Protozoan Parasites of Sarcocystis spp. in Rodents from Commercial Orchards
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Pooling and Muscle Digestion
2.3. Molecular Examination
2.4. Statistical Analysis
3. Results
3.1. Prevalence of Sarcocystis spp. in Small Mammals
3.2. Molecular Characterization of Sarcocystis spp. in Small Mammals
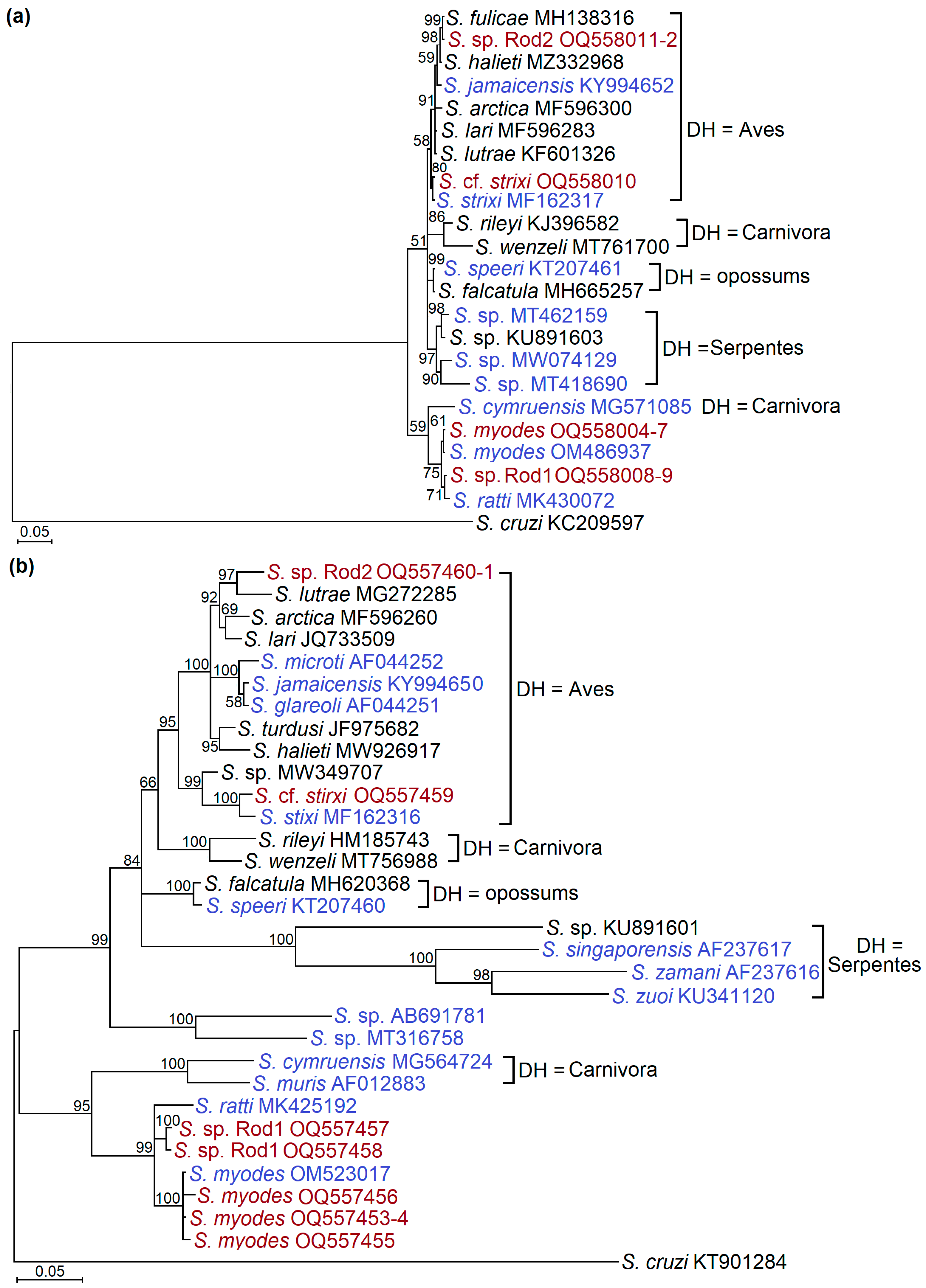
3.3. Phylogenetic Relationships between Identified Sarcocystis Species
4. Discussion
4.1. Evaluation of the Sarcocystis spp. Prevalence in Different Species of Small Mammals
4.2. Sarcocystis Species Identification and Richness in Small Mammals Inhabiting Orchards
4.3. Ecological and Phylogenetic Insights on the Definitive Hosts of Detected Sarcocystis Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, D.E.; Lacher, T.E.; Mittermeier, R.A. Handbook of the Mammals of the World; Lynx Edicions: Barselona, Spain, 2017; Volume 7. [Google Scholar]
- Burgin, C.; Colella, J.; Kahn, P.; Upham, N. How many species of mammals are there? J. Mammal. 2018, 99, 1–14. [Google Scholar] [CrossRef]
- Malecha, A.W.; Antczak, M. Diet of the European polecat Mustela putorius in an agricultural area in Poland. Folia. Zool. 2013, 62, 48–53. [Google Scholar] [CrossRef]
- Grabham, A.A.; Ventress, G.; Hayward, M.W. The diet of denning female European pine martens (Martes martes) in Galloway Forest District, South West Scotland, Great Britain. Mammal. Res. 2019, 64, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Gryz, J.; Krauze-Gryz, D. Changes in the tawny owl Strix aluco diet along an urbanisation gradient. Biologia 2019, 74, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Avotins, A.; Avotins, A., Sr.; Ķerus, V.; Aunins, A. Numerical response of owls to the dampening of small mammal population cycles in Latvia. Life 2023, 13, 572. [Google Scholar] [CrossRef] [PubMed]
- Halle, S. Diel pattern of predation risk in microtine rodents. Oikos 1993, 68, 510–518. [Google Scholar] [CrossRef]
- Fargallo, J.A.; Martinez–Padilla, J.; Vinuela, J.; Blanco, G.; Torre, I.; Vergara, P.; De Neve, L. Kestrel prey dynamic in a Mediterranean region: The effect of generalist predation and climatic factors. PLoS ONE 2009, 4, 4311. [Google Scholar] [CrossRef] [Green Version]
- Terraube, J.; Arroyo, B.; Madders, M.; Mougeot, F. Diet specialisation and foraging efficiency under fluctuating vole abundance: A comparison between generalist and specialist avian predators. Oikos 2011, 120, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Balčiauskienė, L.; Balčiauskas, L.; Vitkauskas, V.; Podėnas, S. Indoor small mammals in Lithuania: Some morphometrical, body condition, and reproductive characteristics. Zool. Ecol. 2015, 25, 305–313. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L. On the doorstep, rodents in homesteads and kitchen gardens. Animals 2020, 10, 856. [Google Scholar] [CrossRef]
- Mazza, V.; Dammhahn, M.; Lösche, E.; Eccard, J.A. Small mammals in the big city: Behavioural adjustments of non-commensal rodents to urban environments. Glob. Chang. Biol. 2020, 26, 6326–6337. [Google Scholar]
- Delattre, P.; Pascal, M.; Le Pesteur, M.H.; Giraudoux, P.; Damange, J.P. Ecological and epidemiological characteristics of Echinococcus multilocularis during a complete population cycle of the secondary host (Microtus arvalis). Can. J. Zool. 1988, 66, 2740–2750. [Google Scholar] [CrossRef]
- Pikula, J.; Treml, F.; Beklova, M.; Holešovská, Z.; Pikulova, J. Geographic information systems in epidemiology–ecology of common vole and distribution of natural foci of Tularaemia. Acta Vet. Brno 2002, 71, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Sinski, E.; Pawelczyk, A.; Bajer, A.; Behnke, J.M. Abundance of wild rodents, ticks and environmental risk of Lyme borreliosis: A longitudinal study in an area of Mazury Lakes district of Poland. Ann. Agr. Env. Med. 2006, 13, 295–300. [Google Scholar]
- Jeske, K.; Schulz, J.; Tekemen, D.; Balčiauskas, L.; Balčiauskienė, L.; Hiltbrunner, M.; Ulrich, R.G. Cocirculation of Leptospira spp. and multiple orthohantaviruses in rodents, Lithuania, Northern Europe. Transbound. Emerg. Dis. 2022, 69, e3196–e3201. [Google Scholar] [CrossRef]
- Prūsaitė, J. Lietuvos fauna. Žinduoliai; Mokslas: Vilnius, Lithuania, 1988. [Google Scholar]
- Balčiauskas, L.; Balčiauskienė, L. Small mammal diversity changes in a Baltic country, 1975–2021: A review. Life 2022, 12, 1887. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L.; Stirkė, V. Mow the grass at the mouse’s peril: Diversity of small mammals in commercial fruit farms. Animals 2019, 9, 334. [Google Scholar] [CrossRef] [Green Version]
- Stirkė, V.; Balčiauskas, L.; Balčiauskienė, L. Spatiotemporal variation of small mammal communities in commercial orchards across the small country. Agriculture 2022, 12, 632. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Stirkė, V.; Balčiauskienė, L. Abundance and population structure of small rodents in fruit and berry farms. Life 2023, 13, 375. [Google Scholar] [CrossRef]
- Lerner, H.; Berg, C. The concept of health in One Health and some practical implications for research and education: What is One Health? Infect. Ecol. Epidemiology 2015, 5, 25300. [Google Scholar] [CrossRef]
- Galán-Puchades, M.T.; Trelis, M.; Sáez-Durán, S.; Cifre, S.; Gosálvez, C.; Sanxis-Furió, J.; Pascual, J.; Bueno-Marí, R.; Franco, S.; Peracho, V.; et al. One health approach to zoonotic parasites: Molecular detection of intestinal protozoans in an urban population of Norway rats, Rattus norvegicus, in Barcelona, Spain. Pathogens 2021, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Perec-Matysiak, A.; Bunkowska-Gawlik, K.; Zalesny, G.; Hildebrand, J. Small rodents as reservoirs of Cryptosporidium spp. and Giardia spp. in south-western Poland. Ann. Agr. Env. Med. 2015, 22, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.P.; Macdonald, D.W. Cryptosporidiosis reservoir in wild brown rats (Rattus norvegicus) in the UK. Epidemiol. Infect. 1995, 115, 207–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lõhmus, M.; Albihn, A. Gastrointestinal pathogens in rodents overwintering in human facilities around Uppsala, Sweden. J. Wildlife Dis. 2013, 49, 747–749. [Google Scholar] [CrossRef] [Green Version]
- Kilonzo, C.; Li, X.; Vivas, E.J.; Jay-Russell, M.T.; Fernandez, K.L.; Atwill, E.R. Fecal shedding of zoonotic food-borne pathogens by wild rodents in a major agricultural region of the central California coast. Appl. Environ. Microb. 2013, 79, 6337–6344. [Google Scholar]
- Odening, K. The Present State of Species-Systematics in Sarcocystis Lankester, 1882 (Protista, Sporozoa, Coccidia). Syst. Parasitol. 1998, 41, 209–233. [Google Scholar] [CrossRef]
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Antunes Murata, F.H.; Cerqueira-Cézar, C.K.; Thompson, P.C.; Tiwari, K.; Mowery, J.D.; Verma, S.K.; Rosenthal, B.M.; Sharma, R.N.; Dubey, J.P. Sarcocystis cymruensis: Discovery in western hemisphere in the brown rat (Rattus norvegicus) from Grenada, West Indies: Redescription, molecular characterization, and transmission to IFN- gene knockout mice via sporocysts from experimentally infected domestic cat (Felis catus). Parasitol. Res. 2018, 117, 1195–1204. [Google Scholar] [CrossRef]
- Ortega Pérez, P.; Wibbelt, G.; Brinkmann, A.; Galindo Puentes, J.A.; Tuh, F.Y.Y.; Lakim, M.B.; Nitsche, A.; Wells, K.; Jäkel, T. Description of Sarcocystis scandentiborneensis sp. nov from treeshrews (Tupaia minor, T. tana) in Northern Borneo with annotations on the utility of COI and 18S rDNA sequences for species delineation. Int. J. Parasitol. Parasites Wildl. 2020, 12, 220–231. [Google Scholar] [CrossRef]
- Rudaitytė-Lukošienė, E.; Jasiulionis, M.; Balčiauskas, L.; Prakas, P.; Stirkė, V.; Butkauskas, D. Morphological and Molecular Description of Sarcocystis myodes n. sp. from the Bank Vole (Clethrionomys glareolus) in Lithuania. Biology. 2022, 1, 512. [Google Scholar] [CrossRef]
- Doležel, D.; Koudela, B.; Jirků, M.; Hypsa, V.; Oborník, M.; Votýpka, J.; Modrý, D.; Slapeta, J.R.; Lukes, J. Phylogenetic analysis of Sarcocystis spp. of mammals and reptiles supports the coevolution of Sarcocystis spp. with their final hosts. Int. J. Parasitol. 1999, 29, 795–798. [Google Scholar] [CrossRef]
- Hu, J.J.; Liu, T.T.; Liu, Q.; Esch, G.W.; Chen, J.Q. Sarcocystis clethrionomyelaphis Matuschka, 1986 (Apicomplexa: Sarcocystidae) infecting the large oriental vole Eothenomys miletus (Thomas) (Cricetidae: Microtinae) and its phylogenetic relationships with other species of Sarcocystis Lankester, 1882. Syst. Parasitol. 2015, 91, 273–279. [Google Scholar] [CrossRef]
- Zeng, H.; Guo, Y.; Ma, C.; Deng, S.; Hu, J.; Zhang, Y. Redescription and molecular characterization of sarcocysts of Sarcocystis cymruensis from Norway rats (Rattus norvegicus) and Sarcocystis ratti from black rats (R. rattus) in China. Parasitol. Res. 2020, 119, 3785–3791. [Google Scholar] [CrossRef]
- Votýpka, J.; Hypsa, V.; Jirků, M.; Flegr, J.; Vávra, J.; Lukes, J. Molecular phylogenetic relatedness of Frenkelia spp. (Protozoa, Apicomplexa) to Sarcocystis falcatula Stiles 1893: Is the genus Sarcocystis paraphyletic? J. Eukaryot. Microbiol. 1998, 45, 137–141. [Google Scholar] [CrossRef]
- Mugridge, N.B.; Morrison, D.A.; Johnson, A.M.; Luton, K.; Dubey, J.P.; Votýpka, J.; Tenter, A.M. Phylogenetic relationships of the genus Frenkelia: A review of its history and new knowledge gained from comparison of large subunit ribosomal ribonucleic acid gene sequences. Int. J. Parasitol. 1999, 29, 957–972. [Google Scholar] [CrossRef]
- Gajadhar, A.A.; Marquardt, W.C.; Hall, R.; Gunderson, J.; Ariztia-Carmona, E.V.; Sogin, M.L. Ribosomal RNA sequences of Sarcocystis muris, Theileria annulata and Crypthecodinium cohnii reveal evolutionary relationships among apicomplexans, dinoflagellates, and ciliates. Mol. Biochem. Parasitol. 1991, 45, 147–154. [Google Scholar] [CrossRef]
- Verma, S.K.; Lindsay, D.S.; Rosenthal, B.M.; Dubey, J.P. Ancient, globally distributed lineage of Sarcocystis from sporocysts of the eastern rat snake (Pantherophis alleghaniensis) and its relation to neurological sequalae in intermediate hosts. Parasitol. Res. 2016, 115, 2697–2704. [Google Scholar] [CrossRef]
- Verma, S.K.; Lindsay, D.S.; Mowery, J.D.; Rosenthal, B.M.; Dubey, J.P. Sarcocystis pantherophisi n. sp., from eastern rat snakes (Pantherophis alleghaniensis) as definitive hosts and interferon gamma gene knockout mice as experimental intermediate hosts. J. Parasitol. 2017, 103, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Prakas, P.; Kirillova, V.; Gavarāne, I.; Grāvele, E.; Butkauskas, D.; Rudaitytė-Lukošienė, E.; Kirjušina, M. Morphological and molecular description of Sarcocystis ratti n. sp. from the black rat (Rattus rattus) in Latvia. Parasitol. Res. 2019, 118, 2689–2694. [Google Scholar] [CrossRef]
- Mugridge, N.B.; Morrison, D.A.; Jäkel, T.; Heckeroth, A.R.; Tenter, A.M.; Johnson, A.M. Effects of sequence alignment and structural domains of ribosomal DNA on phylogeny reconstruction for the protozoan family Sarcocystidae. Mol. Biol. Evol. 2000, 17, 1842–1853. [Google Scholar] [CrossRef]
- Šlapeta, J.R.; Kyselová, I.; Richardson, A.O.; Modrý, D.; Lukeš, J. Phylogeny and sequence variability of the Sarcocystis singaporensis Zaman and Colley, (1975) 1976 ssrDNA. Parasitol. Res. 2002, 88, 810–815. [Google Scholar] [CrossRef]
- Hu, J.J.; Meng, Y.; Guo, Y.M.; Liao, J.Y.; Song, J.L. Completion of the life cycle of Sarcocystis zuoi, a parasite from the Norway rat, Rattus norvegicus. J. Parasitol. 2012, 98, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.L.; Chang, P.Y.; Subramaniam, V.; Ng, Y.H.; Mahmud, R.; Ahmad, A.F.; Fong, M.Y. Genetic assemblage of Sarcocystis spp. in Malaysian snakes. Parasit. Vectors. 2013, 6, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, N.; Matsubara, K.; Tamukai, K.; Miwa, Y.; Takami, K. Molecular evidence of Sarcocystis species in captive snakes in Japan. Parasitol. Res. 2015, 114, 3175–3179. [Google Scholar] [CrossRef] [PubMed]
- Watthanakaiwan, V.; Sukmak, M.; Hamarit, K.; Kaolim, N.; Wajjwalku, W.; Muangkram, Y. Molecular characterization of the ribosomal DNA unit of Sarcocystis singaporensis, Sarcocystis zamani and Sarcocystis zuoi from rodents in Thailand. J. Vet. Med. Sci. 2017, 79, 1412–1418. [Google Scholar] [CrossRef] [Green Version]
- Mohd Fadil, N.F.; Tengku-Idris, T.I.N.; Shahari, S.; Fong, M.Y.; Lau, Y.L. Molecular evidence of Sarcocystis species infecting reptiles in peninsular Malaysia. Iran. J. Parasitol. 2019, 14, 623–630. [Google Scholar]
- Xiang, Z.; Rosenthal, B.M.; He, Y.; Wang, W.; Wang, H.; Song, J.; Shen, P.Q.; Li, M.L.; Yang, Z. Sarcocystis tupaia, sp. nov., a new parasite species employing treeshrews (Tupaiidae, Tupaia belangeri chinensis) as natural intermediate hosts. Parasitol. Int. 2010, 59, 128–132. [Google Scholar] [CrossRef]
- Hu, J.; Sun, J.; Guo, Y.; Zeng, H.; Zhang, Y.; Tao, J. Infection of the Asian gray shrew Crocidura attenuata (Insectivora: Soricidae) with Sarcocystis attenuati n. sp. (Apicomplexa: Sarcocystidae) in China. Parasite. Vector. 2022, 15, 13. [Google Scholar] [CrossRef]
- Dissanaike, A.S.; Poopalachelvam, M. Sarcocystis booliati n. sp. and a parasite of undetermined taxonomic position, Octoplasma garnhami n. gen. n. sp., from the Moonrat, Echinosorex gymnurus. Southeast Asian J. Trop. Med. Public. Health 1975, 6, 175–185. [Google Scholar]
- Pak, S.M.; Sklyarova, O.N.; Dymkova, N.D. Sarcocysts (Sporozoa, Apicomplexa) of some wild mammals. Izvest. Akad. Nauk. Kazakh. Ser. Biol. 1991, 5, 35–40. (In Russian) [Google Scholar]
- Grikienienė, J.; Mažeikytė, R. Investigation of sarcosporidians (Sarcocystis) of small mammals in Kamasta landscape reserve and its surroundings. Acta Zool. Litu. 2000, 10, 55–68. [Google Scholar] [CrossRef]
- Grikienienė, J.; Malakauskas, M.; Mažeikytė, R.; Balčiauskas, L.; Senutaitė, J. Muscle parasites (Sarcocystis, Trichinella, Alaria) of wild mammals in Lithuania. Theriol. Litu. 2001, 1, 29–46. [Google Scholar]
- Grikienienė, J. Investigations into Endoparasites of Small Mammals in the Environs of Lake Drūkšiai. Acta Zool. Litu. 2005, 15, 109–114. [Google Scholar] [CrossRef]
- Prakas, P.; Rehbein, S.; Rudaitytė-Lukošienė, E.; Butkauskas, D. Molecular identification of Sarcocystis species in diaphragm muscle tissue of European mouflon (Ovis gmelini musimon) from Austria. Parasitol. Res. 2021, 20, 2695–2702. [Google Scholar] [CrossRef]
- Prakas, P.; Bea, A.; Juozaitytė-Ngugu, E.; Olano, I.; Villanúa, D.; Švažas, S.; Butkauskas, D. Molecular identification of Sarcocystis halieti in the muscles of two species of birds of prey from Spain. Parasite. Vector. 2021, 14, 414. [Google Scholar] [CrossRef]
- Balčiauskas, L. Methods of Investigation of Terrestrial Ecosystems, Part. I. Animal Surveys; VU Leidykla: Vilnius, Lithuania, 2004; p. 183. [Google Scholar]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, 115. [Google Scholar] [CrossRef] [Green Version]
- Holmdahl, O.J.; Morrison, D.A.; Ellis, J.T.; Huong, L.T. Evolution of ruminant Sarcocystis (Sporozoa) parasites based on small subunit rDNA sequences. Mol. Phylogenet. Evol. 1999, 11, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Gjerde, B. Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int. J. Parasitol. 2013, 43, 579–591. [Google Scholar] [CrossRef]
- Gjerde, B. Molecular characterisation of Sarcocystis rileyi from a Common Eider (Somateria mollissima) in Norway. Parasitol. Res. 2014, 113, 3501–3509. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic. Acids. Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Milne, I.; Wright, F.; Rowe, G.; Marshall, D.; Husmeier, D.; McGuire, G. TOPALi: Software for automatic identification of recombinant sequences Within DNA multiple alignments. Bioinformatics. 2004, 20, 1806–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biggerstaff, B.J. Confidence intervals for the difference of two proportions estimated from pooled samples. JABES 2008, 13, 478–496. [Google Scholar] [CrossRef]
- Biggerstaff, B.J. PooledInfRate, Version 4.0: A Microsoft® Office Excel© Add-In to Compute Prevalence Estimates from Pooled Samples. Centers for Disease Control and Prevention, Fort Collins, CO, U.S.A. 2009. Available online: https://www.cdc.gov/westnile/resourcepages/mosqSurvSoft.html (accessed on 10 January 2023).
- Hepworth, G. Confidence intervals for proportions estimated by group testing with groups of unequal size. J. Agr. Biol. Envir. St. 2005, 10, 478–497. [Google Scholar] [CrossRef]
- Abramson, J.H. WINPEPI updated: Computer programs for epidemiologists, and their teaching potential. Epidemiol. Perspect. Innov. 2011, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Sawilowsky, S.S. New effect size rules of thumb. J. Mod. Appl. Stat. Methods. 2009, 8, 597–599. [Google Scholar] [CrossRef]
- Verma, S.K.; von Dohlen, A.R.; Mowery, J.D.; Scott, D.; Cerqueira-Cézar, C.K.; Rosenthal, B.M.; Dubey, J.P.; Lindsay, D.S. Sarcocystis strixi n. sp. from a Barred Owl (Strix varia) definitive host and interferon gamma gene knockout mice as experimental intermediate host. J. Parasitol. 2017, 103, 768–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Máca, O. Molecular Identification of Sarcocystis lutrae (Apicomplexa: Sarcocystidae) from the Raccoon Dog, Nyctereutes procyonoides, and the Common Raccoon, Procyon lotor, in the Czech Republic. Parasit. Vector. 2020, 13, 231. [Google Scholar] [CrossRef]
- Prakas, P.; Kutkienė, L.; Butkauskas, D.; Sruoga, A.; Žalakevičius, M. Description of Sarcocystis lari sp. n. (Apicomplexa: Sarcocystidae) from the great black-backed gull, Larus marinus (Charadriiformes: Laridae), on the basis of cyst morphology and molecular data. Folia Parasitol. 2014, 61, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Máca, O.; Kouba, M.; Korpimäki, E.; González-Solís, D. Molecular identification of Sarcocystis sp. (Apicomplexa, Sarcocystidae) in offspring of Tengmalm’s Owls, Aegolius funereus (Aves, Strigidae). Front. Vet. Sci. 2021, 8, 804096. [Google Scholar] [CrossRef]
- Fernández-Escobar, M.; Millán, J.; Chirife, A.D.; Ortega-Mora, L.M.; Calero-Bernal, R. Molecular survey for cyst-forming coccidia (Toxoplasma gondii, Neospora caninum, Sarcocystis spp.) in Mediterranean periurban micromammals. Parasitol. Res. 2020, 119, 2679–2686. [Google Scholar] [CrossRef]
- Mayr, S.L.; Maier, K.; Müller, J.; Enderlein, D.; Gruber, A.D.; Lierz, M. Accipiter hawks (Accipitridae) confirmed as definitive Hosts of Sarcocystis turdusi, Sarcocystis cornixi and Sarcocystis sp. ex Phalacrocorax carbo. Parasitol. Res. 2016, 115, 3041–3047. [Google Scholar] [CrossRef]
- Kirillova, V.; Prakas, P.; Calero-Bernal, R.; Gavarāne, I.; Fernández-García, J.L.; Martínez-González, M.; Rudaitytė-Lukošienė, E.; Martínez-Estéllez, M.Á.H.; Butkauskas, D.; Kirjušina, M. Identification and genetic characterization of Sarcocystis arctica and Sarcocystis lutrae in red foxes (Vulpes vulpes) from Baltic States and Spain. Parasit. Vector. 2018, 11, 173. [Google Scholar] [CrossRef] [Green Version]
- Juozaitytė-Ngugu, E.; Švažas, S.; Šneideris, D.; Rudaitytė-Lukošienė, E.; Butkauskas, D.; Prakas, P. The role of birds of the family Corvidae in transmitting Sarcocystis protozoan parasites. Animals. 2021, 11, 3258. [Google Scholar] [CrossRef]
- Rogers, K.H.; Arranz-Solís, D.; Saeij, J.P.J.; Lewis, S.; Mete, A. Sarcocystis calchasi and other Sarcocystidae detected in predatory birds in California, USA. Int. J. Parasitol. Parasites Wildl. 2021, 17, 91–99. [Google Scholar] [CrossRef]
- Máca, O.; González-Solís, D. Role of three bird species in the life cycle of two Sarcocystis spp. (Apicomplexa, Sarcocystidae) in the Czech Republic. Int. J. Parasitol. Parasites Wildl. 2022, 17, 133–137. [Google Scholar] [CrossRef]
- Máca, O.; González-Solís, D. White-Tailed Eagle (Haliaeetus albicilla) as the definitive Host of Sarcocystis lutrae in the Czech Republic. Front. Vet. Sci. 2022, 9, 981829. [Google Scholar] [CrossRef]
- Šukytė, T.; Butkauskas, D.; Juozaitytė-Ngugu, E.; Švažas, S.; Prakas, P. Molecular confirmation of Accipiter birds of prey as definitive hosts of numerous Sarcocystis species, including Sarcocystis sp., closely related to pathogenic S. calchasi. Pathogens 2023, 12, 752. [Google Scholar] [CrossRef]
- Kim, T.H.; Han, J.H.; Chang, S.N.; Kim, D.S.; Abdelkader, T.S.; Seok, S.H.; Park, J.H.; Oh, H.S.; Kim, J.T.; Lee, B.H.; et al. Detection of sarcocystic infection in a wild rodent (Apodemus agrarius chejuensis) captured on Jeju island. Lab. Anim. Res. 2011, 27, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Nevo, E. Adaptive convergence and divergence of subterranean mammals. Annu. Rev. Ecol. Syst. 1979, 10, 269–308. [Google Scholar] [CrossRef]
- Šlapeta, J.R.; Modrý, D.; Votýpka, J.; Jirků, M.; Lukeš, J.; Koudela, B. Evolutionary relationships among Cyst-Forming Coccidia Sarcocystis spp. (Alveolata: Apicomplexa: Coccidea) in endemic African tree vipers and perspective for evolution of heteroxenous life cycle. Mol. Phylogenet. Evol. 2003, 27, 464–475. [Google Scholar] [CrossRef]
- Moré, G.; Regensburger, C.; Gos, M.L.; Pardini, L.; Verma, S.K.; Ctibor, J.; Serrano-Martínez, M.E.; Dubey, J.P.; Venturini, M.C. Sarcocystis masoni, n. sp. (Apicomplexa: Sarcocystidae), and redescription of Sarcocystis aucheniae from Lama (Lama glama), Guanaco (Lama guanicoe) and Alpaca (Vicugna pacos). Parasitology 2016, 143, 617–626. [Google Scholar] [CrossRef]
- Prakas, P.; Butkauskas, D.; Juozaitytė-Ngugu, E. Molecular and morphological description of Sarcocystis kutkienae sp. nov. from the common raven (Corvus corax). Parasitol. Res. 2020, 119, 4205–4210. [Google Scholar] [CrossRef]
- Máca, O.; González-Solís, D. Sarcocystis cristata sp. nov. (Apicomplexa, Sarcocystidae) in the imported great blue turaco Corythaeola cristata (Aves, Musophagidae). Parasit. Vector. 2021, 14, 56. [Google Scholar] [CrossRef]
- Berra, Y.; Moré, G.; Helman, E.; Argibay, H.D.; Orozco, M.M. Identification of a new Sarcocystis sp. in marsh deer (Blastocerus dichotomus) from wetlands of Argentina. Int. J. Parasitol. Parasites Wildl. 2023, 20, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Balčiauskas, L.; Trakimas, G.; Juškaitis, R.; Ulevičius, A.; Balčiauskienė, L. Atlas of Lithuanian Mammals, Amphibians and Reptiles, 2nd ed.; Akstis: Vilnius, Lithuania, 1999; p. 112. [Google Scholar]


| Sample Site | Host Species | |||||||
|---|---|---|---|---|---|---|---|---|
| Apodemus agrarius | Apodemus flavicollis | Clethrionomys glareolus | Microtus agrestis | Microtus arvalis | Microtus oeconomus | Sorex araneus | Sorex minutus | |
| Aukštikalniai | 11 (2) | 4 (1) | 36 (5) | 2 (1) | ||||
| Naradava | 42 (5) | 36 (5) | 5 (1) | 6 (1) | ||||
| Mieliūnai | 16 (2) | |||||||
| Užpaliai | 24 (3) | 3 (1) | 69 (7) | 2 (1) | ||||
| Kalpokai | 3 (1) | |||||||
| Ažuožeriai | 7 (1) | 29 (3) | 40 (5) | 2 (1) | ||||
| Tytuvėnai | 28 (3) | 28 (3) | 5 (1) | |||||
| Taujėnai | 7 (1) | 6 (1) | ||||||
| Dembava | 17 (2) | |||||||
| Barčiai | 10 (1) | 9 (1) | 8 (1) | 8 (1) | ||||
| Luksnėnai | 7 (2) | 42 (6) | 22 (2) | 10 (2) | 3 (1) | |||
| Gaurė | 5 (1) | |||||||
| Šešuolėliai | 13 (2) | 9 (1) | ||||||
| Žiežmariai | 45 (5) | 54 (6) | 6 (1) | |||||
| Primer Name | Sequence | Region | Round of Nested PCR | Ta, °C | Approximate Length of PCR Product a |
|---|---|---|---|---|---|
| Sgrau181 PS | AAGTATAAGCTTTTATACGGCGAAA | 18S rDNA | First | 61 | 900 |
| Sgrau182 PS | TCGCAGTAGTTCGTCTTTAACAAA | ||||
| Sgrau183 PS | TGGATAACCGTGGTAATTCTATG | Second | 59 | 750 | |
| Sgrau184 PS | TCCCTATTAATCATTACTTCAGTCCTA | ||||
| Sgrau281 PS | GCGGAGGAAAAGAAAATAACAAT | 28S rDNA | First | 61 | 900 |
| Sgrau282 PS | CTATCGCTTAGGACCGGCTA | ||||
| Sgrau283 PS | GTGAACAGGGAAGAGCTCAA | Second | 59 | 800 | |
| Sgrau284 PS | CTCCACGTCTTCCTACTCATTG | ||||
| SU1F b | GATTGAGTGTTCCGGTGAATTATT | ITS1 d | First | 59 | 1100 |
| 5.8SR2 b | AAGGTGCCATTTGCGTTCAGAA | ||||
| SgrauITS3 PS | GGGAAGTTTTGTGAACCTTAACACT | Second | 57 | 950 | |
| SgrauITS4 PS | ATTCTGCAATTCACATTGCGTTT | ||||
| SF1 c | ATGGCGTACAACAATCATAAAGAA | cox1 | First | 59 | 1100 |
| SR5 c | TAGGTATCATGTAACGCAATATCCAT | ||||
| SgraucoF1 PS | GGTTTTGGTAACTACTTTGTACCG | Second | 59 | 660 | |
| SgraucoR1 PS | ACCTCTAATCCTACGGTCATCA |
| Sample | Number of Individuals Screened | Number of Pools Analyzed | Number of Positive Pools | Prevalence (95% Confidence Intervals) |
|---|---|---|---|---|
| Species | ||||
| Apodemus agrarius | 146 | 19 | 1 | 0.68 (0.04–3.26) |
| Apodemus flavicollis | 242 | 31 | 2 | 0.84 (0.15–2.75) |
| Mice | 388 | 50 | 3 | 0.79 (0.21–2.12) |
| Clethrionomys glareolus | 73 | 9 | 1 | 1.34 (0.08–6.43) |
| Microtus agrestis | 10 | 2 | 0 | 0 |
| Microtus arvalis | 199 | 26 | 4 | 2.16 (0.71–5.18) |
| Microtus oeconomus | 4 | 2 | 1 | 24.87 (1.64–81.95) |
| Voles | 292 | 39 | 6 | 2.23 (0.92–4.59) |
| Sorex araneus | 2 | 1 | 0 | 0 |
| Sorex minutus | 3 | 1 | 0 | 0 |
| Shrews | 5 | 2 | 0 | 0 |
| Total | 679 | 91 | 9 | 1.38 (0.68–2.52) |
| Sites | ||||
| 1 | 53 | 9 | 2 | 3.77 (0.73–11.86) |
| 2 | 78 | 10 | 1 | 1.27 (0.08–6.09) |
| 3 | 35 | 4 | 0 | 0 |
| 4 | 17 | 2 | 1 | 5.32 (0.39–31.68) |
| 5 | 5 | 1 | 0 | 0 |
| 6 | 3 | 1 | 0 | 0 |
| 7 | 84 | 14 | 1 | 1.18 (0.07–5.67) |
| 8 | 16 | 2 | 0 | 0 |
| 9 | 89 | 12 | 0 | 0 |
| 10 | 22 | 3 | 0 | 0 |
| 11 | 13 | 2 | 0 | 0 |
| 12 | 61 | 7 | 1 | 1.65 (0.10–8.11) |
| 13 | 98 | 12 | 3 | 3.49 (0.94–9.57) |
| 14 | 105 | 12 | 0 | 0 |
| Feature | Sarcocystis Species | |||
|---|---|---|---|---|
| S. myodes | Sarcocystis cf. strixi | Sarcocystis sp. Rod1 | Sarcocystis sp. Rod2 * | |
| IH | Apodemus agrarius, Apodemus flavicollis, Clethrionomys glareolus, Microtus arvalis | Apodemus flavicollis | Microtus arvalis, Microtus oeconomus | Microtus arvalis |
| Sequence similarity | ||||
| Cox1 | 100% S. myodes, 99.68% Sarcocystis sp. Rod1, 99.19% S. ratti, 95.80% S. strixi | 100% S. strixi, 99.52% S. lutrae, 99.52% S. lari | 99.68% S. myodes, 99.52% S. ratti, 95.48% S. strixi | 100% S. fulicae, 100% S. cornixi, 99.82% S. columbae, 99.82% S. corvusi, 99.82% S. turdusi, 99.82% S. halieti |
| 28S rDNA | 99.18–100% S. myodes, 97.28–97.82% Sarcocystis sp. Rod1, 95.92–96.46% S. ratti, 88.36–88.90% S. cymruensis, 88.35–88.89% S. muris | 98.91% S. strixi, 95.37% Sarcocystis sp. (MW349707), 95.24% S. lari, 94.97% S. turdusi | 97.28–97.82% S. myodes, 97.28–97.55% S. ratti, 90.24–90.26% S. cymruensis, 89.17% S. muris | 97.11–97.25% S. arctica, 97.12% S. lari, 97.12% S. lutrae |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakas, P.; Stirkė, V.; Šneideris, D.; Rakauskaitė, P.; Butkauskas, D.; Balčiauskas, L. Protozoan Parasites of Sarcocystis spp. in Rodents from Commercial Orchards. Animals 2023, 13, 2087. https://doi.org/10.3390/ani13132087
Prakas P, Stirkė V, Šneideris D, Rakauskaitė P, Butkauskas D, Balčiauskas L. Protozoan Parasites of Sarcocystis spp. in Rodents from Commercial Orchards. Animals. 2023; 13(13):2087. https://doi.org/10.3390/ani13132087
Chicago/Turabian StylePrakas, Petras, Vitalijus Stirkė, Donatas Šneideris, Paulina Rakauskaitė, Dalius Butkauskas, and Linas Balčiauskas. 2023. "Protozoan Parasites of Sarcocystis spp. in Rodents from Commercial Orchards" Animals 13, no. 13: 2087. https://doi.org/10.3390/ani13132087
APA StylePrakas, P., Stirkė, V., Šneideris, D., Rakauskaitė, P., Butkauskas, D., & Balčiauskas, L. (2023). Protozoan Parasites of Sarcocystis spp. in Rodents from Commercial Orchards. Animals, 13(13), 2087. https://doi.org/10.3390/ani13132087








