Effects of Various LED Light Spectra on Growth, Gonadal Development, and Growth-/Reproduction-Related Hormones in the Juvenile Red Spotted Grouper, Epinephelus akaara
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
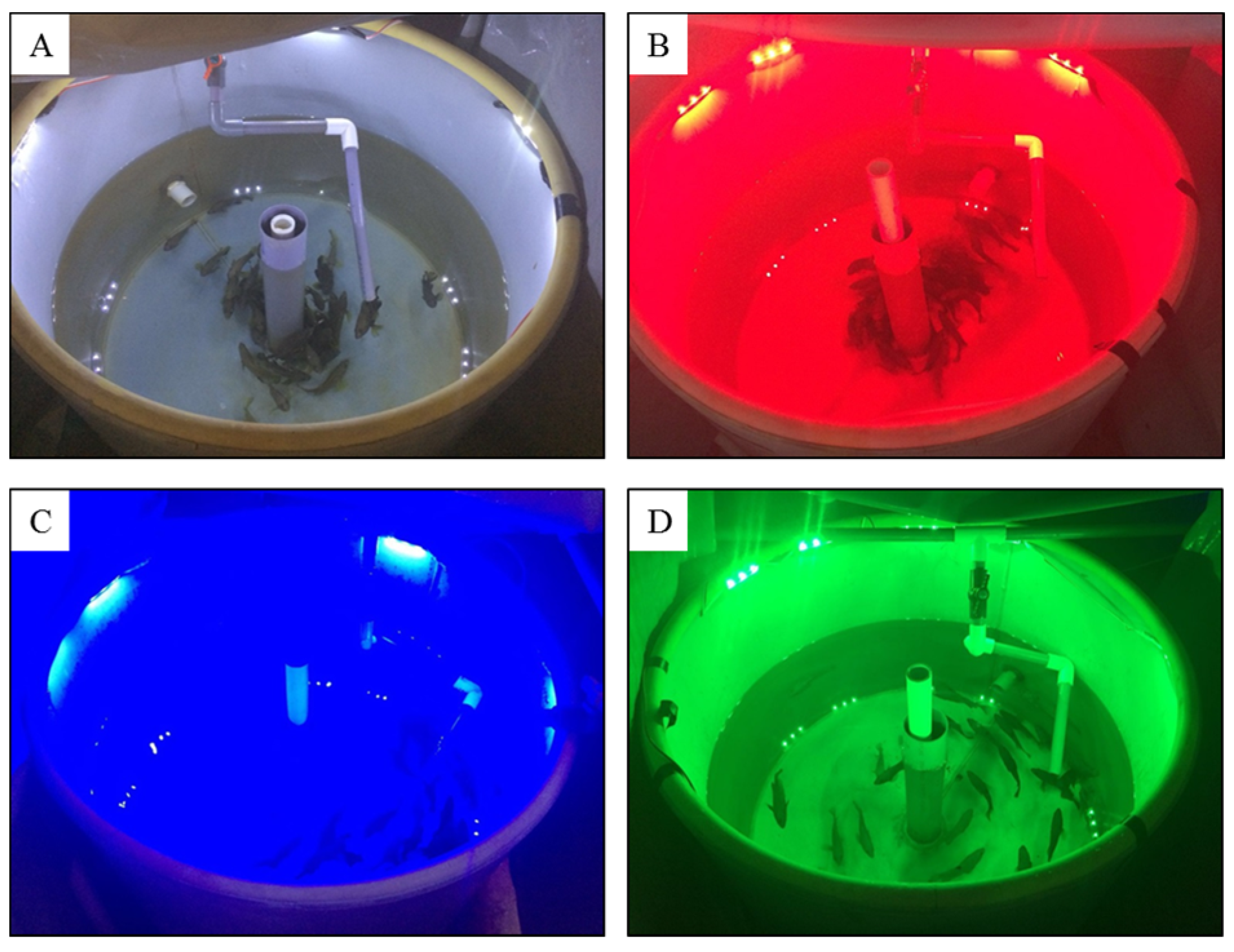
2.1. Experimental Fish and Conditions
2.2. Calculations of Growth Parameters
2.3. Sampling Procedures and Sample Preparation
2.4. Histological Procedures on Gonads
2.5. Total RNA Extraction and cDNA Synthesis
2.6. Quantitative Real-Time PCR
2.7. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Gonadal Development
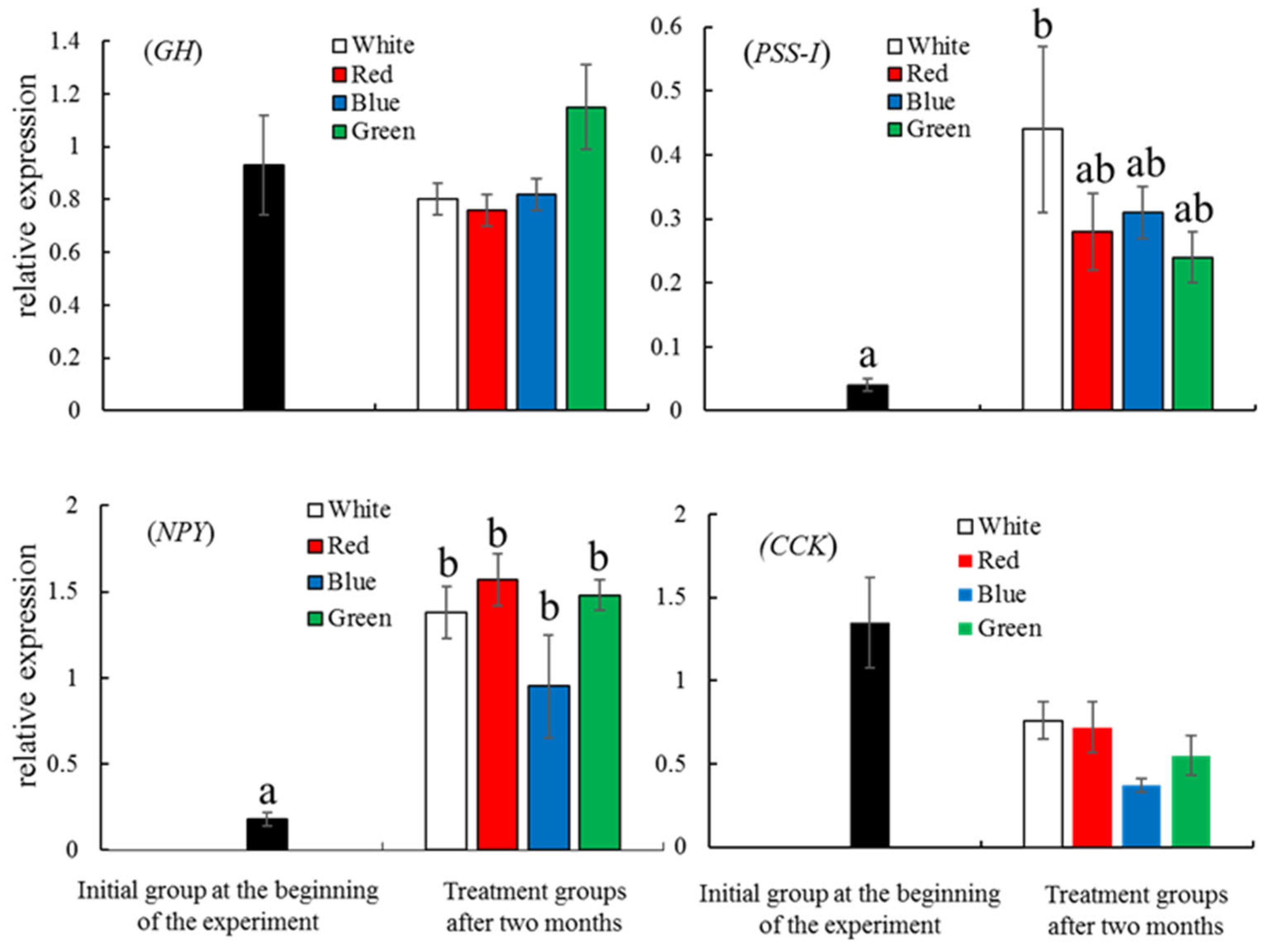
3.3. Expression of Growth-Related and Reproduction-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tupper, M.; Sheriff, N. Capture-based aquaculture of groupers. In Capture-Based Aquaculture: Global Overview; Lovatelli, A., Holthus, P.F., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008; pp. 217–253. [Google Scholar]
- Lee, C.H.; Hur, S.W.; Kim, B.H.; Soyano, K.; Lee, Y.D. Induced maturation and fertilized egg production of the red-spotted grouper, Epinephelus akaara, using adaptive physiology of photoperiod and water temperature. Aquac. Res. 2020, 51, 2084–2090. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.H.; Choi, S.H.; Lee, Y.D.; Lee, C.H. Annual changes in ovarian development stages of farmed female red-spotted grouper, Epinephelus akaara, and inducing sexual maturity using photoperiod manipulation. J. World Aquac. Soc. 2022, 53, 1–14. [Google Scholar] [CrossRef]
- Ruchin, A.B. Influence of colored light on growth rate of juveniles of fish. Fish Physiol. Biochem. 2004, 30, 175–178. [Google Scholar] [CrossRef]
- Migaud, H.; Davie, A.; Taylor, J.F. Current knowledge on the photoneuroendocrine regulation of reproduction in temperate fish species. J. Fish Biol. 2010, 76, 27–68. [Google Scholar] [CrossRef]
- Villamizar, N.; Blanco-Vives, B.; Migaud, H.; Davie, A.; Carboni, S.; Sánchez-Vázquez, F.J. Effects of light during early larval development of some aquacultured teleosts: A review. Aquaculture 2011, 315, 86–94. [Google Scholar] [CrossRef]
- Xu, W.G.; Liu, L.M.; Wang, J.L.; Yu, W.S.; Zhang, J.B.; Soyano, K.; Tang, Y.Z. Effects of four LED light sources on growth and gonadal development in one-year-old red spotted grouper Epinephelus akaara. Fish. Sci. 2022, 41, 343–351, (In Chinese with English Abstract). [Google Scholar]
- Villamizar, N.; García-Alcazar, A.; Sánchez-Vázquez, F.J. Effect of light spectrum and photoperiod on the growth, development and survival of European sea bass (Dicentrarchus labrax) larvae. Aquaculture 2009, 292, 80–86. [Google Scholar] [CrossRef]
- Karakatsouli, N.; Papoutsoglou, S.E.; Pizzonia, G.; Tsatsos, G.; Tsopelakos, A.; Chadio, S.; Kalogiannis, D.; Dalla, C.; Polissidis, A.; Papadopoulou-Daifoti, Z. Effects of light spectrum on growth and physiological status of gilthead seabream Sparus aurata and rainbow trout Oncorhynchus mykiss reared under recirculating system conditions. Aquacult. Eng. 2007, 36, 302–309. [Google Scholar] [CrossRef]
- Bapary, M.A.J.; Amin, M.N.; Takeuchi, Y.; Takemura, A. The stimulatory effects of long wavelengths of light on the ovarian development in the tropical damselfish, Chrysiptera cyanea. Aquaculture 2011, 314, 188–192. [Google Scholar] [CrossRef]
- Job, S.D.; Shand, J. Spectral sensitivity of larval and juvenile coral reef fishes: Implications for feeding in a variable light environment. Mar. Ecol. Prog. Ser. 2001, 214, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Siebeck, U.E.; Wallis, G.M.; Litherland, L. Colour vision in coral reef fish. J. Exp. Biol. 2008, 211, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Canosa, L.F.; Chang, J.P.; Peter, R.E. Neuroendocrine control of growth hormone in fish. Gen. Comp. Endocrinol. 2007, 151, 1–26. [Google Scholar] [CrossRef]
- Jeon, E.J.; Kim, B.H.; Lee, C.H.; Lee, Y.D. Response of appetite-related genes in relation to the rearing water temperature in red spotted grouper (Epinephelus akaara). Fish. Aquat. Sci. 2020, 23, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zimanyi, I.A.; Fathi, Z.; Poindexter, G.S. Central control of feeding behavior by neuropeptide Y. Curr. Pharm. Des. 1998, 4, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H.; Canosa, L.F.; Unniappan, S.; Cerdá-Reverter, J.M.; Bernier, N.J.; Kelly, S.P.; Peter, R.E. Neuropeptides and the control of food intake in fish. Gen. Comp. Endocrinol. 2005, 142, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Dockray, G.J. Cholecystokinin and gut-brain signalling. Regul. Pept. 2009, 155, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Humphries, S.; Peter, R.E.; Rivier, J.E.; Blomqvist, A.G.; Larhammar, D. Actions of goldfish neuropeptide Y on the secretion of growth hormone and gonadotropin-II in female goldfish. Gen. Comp. Endocrinol. 1993, 90, 306–317. [Google Scholar] [CrossRef]
- Shin, H.S.; Lee, J.; Choi, C.Y. Effects of LED light spectra on the growth of the yellowtail clownfish Amphiprion clarkii. Fish. Sci. 2012, 78, 549–556. [Google Scholar] [CrossRef]
- Brüning, A.; Hölker, F.; Franke, S.; Kleiner, W.; Kloas, W. Impact of different colours of artificial light at night on melatonin rhythm and gene expression of gonadotropins in European perch. Sci. Total. Environ. 2016, 543, 214–222. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, B.H.; Hur, S.P.; Lee, C.H.; Lee, Y.D. Effects of different light spectra on the oocyte maturation in grass puffer Takifugu niphobles. Dev. Reprod. 2018, 22, 175–182. [Google Scholar] [CrossRef]
- Pawson, A.J.; McNeilly, A.S. The pituitary effects of GnRH. Anim. Reprod. Sci. 2005, 88, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Akazome, Y.; Kanda, S.; Okubo, K.; Oka, Y. Functional and evolutionary insights into vertebrate kisspeptin systems from studies of fish brain. J. Fish. Biol. 2010, 76, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Parhar, I.S.; Ogawa, S.; Sakuma, Y. Laser-captured single digoxigenin-labeled neurons of gonadotropin-releasing hormone types reveal a novel G protein-coupled receptor (Gpr54) during maturation in cichlid fish. Endocrinology 2004, 145, 3613–3618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.S.; Habibi, H.R.; Choi, C.Y. The environmental regulation of maturation in goldfish, Carassius auratus: Effects of various LED light spectra. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 168, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.Y.; Shin, H.S.; Kim, N.N.; Yang, S.G.; Kim, B.S.; Yu, Y.M. Time-related effects of various LED light spectra on reproductive hormones in the brain of the goldfish Carassius auratus. Biol. Rhythm Res. 2015, 46, 671–682. [Google Scholar] [CrossRef]
- Kim, B.H.; Hur, S.P.; Hur, S.W.; Lee, C.H.; Lee, Y.D. Relevance of light spectra to growth of the rearing tiger puffer Takifugu rubripes. Dev. Reprod. 2016, 20, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Weber, R.A.; Peleteiro, J.B.; García Martín, L.O.; Aldegunde, M. The efficacy of 2-phenoxyethanol, metomidate, clove oil and MS-222 as anaesthetic agents in the Senegalese sole (Solea senegalensis Kaup 1858). Aquaculture 2009, 288, 147–150. [Google Scholar] [CrossRef]
- Kim, B.H.; Choi, S.H.; Lee, C.H.; Lee, Y.D. Effects of Light Spectra on Body Growth in Red Spotted Grouper, Epinephelus akaara. In Proceedings of the 36th Annual Meeting of the Korean Society of Developmental Biology, Seoul, South Korea, 31 August 2017; Available online: https://db.koreascholar.com/article/Detail/354563 (accessed on 31 August 2017).
- Hur, S.W.; Lee, C.H.; Baek, H.J.; Kim, H.B.; Lee, Y.D. Effects of Light Spectrum on Growth Performance, Goblet Cell Activation, and CCK Expression in the LONGTOOTH GROuper, Epinephelus bruneus. In Proceedings of the 30th Annual Meeting of the Korean Society of Developmental Biology, Seoul, South Korea, 30 September 2011; Available online: https://db.koreascholar.com/Article/Detail/354882 (accessed on 30 September 2011).
- Takeshita, A.; Soyano, K. Effects of light intensity and color of rearing tank on cannibalism in the juvenile orange-spotted grouper (Epinephelus coioides). Aquacult. Sci. 2008, 56, 175–180. [Google Scholar] [CrossRef]
- Luchiari, A.C.; Freire, F.A.M. Effects of environmental colour on growth of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758), maintained individually or in groups. J. Appl. Ichthyol. 2009, 25, 162–167. [Google Scholar] [CrossRef]
- Volpato, G.L.; Bovi, T.S.; de Freitas, R.H.; da Silva, D.F.; Delicio, H.C.; Giaquinto, P.C.; Barreto, R.E. Red light stimulates feeding motivation in fish but does not improve growth. PLoS ONE 2013, 8, e59134. [Google Scholar] [CrossRef] [Green Version]
- Volpato, G.L.; Barreto, R.E. Environmental blue light prevents stress in the fish Nile tilapia. Braz. J. Med. Biol. Res. 2001, 34, 1041–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakatsouli, N.; Papoutsoglou, S.E.; Panopoulos, G.; Papoutsoglou, E.S.; Chadio, S.; Kalogiannis, D. Effects of light spectrum on growth and stress response of rainbow trout Oncorhynchus mykiss reared under recirculating system conditions. Aquacult. Eng. 2008, 38, 36–42. [Google Scholar] [CrossRef]
- Güller, U.; Önalan, S.; Arabacı, M.; Karataş, B.; Yaşar, M.; Küfrevioğlu, Ö.İ. Effects of different LED light spectra on rainbow trout (Oncorhynchus mykiss): In vivo evaluation of the antioxidant status. Fish Physiol. Biochem. 2020, 46, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Yamanome, T.; Mizusawa, K.; Hasegawa, E.I.; Takahashi, A. Green light stimulates somatic growth in the barfin flounder Verasper moseri. J. Exp. Zool. Part A 2009, 311, 73–79. [Google Scholar] [CrossRef]
- Cho, H.C.; Kim, J.E.; Kim, H.B.; Baek, H.J. Effects of water temperature change on the hematological responses and plasma cortisol levels in growing of red spotted grouper, Epinephelus akaara. Dev. Reprod. 2015, 19, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Buentello, J.A.; Gatlin III, D.M.; Neill, W.H. Effects of water temperature and dissolved oxygen on daily feed consumption, feed utilization and growth of channel catfish (Ictalurus punctatus). Aquaculture 2000, 182, 339–352. [Google Scholar] [CrossRef]
- Britton, J.R.; Harper, D.M.; Oyugi, D.O. Is the fast growth of an equatorial Micropterus salmoides population explained by high water temperature? Ecol. Freshw. Fish 2010, 19, 228–238. [Google Scholar] [CrossRef]
- Oh, S.B.; Lee, C.H.; Lee, Y.D. Induction of puberty in red spotted grouper, Epinephelus akaara by water temperature. J. Aquac. Res. Dev. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Sakamoto, T.; McCormick, S.D.; Hirano, T. Osmoregulatory actions of growth hormone and its mode of action in salmonids: A review. Fish Physiol. Biochem. 1993, 11, 155–164. [Google Scholar] [CrossRef]
- Johnsson, J.I.; Björnsson, B.T. Growth hormone increases growth rate, appetite and dominance in juvenile rainbow trout, Oncorhynchus mykiss. Anim. Behav. 1994, 48, 177–186. [Google Scholar] [CrossRef]
- Björnsson, B.T. The biology of salmon growth hormone: From daylight to dominance. Fish Physiol. Biochem. 1997, 17, 9–24. [Google Scholar] [CrossRef]
- Gahete, M.D.; Cordoba-Chacón, J.; Duran-Prado, M.; Malagón, M.M.; Martinez-Fuentes, A.J.; Gracia-Navarro, F.; Luque, R.M.; Castaño, J.P. Somatostatin and its receptors from fish to mammals. Ann. Ny. Acad. Sci. 2010, 1200, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Shlomo, A.; Melmed, S. Pituitary somatostatin receptor signaling. Trends Endocrin. Met. 2010, 21, 123–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björnsson, B.T.; Johansson, V.; Benedet, S.; Einarsdottir, I.E.; Hildahl, J.; Agustsson, T.; Jönsson, E. Growth hormone endocrinology of salmonids: Regulatory mechanisms and mode of action. Fish Physiol. Biochem. 2002, 27, 227–242. [Google Scholar] [CrossRef]
- Shahjahan, M.; Motohashi, E.; Doi, H.; Ando, H. Elevation of Kiss2 and its receptor gene expression in the brain and pituitary of grass puffer during the spawning season. Gen. Comp. Endocrinol. 2010, 169, 48–57. [Google Scholar] [CrossRef]
- Alvarado, M.V.; Carrillo, M.; Felip, A. Expression of kisspeptins and their receptors, gnrh-1/gnrhr-II-1a and gonadotropin genes in the brain of adult male and female European sea bass during different gonadal stages. Gen. Comp. Endocrinol. 2013, 187, 104–116. [Google Scholar] [CrossRef]
- Espigares, F.; Carrillo, M.; Gómez, A.; Zanuy, S. The forebrain-midbrain acts as functional endocrine signaling pathway of Kiss2/Gnrh1 system controlling the gonadotroph activity in the teleost fish European sea bass (Dicentrarchus labrax). Biol. Reprod. 2015, 92, 1–13. [Google Scholar] [CrossRef]
- Zmora, N.; Stubblefield, J.; Golan, M.; Servili, A.; Levavi-Sivan, B.; Zohar, Y. The medio-basal hypothalamus as a dynamic and plastic reproduction-related kisspeptin-gnrh-pituitary center in fish. Endocrinology 2014, 155, 1874–1886. [Google Scholar] [CrossRef] [Green Version]
- Prat, F.; Sumpter, J.P.; Tyler, C.R. Validation of radioimmunoassays for two salmon gonadotropins (GTH I and GTH II) and their plasma concentrations throughout the reproductive cycle in male and female rainbow trout (Oncorhynchus mykiss). Biol. Reprod. 1996, 54, 1375–1382. [Google Scholar] [CrossRef] [Green Version]
- Tyler, C.R.; Pottinger, T.G.; Coward, K.; Prat, F.; Beresford, N.; Maddix, S. Salmonid follicle-stimulating hormone (GtH I) mediates vitellogenic development of oocytes in the rainbow trout, Oncorhynchus mykiss. Biol. Reprod. 1997, 57, 1238–1244. [Google Scholar] [CrossRef]
- Planas, J.V.; Athos, J.; Goetz, F.W.; Swanson, P. Regulation of ovarian steroidogenesis in vitro by follicle-stimulating hormone and luteinizing hormone during sexual maturation in salmonid fish. Biol. Reprod. 2000, 62, 1262–1269. [Google Scholar] [CrossRef] [Green Version]
- Kajimura, S.; Yoshiura, Y.; Suzuki, M.; Utoh, T.; Horie, N.; Oka, H.; Aida, K. Changes in the levels of mRNA coding for gonadotropin Iβ and IIβ subunits during vitellogenesis in the common Japanese conger Conger myriaster. Fish. Sci. 2001, 67, 1053–1062. [Google Scholar] [CrossRef]
- Suetake, H.; Okubo, K.; Sato, N.; Yoshiura, Y.; Suzuki, Y.; Aida, K. Differential expression of two gonadotropin (GTH) β subunit genes during ovarian maturation induced by repeated injection of salmon GTH in the Japanese eel Anguilla japonica. Fish. Sci. 2002, 68, 290–298. [Google Scholar] [CrossRef]
- Molés, G.; Gómez, A.; Carrillo, M.; Rocha, A.; Mylonas, C.C.; Zanuy, S. Determination of Fsh quantity and bioactivity during sex differentiation and oogenesis in European sea bass. Biol. Reprod. 2011, 85, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Hirose, K.; Nogami, K.; Hattori, K.; Ishibashi, N. Sexual maturation and sex reversal in red spotted grouper, Epinephelus akaara. Bull. Natl. Res. Inst. Aquacult. 1990, 17, 1–15, (In Japanese with English Abstract). [Google Scholar]




| Gene | Primer | Sequence (5′-3′) | Accession No. | Amplicon (bp) |
|---|---|---|---|---|
| GH | Forward | CGATCTCCTATCGGTTGGTGG | AY326406 | 128 |
| Reverse | CAGCAGTTCGTACGTTCGTC | |||
| PSS-I | Forward | CTCCTCTCTGACCTCCTGCA | AY677120 | 165 |
| Reverse | GTTCTTGCAGCCAGCTTTCC | |||
| NPY | Forward | TGCATCCTAACTTGGTGAGC | LC260519 | 204 |
| Reverse | TGGACCTCTTCCCATACCTC | |||
| CCK | Forward | GACACCCACACCCTAGGAGA | LC260518 | 186 |
| Reverse | TCCGTTGACTCTGCTGTTTG | |||
| Kiss1 | Forward | TGCCACGACTCATTGTTGC | LC102811 | 225 |
| Reverse | AGATCCACCATCCTGACCTG | |||
| Kiss2 | Forward | GGCCTGATTGTTGGACAGGA | GU984383 | 166 |
| Reverse | TCTCGCTCAGGGACAAACAC | |||
| GPR54 | Forward | TCTCCCTGGATGGATCTTTG | MH791444 | 198 |
| Reverse | GAGCCAATCCAAATGCAGAT | |||
| sbGnRH | Forward | ACTGTGTCTGCTGCTTGTGG | MF092862 | 192 |
| Reverse | TTGGCAAAAGGTGATTCCTC | |||
| FSHβ | Forward | ACGTGAGACCTGCAGACGAT | KJ534537 | 301 |
| Reverse | AGTTTCTGGCCACAGGGTAG | |||
| LHβ | Forward | TACAGGTCGGCAGAGTGATG | KJ534538 | 389 |
| Reverse | CTCGAAGGTGCAGTCAGATG | |||
| β-actin | Forward | GAGCGTGGCTACTCCTTCAC | HQ007251 | 390 |
| Reverse | AGGAAGGAAGGCTGGAAGAG |
| Light Spectra | ||||
|---|---|---|---|---|
| White (N = 40) | Red (N = 40) | Blue (N = 40) | Green (N = 40) | |
| Initial BW (g) | 36.62 ± 0.82 | 38.66 ± 0.75 | 38.26 ± 0.81 | 38.97 ± 0.57 |
| The first-month BW (g) | 67.42 ± 1.76 a | 57.69 ± 1.52 b | 54.89 ± 1.61 bc | 50.96 ± 0.83 c |
| Final BW (g) | 72.38 ± 2.41 a | 69.78 ± 2.1 ab | 65.63 ± 1.94 ab | 63.2 ± 1.53 b |
| Initial TL (cm) | 15.04 ± 0.15 | 15.04 ± 0.1 | 15.51 ± 0.12 | 15.42 ± 0.09 |
| The first-month TL (cm) | 16.45 ± 0.13 a | 16.11 ± 0.13 ab | 15.83 ± 0.16 b | 15.64 ± 0.09 b |
| Final TL (cm) | 17.36 ± 0.15 a | 17.01 ± 0.14 ab | 16.82 ± 0.16 b | 16.77 ± 0.09 b |
| Initial CF (g/cm3) | 1.08 ± 0.02 | 1.13 ± 0.01 | 1.03 ± 0.01 | 1.07 ± 0.02 |
| The first-month CF (g/cm3) | 1.51 ± 0.03 a | 1.37 ± 0.02 b | 1.37 ± 0.02 b | 1.33 ± 0.02 b |
| Final CF (g/cm3) | 1.37 ± 0.02 | 1.4 ± 0.02 | 1.37 ± 0.02 | 1.34 ± 0.03 |
| WGR (%) | 97.65 | 80.50 | 71.54 | 62.18 |
| SGR (% per day) | 1.13 | 0.98 | 0.90 | 0.81 |
| Initial GSI (%) | ND | |||
| Final GSI (%) | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.06 ± 0.01 |
| Initial HSI (%) | 1.46 ± 0.19 a | |||
| Final HSI (%) | 2.41 ± 0.15 b | 2.04 ± 0.16 ab | 2.11 ± 0.24 ab | 1.78 ± 0.21 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Zou, H.; Zeng, J.; Mei, W.; Choi, S. Effects of Various LED Light Spectra on Growth, Gonadal Development, and Growth-/Reproduction-Related Hormones in the Juvenile Red Spotted Grouper, Epinephelus akaara. Animals 2023, 13, 2047. https://doi.org/10.3390/ani13132047
Xu W, Zou H, Zeng J, Mei W, Choi S. Effects of Various LED Light Spectra on Growth, Gonadal Development, and Growth-/Reproduction-Related Hormones in the Juvenile Red Spotted Grouper, Epinephelus akaara. Animals. 2023; 13(13):2047. https://doi.org/10.3390/ani13132047
Chicago/Turabian StyleXu, Wengang, Huafeng Zou, Jun Zeng, Weiping Mei, and SongHee Choi. 2023. "Effects of Various LED Light Spectra on Growth, Gonadal Development, and Growth-/Reproduction-Related Hormones in the Juvenile Red Spotted Grouper, Epinephelus akaara" Animals 13, no. 13: 2047. https://doi.org/10.3390/ani13132047
APA StyleXu, W., Zou, H., Zeng, J., Mei, W., & Choi, S. (2023). Effects of Various LED Light Spectra on Growth, Gonadal Development, and Growth-/Reproduction-Related Hormones in the Juvenile Red Spotted Grouper, Epinephelus akaara. Animals, 13(13), 2047. https://doi.org/10.3390/ani13132047





