Impact of Red Imported Fire Ant Nest-Building on Soil Properties and Bacterial Communities in Different Habitats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
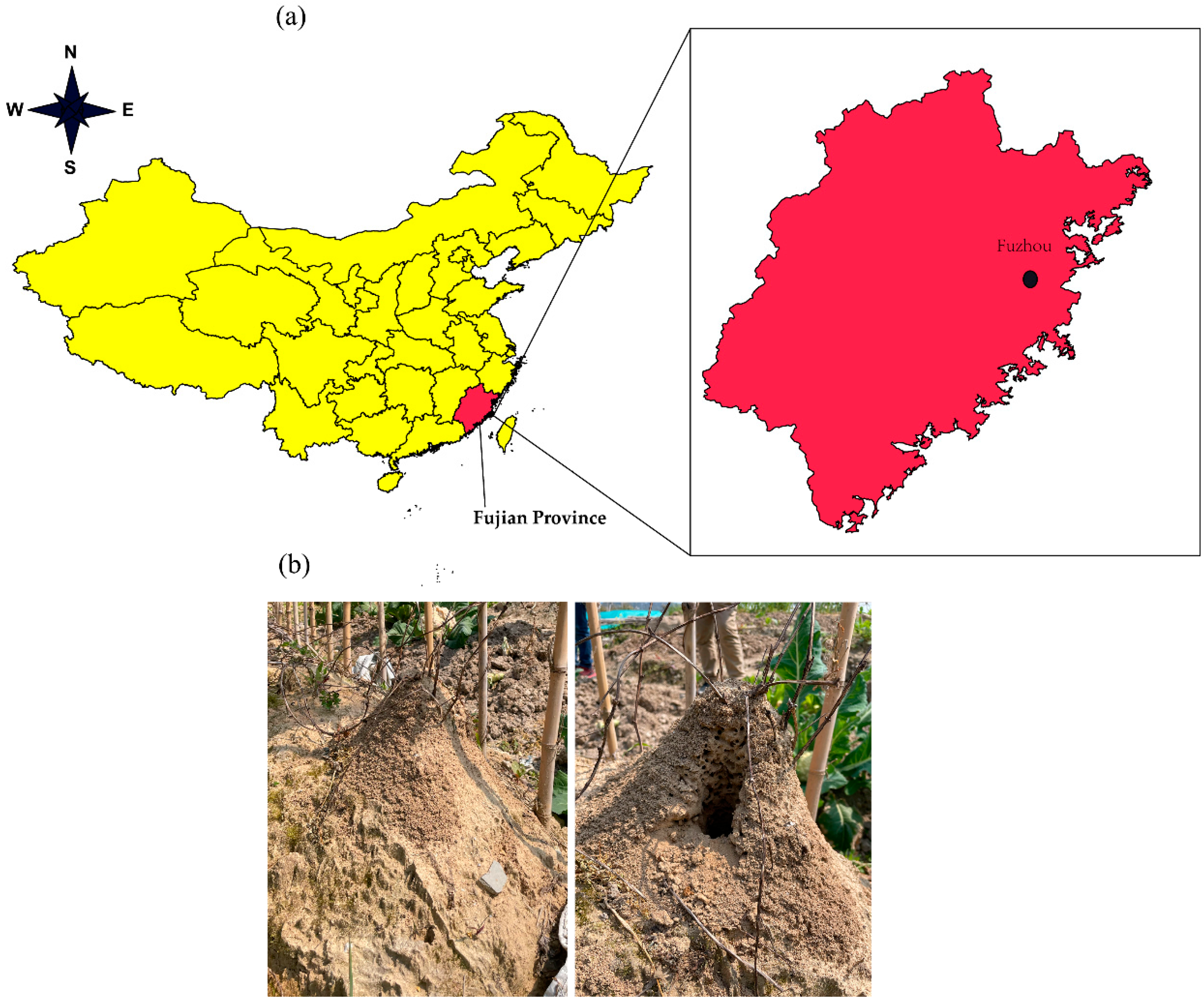
2.1. Experimental Materials
2.2. Soil Physicochemical Analysis
2.3. Extraction and Purification of Soil Microbial DNA
2.4. Analysis of Soil Microbial DNA Diversity
2.5. Data Analysis
3. Results
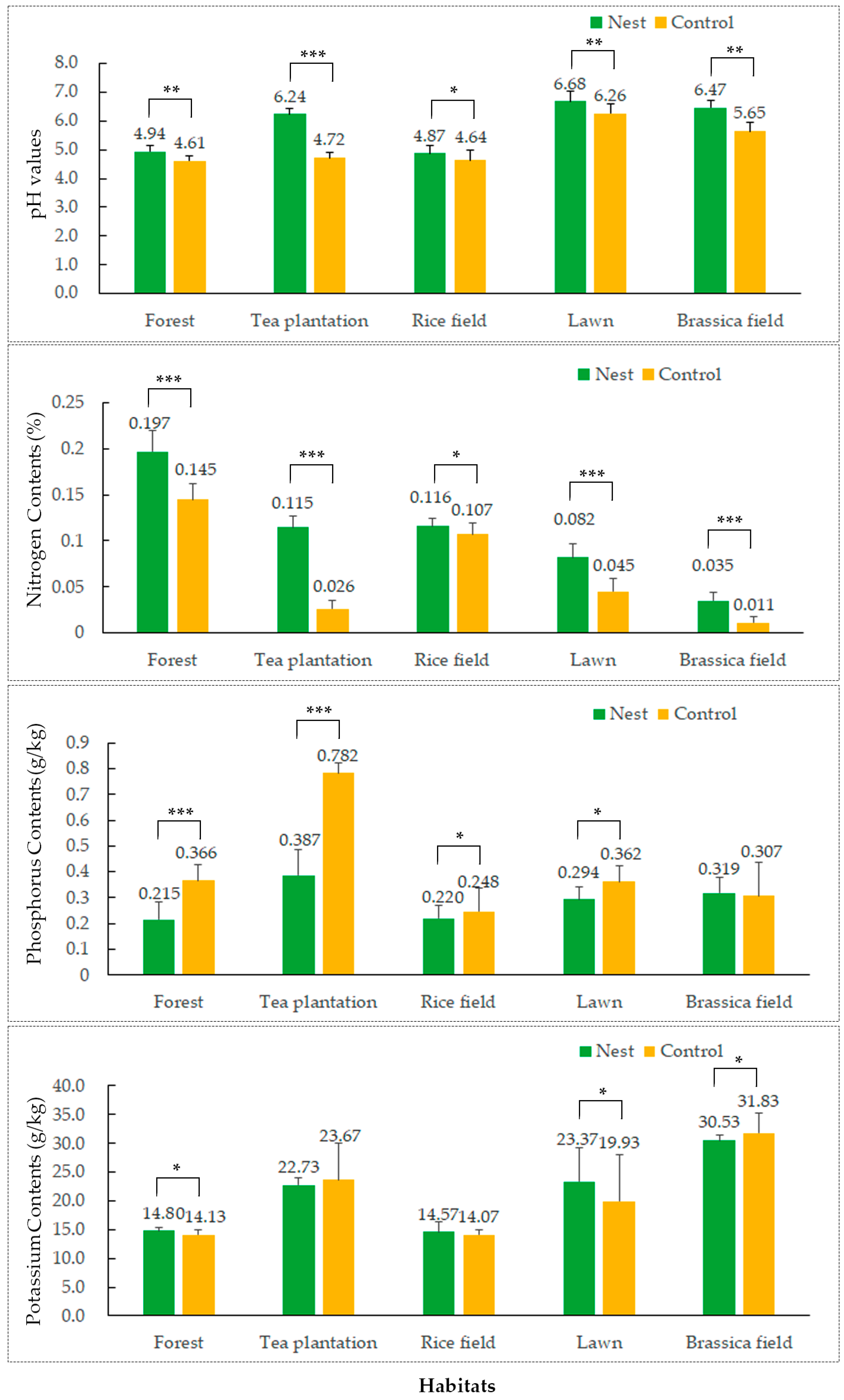
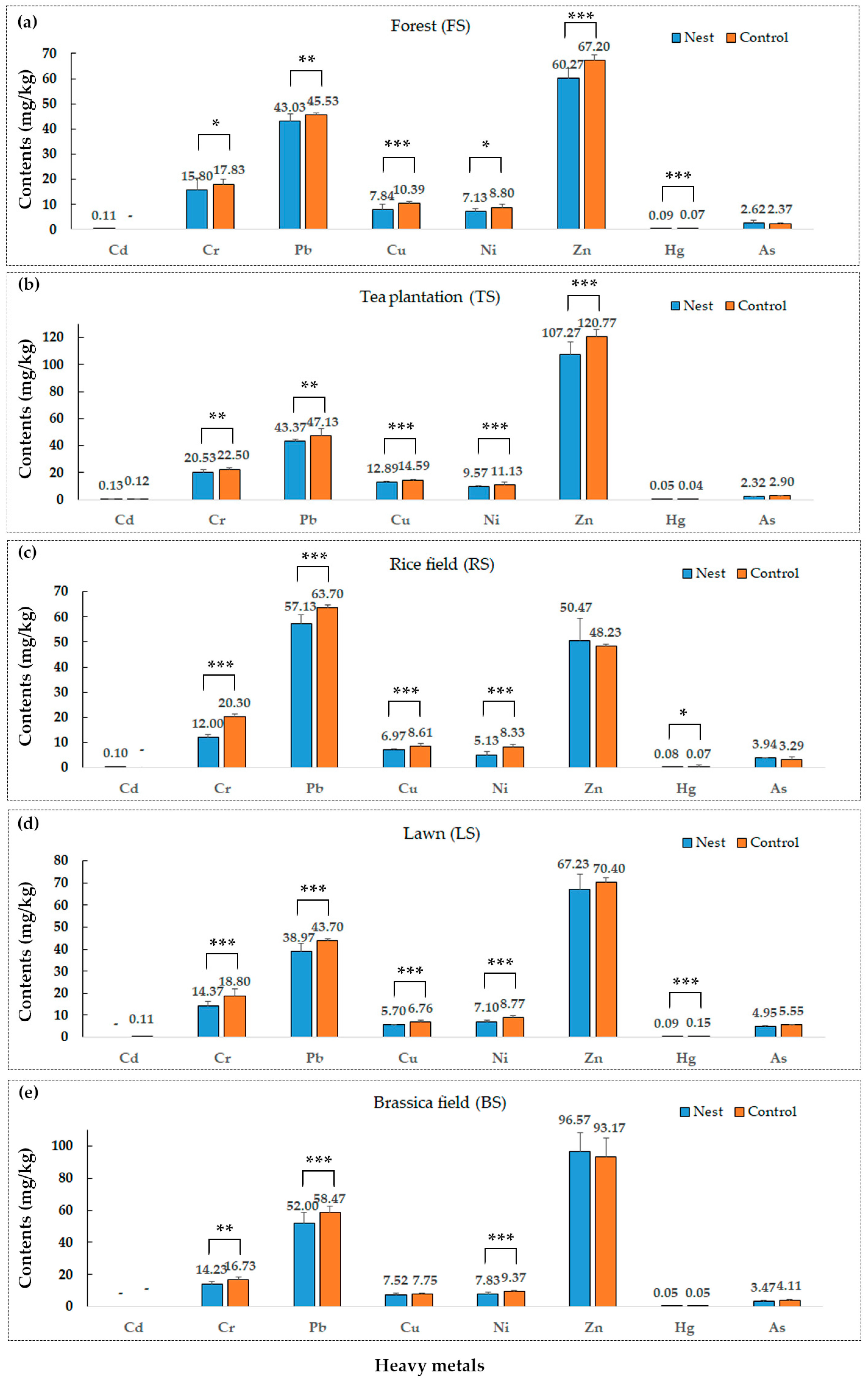
3.1. Impacts of the Red Imported Fire Ant on Soil Physicochemical Properties
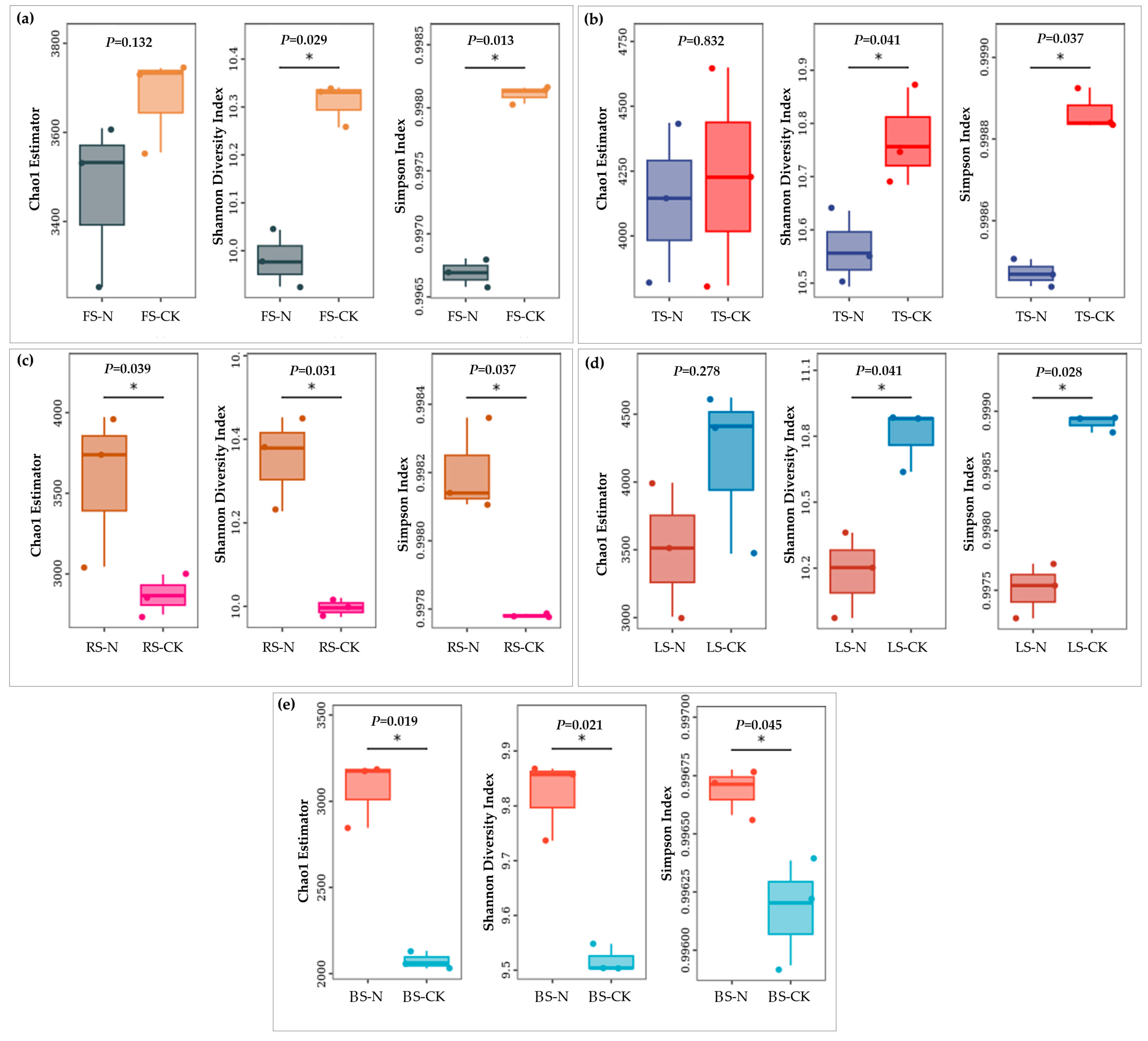
3.2. Effect of the Red Imported Fire Ant on Soil Bacterial Diversity
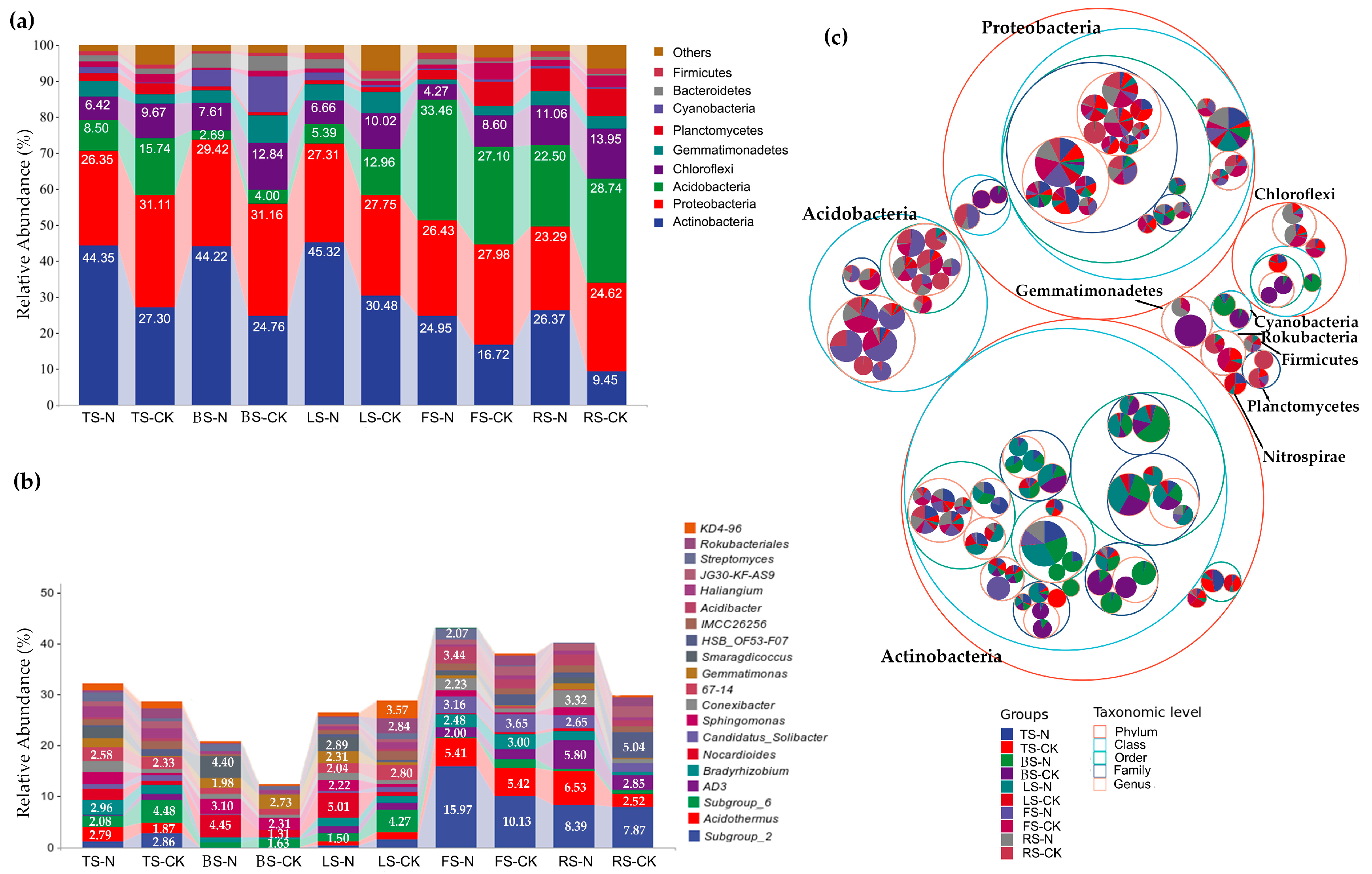
3.3. Effect of RIFAs on Soil Bacterial Species Composition
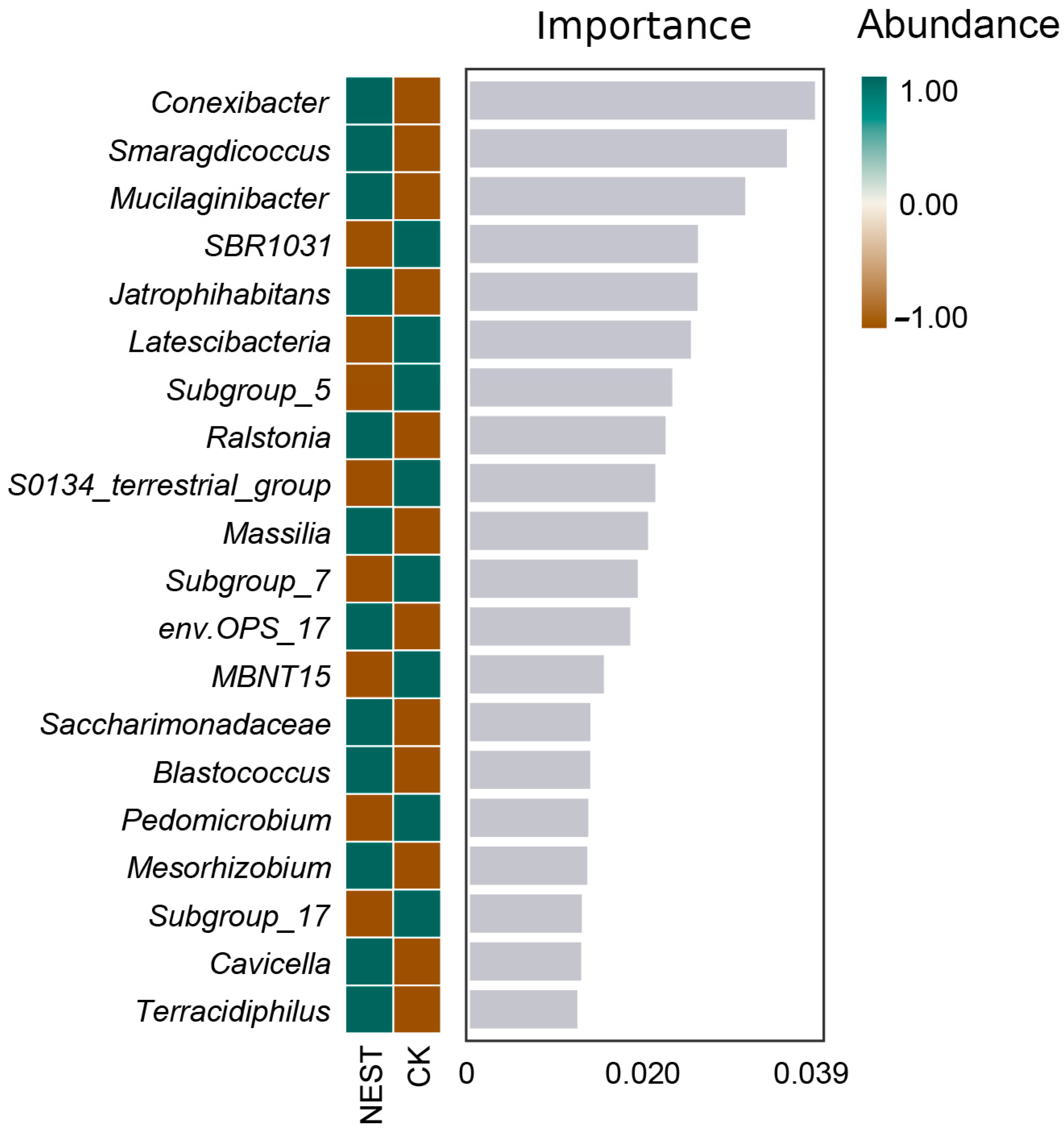
3.4. Biomarker Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ascunce, M.S.; Yang, C.C.; Oakey, J.; Calcaterra, L.; Wu, W.J.; Shih, C.J.; Goudet, J.; Ross, K.G.; Shoemaker, D. Global invasion history of the fire ant Solenopsis invicta. Science 2011, 331, 1066–1068. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-S.; Huang, S.-A.; Lin, I.-L.; Lin, C.-C.; Lai, H.-K.; Yang, C.-H.; Huang, R.-N. Establishment and social impacts of the red imported fire ant, Solenopsis invicta, (Hymenoptera: Formicidae) in Taiwan. Int. J. Environ. Res. Public Health 2021, 18, 5055. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Zeng, L. 10 years after red imported fire ant found to invade China: History, current situation and trend of its infestation. Plant Quar. 2015, 29, 1–6. [Google Scholar]
- Chi, W.-L.; Chen, C.-H.; Lin, H.-M.; Lin, C.-C.; Chen, W.-T.; Chen, Y.-C.; Lien, Y.-Y.; Tsai, Y.-L. Utilizing odor-adsorbed filter papers for detection canine training and off-site fire ant indications. Animals 2021, 11, 2204. [Google Scholar] [CrossRef] [PubMed]
- Tschinkel, W.R.; Adams, E.S.; Macon, T. Territory area and colony size in the fire ant, Solenopsis invicta. J. Anim. Ecol. 1995, 64, 473–480. [Google Scholar] [CrossRef]
- Adams, E.S. Experimental analysis of territory size in a population of the fire ant Solenopsis invicta. Behav. Ecol. 2003, 14, 48–53. [Google Scholar] [CrossRef]
- Vinson, S.B. Insect Life: Invasion of the Red Imported Fire Ant (Hymenoptera: Formicidae). Am. Èntomol. 1997, 43, 23–39. [Google Scholar] [CrossRef]
- Storey, G.K.; Vander Meer, R.K.; Boucias, D.G.; McCoy, C.W. Effect of fire ant (Solenopsis invicta) venom alkaloids on the in vitro germination and development of selected entomogenous fungi. J. Invertebr. Pathol. 1991, 58, 88–95. [Google Scholar] [CrossRef]
- Haight, K.L.; Tschinkel, W.R. Patterns of venom synthesis and use in the fire ant, Solenopsis invicta. Toxicon 2003, 42, 673–682. [Google Scholar] [CrossRef]
- Chen, J.; Cantrell, C.L.; Shang, H.; Rojas, M.G. Piperideine alkaloids from the poison gland of the red imported fire ant (Hymenoptera: Formicidae). J. Agric. Food Chem. 2009, 57, 3128–3133. [Google Scholar] [CrossRef]
- Steward, J.W.; Vinson, S.B. Red imported fire imported fire and damage to commercial cucumber and sunflower plants. Southwest Entomol. 1991, 16, 168–170. [Google Scholar]
- Porter, S.D.; Savignano, D.A. Invasion of polygyne fire ants decimates native ants and disrupts arthropod community. Ecology 1990, 71, 2095–2106. [Google Scholar] [CrossRef]
- Drik, S.; Frank, V.F.J. Ecosystem engineering and predation: The multi-trophic impact of two ant species. J. Anim. Ecol. 2011, 80, 569–576. [Google Scholar]
- Juliane, F.; Jack, H.F.; Alexei, V.T.; Lijbert, B.; Jan, F.; Gerlinde, D.D.; Alexei, V.U.; Matty, P.B.; Patrick, L.; Michel, L.; et al. Soil fauna: Key to new carbon models. Soil 2016, 2, 565–582. [Google Scholar]
- Lafleur, B.; Hooper-Bùi, L.M.; Mumma, E.P.; Geaghan, J.P. Soil fertility and plant growth in soils from pine forests and plantations: Effect of invasive red imported fire ants Solenopsis invicta Buren. Pedobiologia 2005, 49, 415–423. [Google Scholar] [CrossRef]
- Lei, Y.; Jaleel, W.; Faisal Shahzad, M.; Ali, S.; Azad, R.; Muhammad Ikram, R.; Ali, H.; Ghramh, H.A.; Ali Khan, K.; Qiu, X.; et al. Effect of constant and fluctuating temperature on the circadian foraging rhythm of the red imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae). Saudi J. Biol. Sci. 2021, 28, 64–72. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Zeng, L.; Lu, Y.-Y. Negative effects of red imported fire ant (Solenopsis invicta Buren) invasion on arthropod community in the banana plantations. J. Environ. Entomol. 2017, 39, 835–847. [Google Scholar]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. Dada2: High-resolution sample inference from illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Blum, M.S. Biocidal and deterrent activities of nitrogen heterocycles produced by venomous myrmicine ants. ACS Symp. Ser. 1988, 380, 438–449. [Google Scholar]
- Li, X.-L.; Chen, L.; Fang, S.-G. Analysis of alkaloid components in the soil from the nest of the red imported fire ant, Solenopsis invicta (Hymenoptera: Formicidae). Acta Entomol. Sin. 2020, 63, 494–501. [Google Scholar]
- NY 5199–2002; Environmental Conditions of Organic Tea Origin. Standards Press of China: Beijing, China, 2002.
- Yang, X.; Shi, Y.; Yi, X.; Ma, L. Research progress and prospects on soil acidification at tea plantations. Acta Tea Sin. 2015, 56, 189–197. [Google Scholar]
- Wan, Q.; Xu, R.K.; Li, X.H. Proton release by tea plant (Camellia sinensis L.) roots as affected by nutrient solution concentration and pH. Plant Soil Environ. 2012, 58, 429–434. [Google Scholar] [CrossRef]
- Wan, Q.; Xu, R.; Li, X. Proton release from tea plant (Camellia sinensis L.) roots induced by Al (III) under hydroponic conditions. Soil Res. 2012, 50, 482–488. [Google Scholar] [CrossRef]
- Qiu, H.L.; Cheng, D.F. A chemosensory protein gene Si-CSP1 associated with necrophoric behavior in red imported fire ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2017, 110, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.L.; Qin, C.S.; Fox, E.G.P.; Wang, D.S.; He, Y.R. Differential behavioral responses of Solenopsis invicta (Hymenoptera: Formicidae) workers toward nestmate and non-Nestmate Corpses. J. Insect Sci. 2020, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Herzog, D.C.; Reagan, T.E.; Sheppard, D.C.; Hyde, K.M.; Nilakhe, S.S.; Hussein, M.Y.B.; McMahan, M.L.; Thomas, R.C.; Newsom, L.D. Solenopsis invicta Buren: Influence on Louisiana pasture soil chemistry. Environ. Entomol. 1976, 5, 160–162. [Google Scholar] [CrossRef]
- Chen, D.; Ye, X.; Jiang, Y.; Xiao, W.; Zhang, Q.; Zhao, S.; Shao, S.; Gao, N.; Huang, M.; Hu, J. Continuously applying compost for three years alleviated soil acidity and heavy metal bioavailability in a soil-asparagus lettuce system. Front. Plant Sci. 2022, 13, 972789. [Google Scholar] [CrossRef]
- Yang, F.; Shao, R.; Zhao, J.; Li, L.; Wang, M.; Zhou, A. Cadmium exposure disrupts the olfactory sensitivity of fire ants to semiochemicals. Environ. Pollut. 2021, 287, 117359. [Google Scholar] [CrossRef]
- Skaldina, O.; Peräniemi, S.; Sorvari, J. Ants and their nests as indicators for industrial heavy metal contamination. Environ. Pollut. 2018, 240, 574–581. [Google Scholar] [CrossRef]
- Del Toro, I.; Floyd, K.; Gardea-Torresdey, J.; Borrok, D. Heavy metal distribution and bioaccumulation in Chihuahuan Desert Rough Harvester ant (Pogonomyrmex rugosus) populations. Environ. Pollut. 2010, 158, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Young, I.M.; Crawford, J.W. Interactions and self-organization in the soil-microbe complex. Science 2004, 304, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Muneer, M.A.; Hou, W.; Li, J.; Huang, X.; Ur Rehman Kayani, M.; Cai, Y.; Yang, W.; Wu, L.; Ji, B.; Zheng, C. Soil pH: A key edaphic factor regulating distribution and functions of bacterial community along vertical soil profiles in red soil of pomelo orchard. BMC Microbiol. 2022, 22, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chi, Z.; Li, J.; Wu, H.; Yan, B. Bacterial community structure and function in soils from tidal freshwater wetlands in a Chinese delta: Potential impacts of salinity and nutrient. Sci. Total Environ. 2019, 696, 134029. [Google Scholar] [CrossRef]
- Charokopos, N.; Artemiou, P.; Antonitsis, P.; Rouska, E. Repair of aortic regurgitation caused by spontaneous avulsion of aortic valve commissure in a patient with idiopathic thrombocytopenic purpura. Thorac. Cardiovasc. Surg. 2010, 58, 43–44. [Google Scholar] [CrossRef]
- Jouvenaz, D.P.; Blum, M.S.; MacConnell, J.G. Antibacterial activity of venom alkaloids from the imported fire ant, Solenopsis invicta Buren. Antimicrob. Agents Chemother. 1972, 2, 291–293. [Google Scholar] [CrossRef]
- Sullivan, D.C.; Flowers, H.; Rockhold, R.; Herath, H.M.; Nanayakkara, N.P. Antibacterial activity of synthetic fire ant venom: The solenopsins and isosolenopsins. Am. J. Med. Sci. 2009, 338, 287–291. [Google Scholar] [CrossRef]
- Dawadi, S.; Baysal-Gurel, F.; Addesso, K.M.; Liyanapathiranage, P.; Simmons, T. Fire ant venom alkaloids: Possible control measure for soilborne and foliar plant pathogens. Pathogens 2021, 10, 659. [Google Scholar] [CrossRef]
- Huang, H.; Ren, L.; Li, H.; Schmidt, A.; Gershenzon, J.; Lu, Y.; Cheng, D. The nesting preference of an invasive ant is associated with the cues produced by actinobacteria in soil. PLoS Pathog. 2020, 16, e1008800. [Google Scholar] [CrossRef] [PubMed]
- Travanty, N.V.; Vargo, E.L.; Schal, C.; Apperson, C.S.; Ponnusamy, L. Bacterial isolates derived from nest soil affect the attraction and digging behavior of workers of the red imported fire ant, Solenopsis invicta Buren. Insects 2022, 13, 444. [Google Scholar] [CrossRef]
- Kaltenpoth, M. Actinobacteria as mutualists: General healthcare for insects? Trends Microbiol. 2009, 17, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Jakubiec-Krzesniak, K.; Rajnisz-Mateusiak, A.; Guspiel, A.; Ziemska, J.; Solecka, J. Secondary metabolites of actinomycetes and their antibacterial, antifungal and antiviral properties. Pol. J. Microbiol. 2018, 67, 259–272. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Liu, F.; Peng, L. Impact of Red Imported Fire Ant Nest-Building on Soil Properties and Bacterial Communities in Different Habitats. Animals 2023, 13, 2026. https://doi.org/10.3390/ani13122026
Shi L, Liu F, Peng L. Impact of Red Imported Fire Ant Nest-Building on Soil Properties and Bacterial Communities in Different Habitats. Animals. 2023; 13(12):2026. https://doi.org/10.3390/ani13122026
Chicago/Turabian StyleShi, Longqing, Fenghao Liu, and Lu Peng. 2023. "Impact of Red Imported Fire Ant Nest-Building on Soil Properties and Bacterial Communities in Different Habitats" Animals 13, no. 12: 2026. https://doi.org/10.3390/ani13122026
APA StyleShi, L., Liu, F., & Peng, L. (2023). Impact of Red Imported Fire Ant Nest-Building on Soil Properties and Bacterial Communities in Different Habitats. Animals, 13(12), 2026. https://doi.org/10.3390/ani13122026




