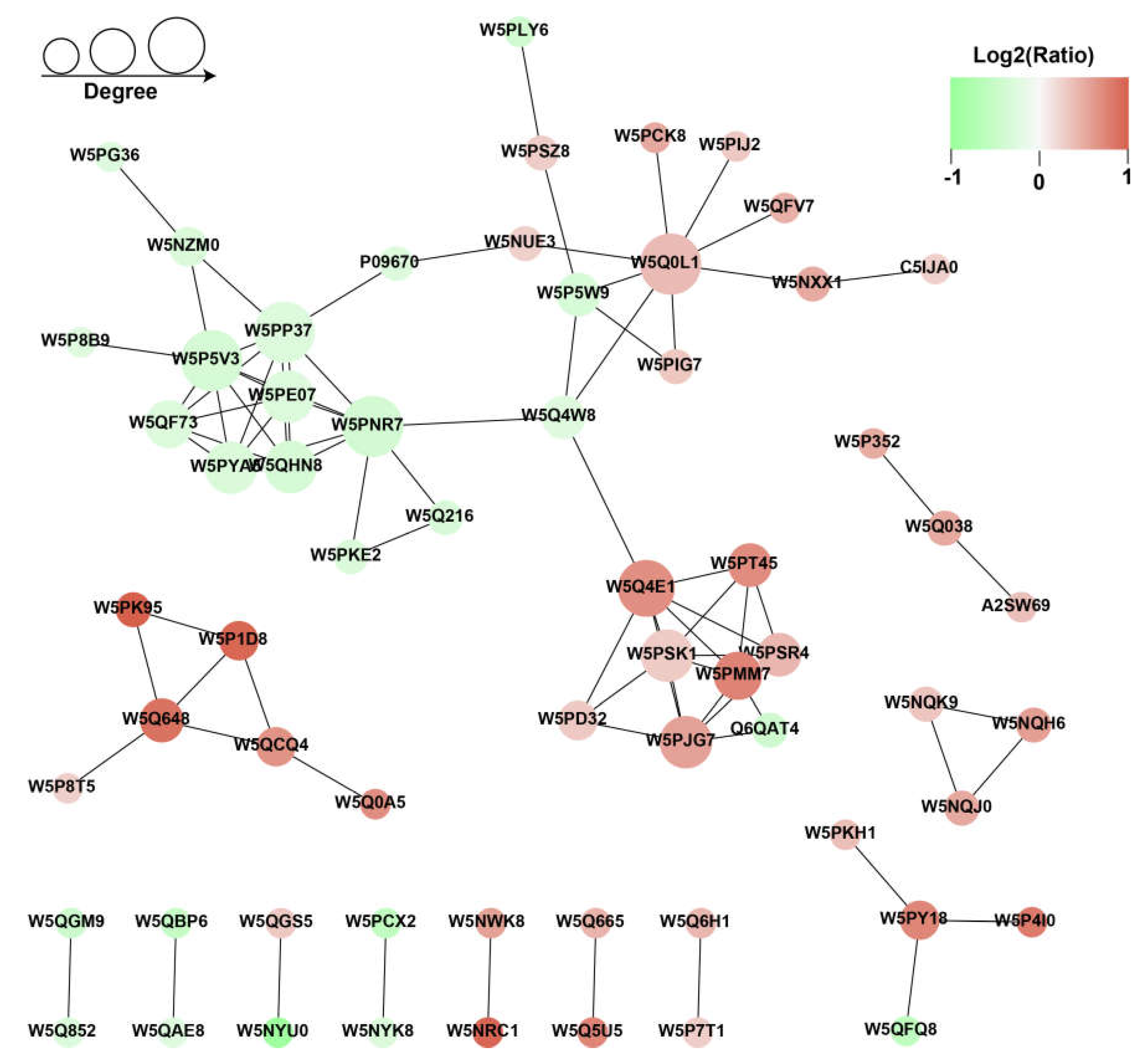
3.5. Protein-Protein Interaction Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boitrelle, F.; Albert, M.; Theillac, C.; Ferfouri, F.; Bergere, M.; Vialard, F.; Wainer, R.; Bailly, M.; Selva, J. Cryopreservation of human spermatozoa decreases the number of motile normal spermatozoa, induces nuclear vacuolization and chromatin decondensation. J. Androl. 2012, 33, 1371–1378. [Google Scholar] [CrossRef]
- Park, Y.S.; Lee, S.H.; Song, S.J.; Jun, J.H.; Koong, M.K.; Seo, J.T. Influence of motility on the outcome of in vitro fertilization/intracytoplasmic sperm injection with fresh vs. frozen testicular sperm from men with obstructive azoospermia. Fertil. Steril. 2003, 80, 526–530. [Google Scholar] [CrossRef]
- Nagy, Z.P.; Verheyen, G.; Tournaye, H.; Van Steirteghem, A.C. Special applications of intracytoplasmic sperm injection: The influence of sperm count, motility, morphology, source and sperm antibody on the outcome of ICSI. Hum. Reprod. 1998, 13, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Sallam, H.N.; Ezzeldin, F.; Sallam, A.; Agameya, A.F.; Farrag, A. Sperm velocity and morphology, female characteristics, and the hypo-osmotic swelling test as predictors of fertilization potential: Experience from the IVF model. Int. J. Fertil. Womens Med. 2003, 48, 88–95. [Google Scholar]
- Casas, I.; Sancho, S.; Briz, M.; Pinart, E.; Bussalleu, E.; Yeste, M.; Bonet, S. Freezability prediction of boar ejaculates assessed by functional sperm parameters and sperm proteins. Theriogenology 2009, 72, 930–948. [Google Scholar] [CrossRef] [PubMed]
- Porambo, J.R.; Salicioni, A.M.; Visconti, P.E.; Platt, M.D. Sperm phosphoproteomics: Historical perspectives and current methodologies. Expert. Rev. Proteom. 2012, 9, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Thurston, L.M.; Siggins, K.; Mileham, A.J.; Watson, P.F.; Holt, W.V. Identification of amplified restriction fragment length polymorphism markers linked to genes controlling boar sperm viability following cryopreservation. Biol. Reprod. 2002, 66, 545–554. [Google Scholar] [CrossRef]
- Yoon, S.J.; Rahman, M.S.; Kwon, W.S.; Ryu, D.Y.; Park, Y.J.; Pang, M.G. Proteomic identification of cryostress in epididymal spermatozoa. J. Anim. Sci. Biotechnol. 2016, 7, 67. [Google Scholar] [CrossRef]
- Kwon, W.S.; Oh, S.A.; Kim, Y.J.; Rahman, M.S.; Park, Y.J.; Pang, M.G. Proteomic approaches for profiling negative fertility markers in inferior boar spermatozoa. Sci. Rep. 2015, 5, 13821. [Google Scholar] [CrossRef]
- Vilagran, I.; Yeste, M.; Sancho, S.; Casas, I.; del Rivera Álamo, M.M.; Bonet, S. Relationship of sperm small heat-shock protein 10 and voltage-dependent anion channel 2 with semen freezability in boars. Theriogenology 2014, 82, 418–426. [Google Scholar] [CrossRef]
- Vilagran, I.; Castillo, J.; Bonet, S.; Sancho, S.; Yeste, M.; Estanyol, J.M.; Oliva, R. Acrosin-binding protein (ACRBP) and triosephosphate isomerase (TPI) are good markers to predict boar sperm freezing capacity. Theriogenology 2013, 80, 443–450. [Google Scholar] [CrossRef]
- He, Y.X.; Wang, K.; Zhao, X.X.; Zhang, Y.; Ma, Y.J.; Hu, J.J. Differential proteome association study of freeze-thaw damage in ram sperm. Cryobiology 2016, 72, 60–68. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Xiong, C.L. Proteins in sperm and seminal plasma associated with human sperm resistance to cryopreservation. Natl. J. Androl. 2013, 19, 214–217. [Google Scholar]
- Li, C.Y.; Ren, C.H.; Chen, Y.L.; Wang, M.M.; Tang, J.; Zhang, Y.; Wang, Q.J.; Zhang, Z.J. Changes on proteomic and metabolomic profiling of cryopreserved sperm effected by melatonin. J. Proteom. 2023, 20, 273. [Google Scholar] [CrossRef]
- Druart, X.; Cognie, J.; Baril, G.; Clement, F.; Dacheux, J.L.; Gatti, J.L. In vivo imaging of in situ motility of fresh and liquid stored ram spermatozoa in the ewe genital tract. Reproduction 2009, 138, 45–53. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, L.; Hanrahan, J.P.; Richardson, L.; Donovan, A.; Fair, S.; Evans, A.C.; Lonergan, P. Effect of storage duration, storage temperature, and diluent on the viability and fertility of fresh ram sperm. Theriogenology 2010, 73, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, Y.; Ren, C.H.; Cheng, X.; Chen, J.H.; Ge, Z.Y.; Sun, Z.P.; Zhuo, X.; Sun, F.F.; Chen, Y.L.; et al. Identification of proteomic markers for ram spermatozoa motility using a tandem mass tag (TMT) approach. J. Proteom. 2020, 210, 103438. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Kuo, Y.H.; Lee, W.C.; Tsou, H.L.; Lee, Y.P.; Chang, H.L.; Wu, J.J.; Yang, P.C. Substantial decrease of heat-shock protein 90 precedes the decline of sperm motility during cooling of boar spermatozoa. Theriogenology 1999, 51, 1007–1016. [Google Scholar] [CrossRef]
- Guimarães, D.B.; Barros, T.B.; van Tilburg, M.F.; Martins, J.A.M.; Moura, A.A.; Moreno, F.B.; Monteiro-Moreira, A.C.; Moreira, R.A.; Toniolli, R. Sperm membrane proteins associated with the boar semen cryopreservation. Anim. Reprod. Sci. 2017, 183, 27–38. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martín-Maestro, A.; Sánchez-Ajofrín, I.; Medina-Chávez, D.A.; Fernández-Santos, M.R.; García-Álvarez, O.; Maroto-Morales, A.; Montoro, V.; et al. Sperm cryodamage in ruminants: Understanding the molecular changes induced by the cryopreservation process to optimize sperm quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef]
- Naaby-Hansen, S.; Herr, J.C. Heat shock proteins on the human sperm surface. J. Reprod. Immunol. 2010, 84, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kemter, E.; Fröhlich, T.; Arnold, G.J.; Wolf, E.; Wanke, R. Mitochondrial dysregulation secondary to endoplasmic reticulum stress in autosomal dominant tubulointerstitial kidney disease-UMOD (ADTKD-UMOD). Sci. Rep. 2017, 7, 42970. [Google Scholar] [CrossRef]
- Anas, A.A.; De Vos, A.F.; Hoogendijk, A.J.; Van Lieshout, M.H.; Van Heijst, J.W.; Florquin, S.; Li, Z.; Van’t Veer, C.; Van der Poll, T. Endoplasmic reticulum chaperone gp96 in macrophages is essential for protective immunity during Gram-negative pneumonia. J. Pathol. 2016, 238, 74–84. [Google Scholar] [CrossRef]
- Casas, I.; Sancho, S.; Ballester, J.; Briz, M.; Pinart, E.; Bussalleu, E.; Yeste, M.; Fàbrega, A.; Rodríguez-Gil, J.E.; Bonet, S. The HSP90AA1 sperm content and the prediction of the boar ejaculate freezability. Theriogenology 2010, 74, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Lingner, J. PRDX1 counteracts catastrophic telomeric cleavage events that are triggered by DNA repair activities post oxidative damage. Cell Rep. 2020, 33, 108347. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Sabharwal, P.; Rao, M.; Sockanathan, S. The antioxidant enzyme Prdx1 controls neuronal differentiation by thiol-redox-dependent activation of GDE2. Cell 2009, 138, 1209–1221. [Google Scholar] [CrossRef]
- Conway, J.P.; Michael, K. Dual role of peroxiredoxin I in macrophage-derived foam cells. J. Biol. Chem. 2006, 281, 27991. [Google Scholar] [CrossRef]
- Brandt, C.; Hansen, R.H.; Hansen, J.B.; Olsen, C.H.; Galle, P.; Mandrup-Poulsen, T.; Gehl, J.; Pedersen, B.K.; Hojman, P. Over-expression of Follistatin-like 3 attenuates fat accumulation and improves insulin sensitivity in mice. Metab. Clin. Exp. 2015, 64, 283–295. [Google Scholar] [CrossRef]
- Mittal, L.; Aryal, U.K.; Camarillo, I.G.; Ferreira, R.M.; Sundararaja, R. Quantitative proteomic analysis of enhanced cellular effects of electrochemotherapy with Cisplatin in triple-negative breast cancer cells. Sci. Rep. 2019, 9, 9–26. [Google Scholar]
- Nakamura, N.; Dai, Q.; Williams, J.; Goulding, E.H.; Willis, W.D.; Brown, P.R.; Eddy, E.M. Disruption of a spermatogenic cell-specific mouse enolase 4 (eno4) gene causes sperm structural defects and male infertility. Biol. Reprod. 2013, 88, 90. [Google Scholar] [CrossRef]
- Li, H.Y.; He, Y.X.; Yan, J.W.; Zhao, Q.Y.; Di, C.X.; Zhang, H. Comparative proteomics reveals the underlying toxicological mechanism of low sperm motility induced by iron ion radiation in mice. Reprod. Toxicol. 2016, 65, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Froman, D.P.; Kirby, J.D. Sperm Mobility: Phenotype in roosters (Gallus domesticus) determined by mitochondrial function. Biol. Reprod. 2005, 72, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Klemme, L.M.; Roberts, K.P.; Hoffman, L.B.; Ensrud, K.M.; Siiteri, J.E.; Hamilton, D.W. Cloning and characterization of the rat Crisp-1 gene. Gene 1999, 240, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Udby, L.; Bjartell, A.; Malm, J.; Egesten, A.; Lundwall, A.; Cowland, J.B.; Borregaard, N.; Kjeldsen, L. Characterization and localization of cysteine-rich secretory protein 3 (CRISP-3) in the human male reproductive tract. J. Androl. 2005, 26, 333–342. [Google Scholar] [CrossRef]
- O’Bryan, M.K.; Sebire, K.; Meinhardt, A.; Edgar, K.; Keah, H.H.; Hearn, M.T.; De Kretser, D.M. Tpx-1 is a component of the outer dense fibers and acrosome of rat spermatozoa. Mol. Reprod. Dev. 2001, 58, 116–125. [Google Scholar] [CrossRef]
- Ellerman, D.A.; Myles, D.G.; Primakoff, P.A. Role for sperm surface protein disulfide isomerase activity in gamete fusion: Evidence for the participation of ERp57. Dev. Cell. 2006, 10, 831–837. [Google Scholar] [CrossRef]
- Jessop, C.E.; Chakravarthi, S.; Garbi, N.; Hämmerling, G.J.; Lovell, S.; Bulleid, N.J. ERp57 is essential for efficient folding of glycoproteins sharing common structural domains. EMBO J. 2006, 26, 28–40. [Google Scholar] [CrossRef]
- Wang, S.W.; Song, R.; Wang, Z.Y.; Jing, Z.C.; Wang, S.X.; Ma, J. S100A8/A9 in inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Ning, X.Y.; Zhang, Y.L.; Yuan, T.T.; Li, Q.B.; Tian, J.; Guan, W.S.; Liu, B.; Zhang, W.; Xu, X.X.; Zhang, Y.H. Enhanced thermostability of glucose oxidase through computer-aided molecular design. Int. J. Mol. Sci. 2018, 19, 425. [Google Scholar] [CrossRef]
- Darszon, A.; Nishigaki, T.; Beltran, C.; Treviño, C.L. Calcium channels in the development, maturation, and function of spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar] [CrossRef]
- Costello, S.; Michelangeli, F.; Nash, K.; Lefievre, L.; Morris, J.; Machado-Oliveira, G.; Barratt, C.; Kirkman-Brown, J.; Publicover, S. Ca2+-stores in sperm: Their identities and functions. Reproduction 2009, 138, 425–437. [Google Scholar] [CrossRef]
- Marquez, B.; Ignotz, G.; Suarez, S.S. Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: Release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Dev. Biol. 2007, 303, 214–221. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, H.Y.; Cui, J.T.; Li, W.M.; An, G.; Pan, Y.M.; Zhang, Q.Y.; Xing, R.; Lu, Y.Y. Calcium-binding protein S100A14 induces differentiation and suppresses metastasis in gastric cancer. Cell. Death Dis. 2017, 8, e2938. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.H.; Xin, J.; Sun, Y.; Li, J.; Wei, D.; Zhao, A.Z. Identification of S100A16 as a novel adipogenesis promoting factor in 3T3-L1 cells. Endocrinology 2011, 152, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.H.; Su, D.M.; Zhu, W.D.; Huang, Q.; Liu, M.L.; Xue, Y.; Zhang, Y.Y.; Li, D.; Zhao, A.; Liu, Y. Estrogen suppresses adipogenesis by inhibiting S100A16 expression. J. Mol. Endocrinol. 2014, 52, 235–244. [Google Scholar] [CrossRef]
- Lizarbe, M.A.; Barrasa, J.I.; Olmo, N.; Gavilanes, F.; Turnay, J. Annexin phospholipid interactions. Functional implications. Int. J. Mol. Sci. 2013, 14, 2652–2683. [Google Scholar] [CrossRef]
- Vcan De Graaf, S.F.J.; Hoenderop, J.G.J.; Gkika, D.; Lamers, D.; Prenen, J.; Rescher, U.; Gerke, V.; Staub, O.; Nilius, B.; Bindels, R.M. Functional expression of the epithelial Ca2+ channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex. EMBO J. 2003, 22, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Koike, M.; Sakata, S.; Takeda, Y.; Nakagawa, T.; Hatano, T.; Ohashi, S.; Funayama, M.; Yoshimi, K.; Asanuma, M.; et al. Possible involvement of iron-induced oxidative insults in neurodegeneration. Neurosci. Lett. 2014, 588, 29–35. [Google Scholar] [CrossRef]
- Philibert, P.; Boizet-Bonhoure, B.; Bashamboo, A.; Paris, F.; Aritake, K.; Urade, Y.; Leger, J.; Sultan, C.; Poulat, F. Unilateral cryptorchidism in mice mutant for Ptgds. Hum. Mutat. 2013, 34, 278–282. [Google Scholar] [CrossRef]
- Xie, H.; Hu, Z.; Chyna, B.; Horrigan, S.K.; Westbrook, C.A. Human mortalin (HSPA9): A candidate for the myeloid leukemia tumor suppressor gene on 5q31. Leukemia 2000, 14, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Hiller, S.; Garces, R.G.; Malia, T.J.; Orekhov, V.Y.; Colombini, M.; Wagner, G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science 2008, 321, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Mannella, C.A.; Kinnally, K.W. Reflections on VDAC as a voltage-gated channel and a mitochondrial regulator. J. Bioenerg. Biomembr. 2008, 40, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Rostovtseva, T.K.; Komarov, A.; Bezrukov, S.M.; Colombini, M. VDAC channels differentiate between natural metabolites and synthetic molecules. J. Membr. Biol. 2002, 187, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Herst, P.M.; Rowe, M.R.; Carson, G.M.; Berridge, M.V. Functional mitochondria in health and disease. Front. Endocrinol. 2017, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.K.; Kitami, T.; Gilbert, T.J.; Peck, D.; Ramanathan, A.; Schreiber, S.L.; Golub, T.R.; Mootha, V.K. Large-scale chemical dissection of mitochondrial function. Nat. Biotechnol. 2008, 26, 343–351. [Google Scholar] [CrossRef]
- Simsek-Duran, F.; Li, F.; Ford, W.; Swanson, R.J.; Jones, H.W.; Castora, F.J. Ageassociated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS ONE 2013, 8, e64955. [Google Scholar] [CrossRef]
- Yin, H.; Baart, E.; Betzendahl, I.; Eichenlaub-Ritter, U. Diazepam induces meiotic delay, aneuploidy and predivision of homologues and chromatids in mammalian oocytes. Mutagenesis 1998, 13, 567–580. [Google Scholar] [CrossRef]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Diaz, F.; Kotarsky, H.; Fellman, V.; Moraes, C.T. Mitochondrial disorders caused by mutations in respiratory chain assembly factors. Semin. Fetal. Neonatal Med. 2011, 16, 197–204. [Google Scholar] [CrossRef]
- Usui, S.; Oveson, B.C.; Iwase, T.; Lu, L.; Lee, S.Y.; Jo, Y.J.; Wu, Z.; Choi, E.Y.; Samulski, R.J.; Campochiaro, P.A. Overexpression of SOD in retina: Need for increase in H2O2-detoxifying enzyme in same cellular compartment. Free Radic. Biol. Med. 2011, 51, 1347–1354. [Google Scholar] [CrossRef]
- Cui, H.; Kong, Y.H.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.A.; Agarwal, A. Oxidative stress and male infertility: From research bench to clinical practice. J. Androl. 2002, 23, 737–752. [Google Scholar] [PubMed]


| Group | Concentration (Million/mL) | Age (Months) | Weight (kg) | Breed |
|---|---|---|---|---|
| HCR | 3861.60 ± 296.11 | 37.00 ± 0.58 | 98.33 ± 0.88 | Dorper sheep |
| LCR | 4191.53 ± 314.09 | 36.33 ± 0.88 | 96.33 ± 0.88 | Dorper sheep |
| Parameters | HCR | LCR | p-Value |
|---|---|---|---|
| Fresh sperm | |||
| MOT (%) | 82.3 ± 0.006 | 80.9 ± 0.02 | 0.41 |
| VCL (μm/s) | 101.3 ± 2.98 | 103.1 ± 3.10 | 0.70 |
| VSL (μm/s) | 43 ± 2.65 | 38.725 ± 2.14 | 0.25 |
| VAP (μm/s) | 60.8 ± 1.33 | 58.1 ± 1.43 | 0.18 |
| LIN (%) | 42.8 ± 2.71 | 37.9 ± 2.34 | 0.21 |
| STR (%) | 63.8 ± 2.17 | 60.6 ± 1.84 | 0.31 |
| ALH (μm) | 3.6 ± 0.16 | 3.8 ± 0.18 | 0.33 |
| BCF (Hz) | 6.2 ± 0.15 | 6.1 ± 0.20 | 0.60 |
| Cryo-recovered sperm | |||
| MOT (%) | 56.6 ± 0.02 *** | 22.1 ± 0.02 *** | <0.001 |
| VCL (μm/s) | 80.2 ± 1.94 *** | 48.5 ± 2.49 *** | <0.001 |
| VSL (μm/s) | 31.5 ± 1.27 *** | 22.2 ± 1.33 *** | <0.001 |
| VAP (μm/s) | 45.4 ± 1.50 *** | 36.9 ± 2.06 *** | <0.001 |
| LIN (%) | 39.7 ± 1.95 * | 45.7 ± 1.72 * | <0.05 |
| STR (%) | 61.8 ± 1.38 * | 67.1 ± 1.42 * | <0.05 |
| ALH (μm) | 3.3 ± 0.12 *** | 2.6 ± 0.09 *** | <0.001 |
| BCF (Hz) | 5.1 ± 0.11 *** | 6.9 ± 0.25 *** | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, C.; Sun, Z.; Chen, Y.; Chen, J.; Wang, S.; Liu, Q.; Wang, P.; Cheng, X.; Zhang, Z.; Wang, Q. Identification of Biomarkers Affecting Cryopreservation Recovery Ratio in Ram Spermatozoa Using Tandem Mass Tags (TMT)-Based Quantitative Proteomics Approach. Animals 2023, 13, 2368. https://doi.org/10.3390/ani13142368
Ren C, Sun Z, Chen Y, Chen J, Wang S, Liu Q, Wang P, Cheng X, Zhang Z, Wang Q. Identification of Biomarkers Affecting Cryopreservation Recovery Ratio in Ram Spermatozoa Using Tandem Mass Tags (TMT)-Based Quantitative Proteomics Approach. Animals. 2023; 13(14):2368. https://doi.org/10.3390/ani13142368
Chicago/Turabian StyleRen, Chunhuan, Zhipeng Sun, Yale Chen, Jiahong Chen, Shijia Wang, Qingqing Liu, Penghui Wang, Xiao Cheng, Zijun Zhang, and Qiangjun Wang. 2023. "Identification of Biomarkers Affecting Cryopreservation Recovery Ratio in Ram Spermatozoa Using Tandem Mass Tags (TMT)-Based Quantitative Proteomics Approach" Animals 13, no. 14: 2368. https://doi.org/10.3390/ani13142368
APA StyleRen, C., Sun, Z., Chen, Y., Chen, J., Wang, S., Liu, Q., Wang, P., Cheng, X., Zhang, Z., & Wang, Q. (2023). Identification of Biomarkers Affecting Cryopreservation Recovery Ratio in Ram Spermatozoa Using Tandem Mass Tags (TMT)-Based Quantitative Proteomics Approach. Animals, 13(14), 2368. https://doi.org/10.3390/ani13142368






