Interference with DGAT Gene Inhibited TAG Accumulation and Lipid Droplet Synthesis in Bovine Preadipocytes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bovine Preadipocytes Isolation, Culture, and Differentiation
2.2. Construction of DGAT-siRNA
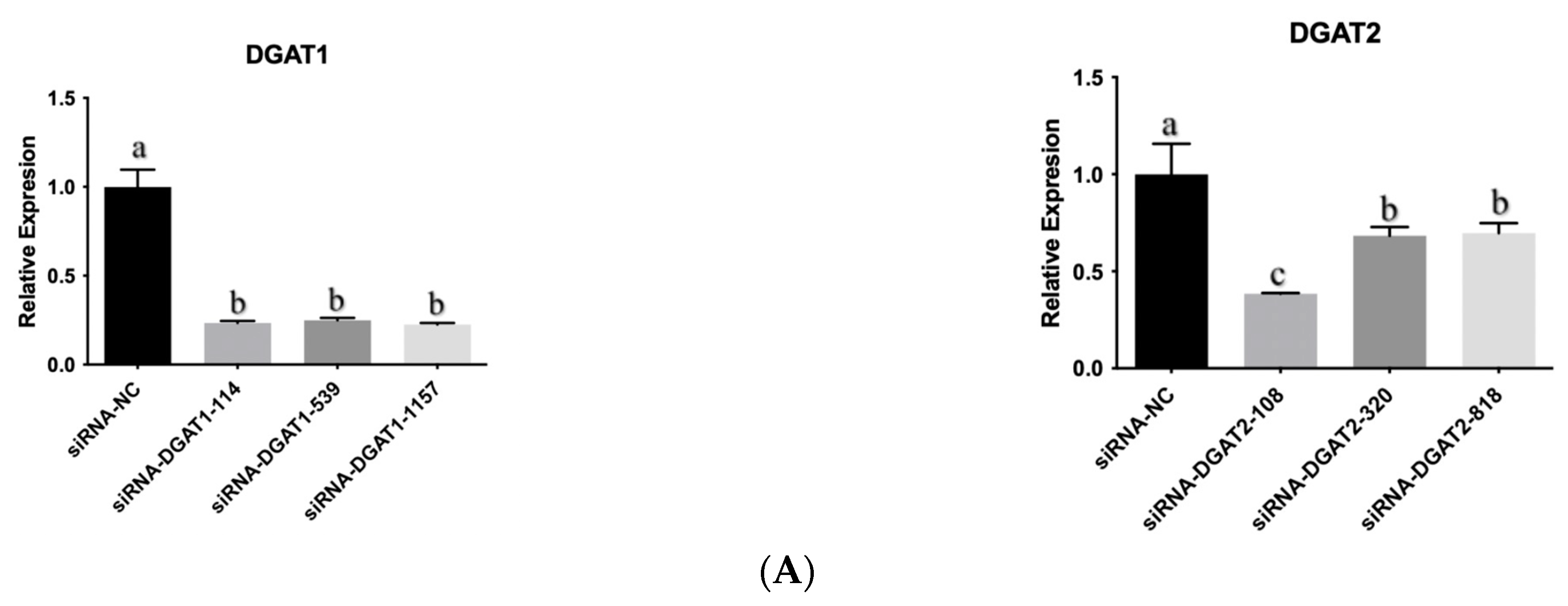
2.3. Detection of DGAT-siRNA Interference Effect
2.4. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR) Detection
2.5. Oil Red O Staining and Triglyceride Determination
2.6. Determination of Aiponectin (ADP) Concentration
2.7. RNA Sequencing (RNA-Seq)
2.8. Gene Ontology (GO) and Kyoto Encyclopaedia of Genes and Genomes (KEGG) Enrichment Analysis
2.9. Statistical Analyses
3. Results
3.1. Interfering DGAT Gene Inhibited the Differentiation of Bovine Adipose Cells Induced by Oleic Acid
3.2. Difference Analysis of Bovine Preadipocytes Infected with sh-DGAT1/sh-DGAT2
3.3. Functional Analysis of Differentially Expressed Genes GO
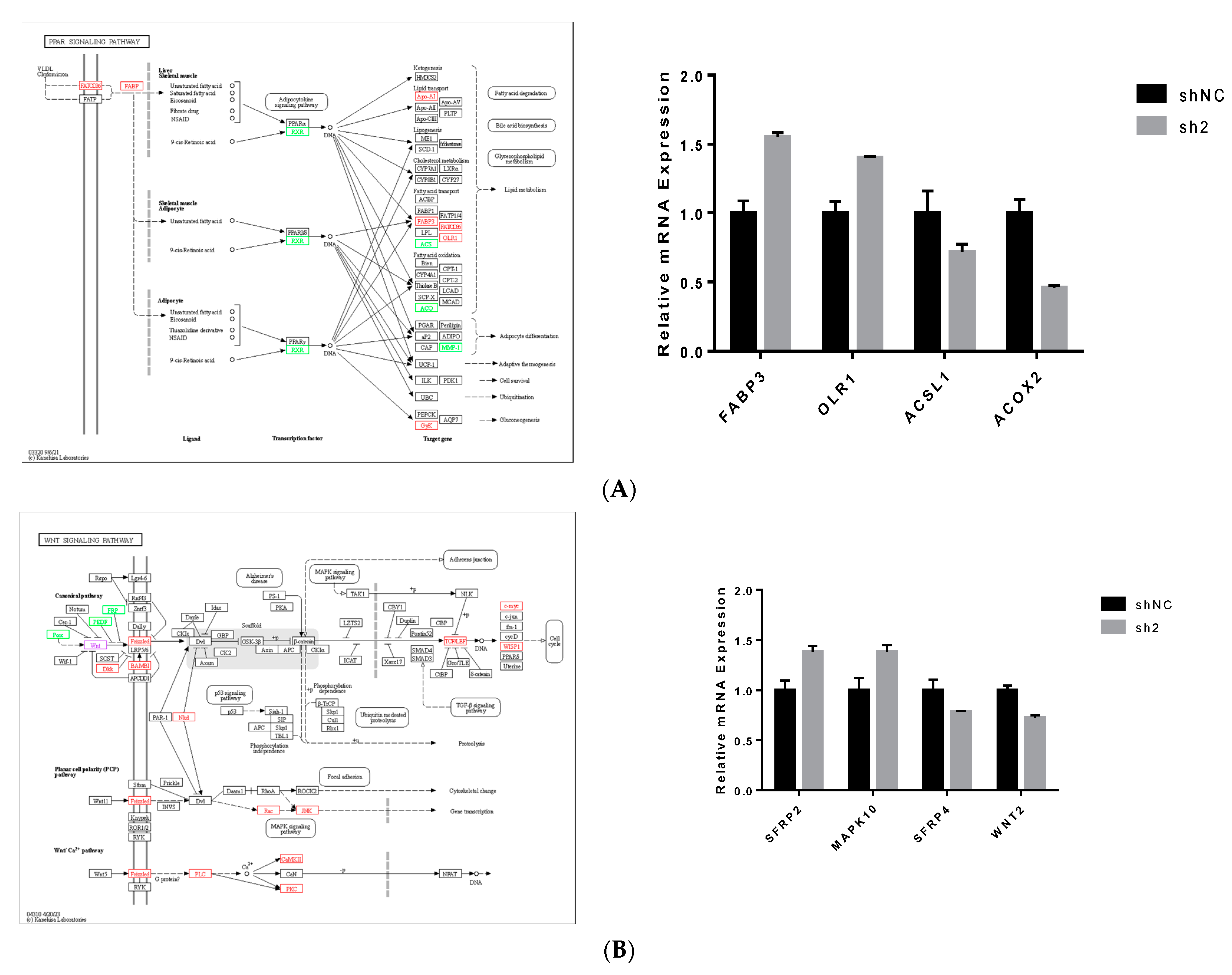
3.4. KEGG Enrichment Analysis of DEGs
3.5. Analysis of Signal Pathways Related to Differentially Expressed Genes and Lipid Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Li, G.P. Beef quality traits and their influencing factors. Chin. J. Anim. Nutr. 2019, 31, 4949–4958. [Google Scholar] [CrossRef]
- Martins, T.S.; Sanglard, L.M.P.; Silva, W.; Chizzotti, M.L.; Rennó, L.N.; Serão, N.V.L.; Silva, F.F.; Guimarães, S.E.F.; Ladeira, M.M.; Dodson, M.V.; et al. Molecular factors underlying the deposition of intramuscular fat and collagen in skeletal muscle of Nellore and Angus Cattle. PLoS ONE 2015, 10, e0139943. [Google Scholar] [CrossRef] [PubMed]
- Billecke, N.; Bosma, M.; Rock, W.; Fleissner, F.; Best, G.; Schrauwen, P.; Kersten, S.; Bonn, M.; Hesselink, M.K.C.; Parekh, S.H. Perilipin 5 mediated lipid droplet remodelling revealed by coherent Raman imaging. Integr. Biol. 2015, 7, 467–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, R.A.; Lee, D.P. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 2004, 43, 134–176. [Google Scholar] [CrossRef] [PubMed]
- Turchetto-Zolet, A.C.; Maraschin, F.S.; de Morais, G.L.; Cagliari, A.; Andrade, C.M.; Margis-Pinheiro, M.; Margis, R. Evolutionary view of acyl-CoA diacylglycerol acyltransferase (DGAT), a key enzyme in neutral lipid biosynthesis. BMC Evol. Biol. 2011, 11, 263. [Google Scholar] [CrossRef] [Green Version]
- Cases, S.; Smith, S.J.; Zheng, Y.W.; Myers, H.M.; Lear, S.R.; Sande, E.; Novak, S.; Collins, C.; Welch, C.B.; Lusis, A.J.; et al. Identification of a gene encoding an acyl Co A: Diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 13018–13023. [Google Scholar] [CrossRef]
- Bhumika, B.W.; William, J.T.; Miller, J.H.; Peng, L. Role of DGAT enzymes in triacylglycerol metabolism. Arch. Biochem. Biophys. 2018, 655, 1–11. [Google Scholar] [CrossRef]
- Song, T.; Wang, D.H.; Liu, X.; An, Q.M.; Wang, X.W.; Ru, B.R.; Xv, Z.J.; Lei, C.C.; Chen, H.; Huang, Y.Z. Biological Function of DGAT1 gene in livestock and its application in Genetic breeding. Chin. Cattle Sci. 2020, 46, 52–56. [Google Scholar]
- Harris, C.A.; Haas, J.T.; Streeper, R.S.; Stone, S.J.; Kumari, M.; Yang, K.; Han, X.L.; Brownell, N.; Gross, R.W.; Zechner, R.; et al. DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J. Lipid Res. 2011, 52, 657–667. [Google Scholar] [CrossRef] [Green Version]
- Yen, C.-L.E. The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J. Lipid Res. 2005, 46, 1502–1511. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, T.G. Porcine preadipocyte proliferation and differentiation: A role for leptin? J. Anim. Sci. 2005, 83, 2066–2074. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.Y.; Hao, J.Y.; Li, H.Q.; Duan, Y.X.; Xie, Z.Y.; Feng, H.Y.; Chen, B.; Wang, J.P. The mechanism of action of small interfering RNA and its application in cancer research. Gansu Med. 2020, 39, 1067–1071. [Google Scholar]
- Zhang, J.F.; Choi, S.H.; Li, Q.; Wang, Y.; Sun, B.; Tang, L.; Wang, E.Z.; Hua, H.; Li, X.Z. Overexpression of DGAT2 stimulates lipid droplet formation and triacylglycerol accumulation in bovine satellite cells. Animals 2022, 12, 1847. [Google Scholar] [CrossRef]
- Kantartzis, K.; Machicao, F.; Machann, J.; Schick, F.; Stefan, N. The DGAT2 gene is a candidate for the dissociation between fatty liver and insulin resistance in humans. Clin. Sci. 2009, 116, 531–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Rojas, P.; Antaramian, A.; González-Dávalos, L.; Villarroya, F.; Shimada, A.; Varela-Echavarría, A.; Mora, O. Induction of peroxisomal proliferator-activated receptor γ and peroxisomal proliferator-activated receptor γ coactivator 1 by unsaturated fatty acids, retinoic acid, and carotenoids in preadipocytes obtained from bovine white adipose tissue. J. Anim. Sci. 2010, 88, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.X. Effects of Oleic Acid-Induced Steatosis on the Expression of Genes Related to the Balance of Lipid Metabolism in Foie Liver Cells. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2008. [Google Scholar]
- Stone, S.J. Lipopenia and Skin Barrier Abnormalities in DGAT2-deficient Mice. J. Biol. Chem. 2004, 279, 11767–11776. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.J.; Cases, S.; Jensen, D.R.; Chen, H.C.; Sande, E.; Tow, B.; Sanan, D.A.; Raber, J.; Eckel, R.H., Jr.; Farese, R.V. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking DGAT. Nat. Genet. 2000, 25, 87–90. [Google Scholar] [CrossRef]
- Yu, Y.H.; Ginsberg, H.N. The role of acyl-CoA: Diacylglycerol acyltransferase (DGAT) in energy metabolism. Ann. Med. 2004, 36, 252–261. [Google Scholar] [CrossRef]
- van Rijn, J.M.; van Hoesel, M.; de Heus, C.; van Vugt, A.H.M.; Klumperman, J.; Nieuwenhuis, E.E.S.; Houwen, R.H.J.; Middendorp, S. DGAT2 partially compensates for lipid-induced ER stress in human DGAT1-defificient intestinal stem cells. J. Lipid Res. 2019, 60, 1787–1800. [Google Scholar] [CrossRef]
- Ibrahimi, A.; Sfeir, Z.; Magharaie, H.; Amri, E.Z.; Grimaldi, P.; Abumrad, N.A. Expression of the CD36 homolog (FAT) in fifibroblast cells: Effects on fatty acid transport. Proc. Natl Acad. Sci. USA 1996, 93, 2646–2651. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.B.; Lu, W.H.; Lin, Y.; Luo, J.Z. Development and application of transcriptome sequencing. Environ. Sci. Technol. 2018, 35, 27–33. [Google Scholar]
- Qin, W.; Liang, C.N.; Guo, X.; Chu, X.; Pei, J.; Bao, P.J.; Wu, X.Y.; Li, T.K.; Yan, P. PPARα signal pathway gene expression is associated with fatty acid content in yak and cattle longissimus dorsi muscle. Genet. Mol. Res. 2015, 14, 14469–14478. [Google Scholar] [CrossRef]
- Lopes-Marques, M.; Cunha, I.; Reis-Henriques, M.A.; Santos, M.M.; Castro, L.F.C. Diversity and history of the long-chain acyl-CoA synthetase (Acsl) gene family in vertebrates. BMC Evol. Biol. 2013, 13, 271. [Google Scholar] [CrossRef] [Green Version]
- Li, L.O.; Ellis, J.M.; Paich, H.A.; Wang, S.; Gong, N.; Altshuller, G.; Thresher, R.J.; Koves, T.R.; Watkins, S.M.; Muoio, D.M.; et al. Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J. Biol. Chem. 2009, 284, 27816–27826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, S.; Wiczer, B.M.; Bernlohr, D.A. Functional analysis of long-chain acyl-CoA synthetase 1 in 3T3-L1 adipocytes. J. Biol. Chem. 2009, 284, 18347–18356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, H.C.; Kovacs, A.; Ford, D.A.; Hsu, F.F.; Garcia, R.; Herrero, P.; Saffitz, J.E.; Schaffer, J.E. A novel mouse model of lipotoxic cardiomyopathy. J. Clin. Investig. 2001, 107, 813–822. [Google Scholar] [CrossRef] [Green Version]
- Grahame Hardie, D.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Y.; Lee, M.H.; Hsu, C.C.; Wei, C.-L.; Tsai, Y.-C. Methyl cinnamate inhibits adipocyte differentiation via activation of the CaMKK2-AMPK pathway in 3T3-L1 preadipocytes. J. Agric. Food Chem. 2012, 60, 955–963. [Google Scholar] [CrossRef]
- Hwang, J.T.; Kwon, D.Y.; Yoon, S.H. AMP-activated protein kinase: A potential target for the diseases prevention by natural occurring polyphenols. New Biotechnol. 2009, 26, 17–22. [Google Scholar] [CrossRef]
- Lu, T.; Sun, L.; Wang, Z.; Zhang, Y.; He, Z.; Xu, C. Fatty acid synthase enhances colorectal cancer cell proliferation and metastasis via regulating AMPK/mTOR pathway. Onco Targets Ther. 2019, 12, 3339–3347. [Google Scholar] [CrossRef] [Green Version]
- Kongsuphol, P.; Cassidy, D.; Hieke, B.; Treharne, K.J.; Schreiber, R.; Mehta, A.; Kunzelmann, K. Mechanistic Insight into Control of CFTR by AMPK*S. J. Biol. Chem. 2009, 284, 5645–5653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, J.S.; Barshishat-Kupper, M.; Feroze-Merzoug, F.; Xi, Z.F. Secreted WNT antagonists as tumor suppressors: Pro and con. Front. Biosci. 2006, 11, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Jomary, C. Secreted Frizzled-related proteins: Searching for relationships and patterns. Bioessays 2002, 24, 811–820. [Google Scholar] [CrossRef]
- Wang, S.; Krinks, M.; Lin, K.; Moose Jr, M. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell 1997, 88, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.; Esteve, P.; Weinl, C.; Ruiz, J.M.; Fermin, Y.; Trousse, F.; Dwivedy, A.; Holt, C.; Bovolenta, P. SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat. Neurosci. 2005, 8, 1301–1309. [Google Scholar] [CrossRef]
- Kress, E.; Rezza, A.; Nadjar, J.; Samarut, J.; Plateroti, M. The frizzled-related sFRP2 gene is a target of thyroid hormone receptor α1 and activates β-catenin signaling in mouse intestine. J. Biol. Chem. 2009, 284, 1234–1241. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, K.; Rubin, J.S.; Higinbotham, K.G.; Uren, A.; Anest, V.; Plisov, S.Y.; Perantoni, A.O. Secreted Frizzled-related proteins can regulate metanephric development. Mech. Dev. 2001, 102, 45–55. [Google Scholar] [CrossRef]
- Mii, Y.; Taira, M. Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development 2009, 136, 4083–4088. [Google Scholar] [CrossRef] [Green Version]
- Muruganandan, S.; Roman, A.A.; Sinal, C.J. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenic program. Cell. Mol. Life Sci. 2009, 66, 236–253. [Google Scholar] [CrossRef] [PubMed]








| Scheme | Sequence | |
|---|---|---|
| Sence (5′ to 3′) | Sence (3′ to 5′) | |
| siRNA-DGAT1-144 | AGACAAGGACGGAGACGUATT | UACGUCUCCGUCCUUGUCUTT |
| siRNA-DGAT1-539 | CCUUUCUCCUCGAGUCUAUTT | AUAGACUCGAGGAGAAAGGTT |
| siRNA-DGAT1-1157 | GCAUCAGACACUUCUACAATT | UUGUAGAAGUGUCUGAUGCTT |
| siRNA-DGAT2-108 | GGUAGAGAAGCAGCUCCAATT | UUGGAGCUGCUUCUCUACCTT |
| siRNA-DGAT2-320 | GCUACUUUCGAGACUACUUTT | AAGUAGUCUCGAAAGUSGCTT |
| siRNA-DGAT2-818 | AGAAGAAGUUCCAGCUCCAATT | UACUUCUGGAACUUCUUCUTT |
| siRNA-NC | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
| Gene | Sense Strand (5′→3′) | Length (bp) | Gene ID |
|---|---|---|---|
| GAPDH | F:ACTCTGGCAAAGTGGATGTTGTC R:GCATCACCCCACTTGATGTTG | 143 | NM_001034034 |
| DGAT1 | F:CTACACCATCCTCTTCCTCAAG R:AGTAGTAGAGATCGCGGTAGGTC | 176 | NM_174693.2 |
| DGAT2 | F:GACCCTCATAGCCTCCTACTCC R:GACCCATTGTAGCACCGAGATGAC | 145 | NM_205793.2 |
| AGPAT4 | F:TGTTCTCGTCTTCTTTGTGGCTTCC R:TCGCTATGTTTCTGCTTGCTGTCC | 111 | NM_001015537.1 |
| MGAT1 | F:AGCCGTGGTGGTAGAGGATGATC R:TGCTCCTTGCCATTGTCGTTCC | 132 | XM_024994376.1 |
| LIPIN1 | F:AGTCCTCGCCACACAAGATG R:AGATGCCCTGACCAGTGTTG | 137 | NM_001206156.2 |
| GPAT4 | F:ATGCGGTCCAGTTTGCCAATAGG R:GCTTCTGCTGCTCCTCCTTGAAC | 129 | NM_001083669.1 |
| PPARγ | F:ATCTGCTGCAAGCCTTGGA R:TGGAGCAGCTTGGCAAAGA | 138 | NM_181024 |
| C/EBPα | F:CCAGAAGAAGGTGGAGCAACTG R:TCGGGCAGCGTCTTGAAC | 69 | NM_176788 |
| PLIN2 | F:GCGTCTGCTGGCTGATTTCT R:TGTAAGCCGAGGAGACCAGA | 139 | NM_173980.2 |
| FABP4 | F:AAACTTAGATGAAGGTGCTCTGG R:CATAAACTCTGGTGGCAGTGA | 134 | NM_174314.2 |
| SCD | F:TGCCCACCACAAGTTTTCAG R:GCCAACCCACGTGAGAGAAG | 80 | NM_173959 |
| CD36 | F:ACTGCGGATGGAATTTACAAAG R:ATGAGGCTGCATCTGTACCATTA | 142 | NM_001278621.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, P.; Yao, X.; Jin, X.; Xv, Y.; Zhang, J.; Li, Q.; Yan, C.; Li, X.; Kim, N. Interference with DGAT Gene Inhibited TAG Accumulation and Lipid Droplet Synthesis in Bovine Preadipocytes. Animals 2023, 13, 2223. https://doi.org/10.3390/ani13132223
Guo P, Yao X, Jin X, Xv Y, Zhang J, Li Q, Yan C, Li X, Kim N. Interference with DGAT Gene Inhibited TAG Accumulation and Lipid Droplet Synthesis in Bovine Preadipocytes. Animals. 2023; 13(13):2223. https://doi.org/10.3390/ani13132223
Chicago/Turabian StyleGuo, Panpan, Xuerui Yao, Xin Jin, Yongnan Xv, Junfang Zhang, Qiang Li, Changguo Yan, Xiangzi Li, and Namhyung Kim. 2023. "Interference with DGAT Gene Inhibited TAG Accumulation and Lipid Droplet Synthesis in Bovine Preadipocytes" Animals 13, no. 13: 2223. https://doi.org/10.3390/ani13132223
APA StyleGuo, P., Yao, X., Jin, X., Xv, Y., Zhang, J., Li, Q., Yan, C., Li, X., & Kim, N. (2023). Interference with DGAT Gene Inhibited TAG Accumulation and Lipid Droplet Synthesis in Bovine Preadipocytes. Animals, 13(13), 2223. https://doi.org/10.3390/ani13132223




