Camera Trap Methods and Drone Thermal Surveillance Provide Reliable, Comparable Density Estimates of Large, Free-Ranging Ungulates
Abstract
Simple Summary
Abstract
1. Introduction
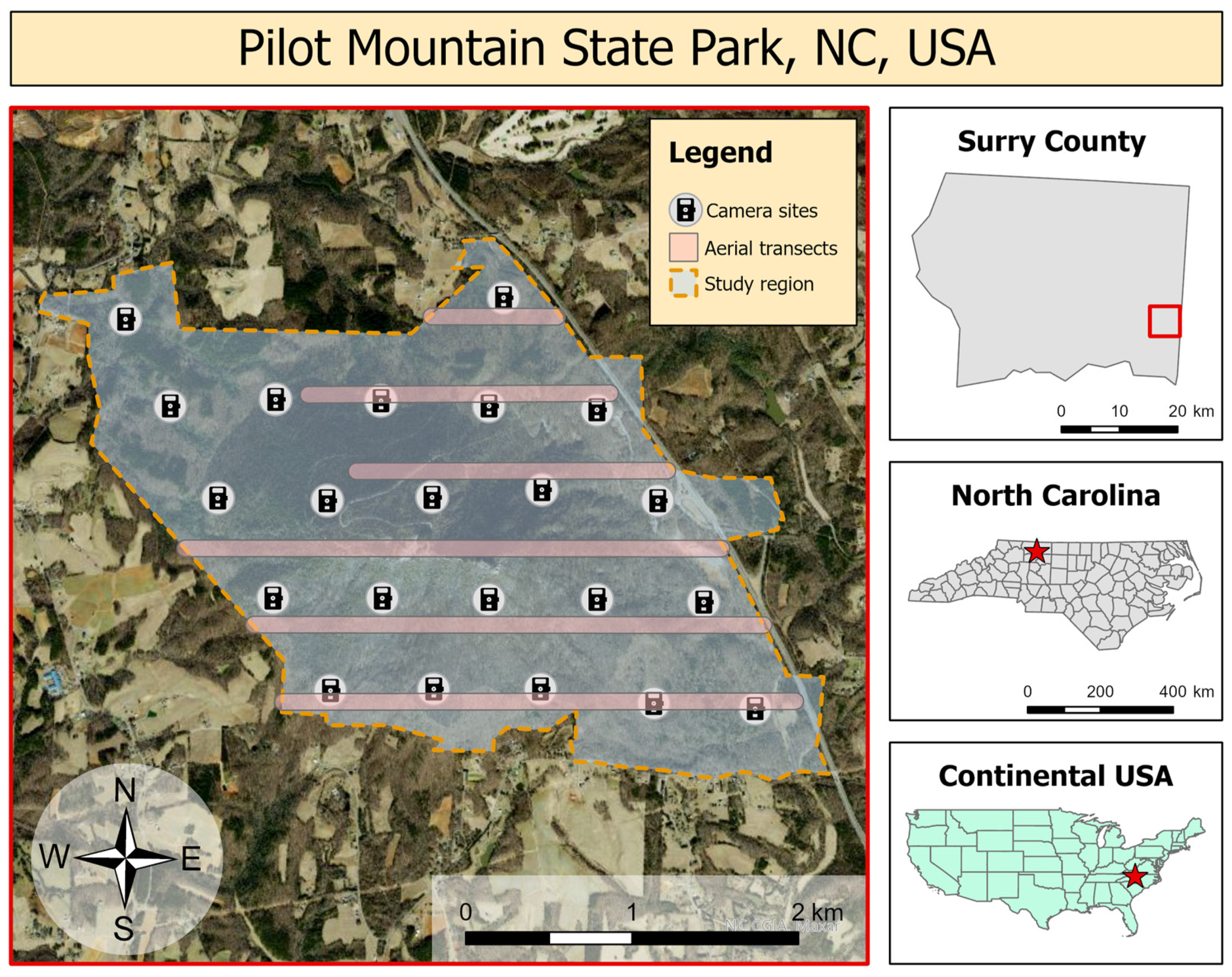
2. Materials and Methods
Cij|Ni ~ Binomial(Ni,pij); with log(pij) = α0 + α1
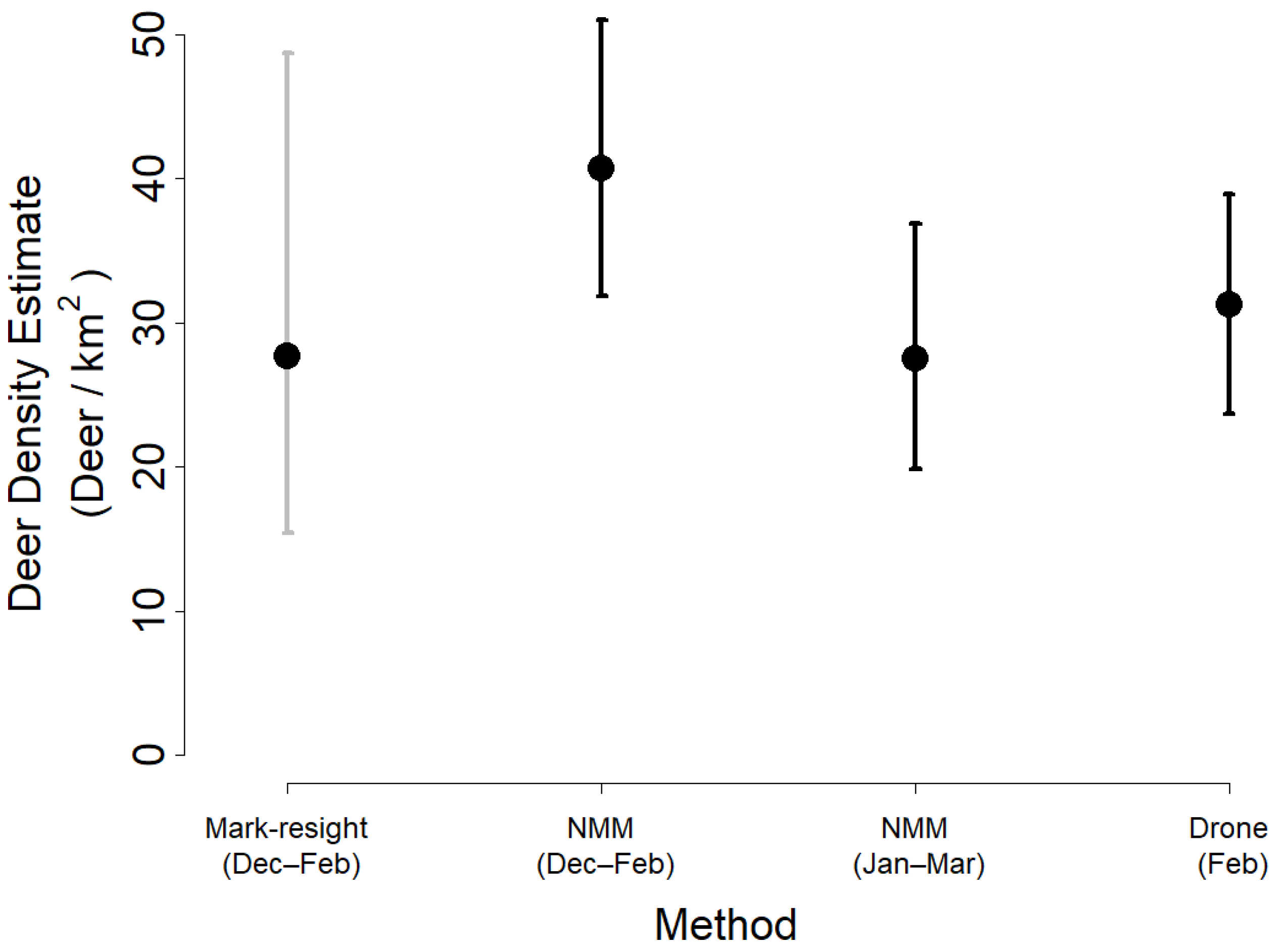
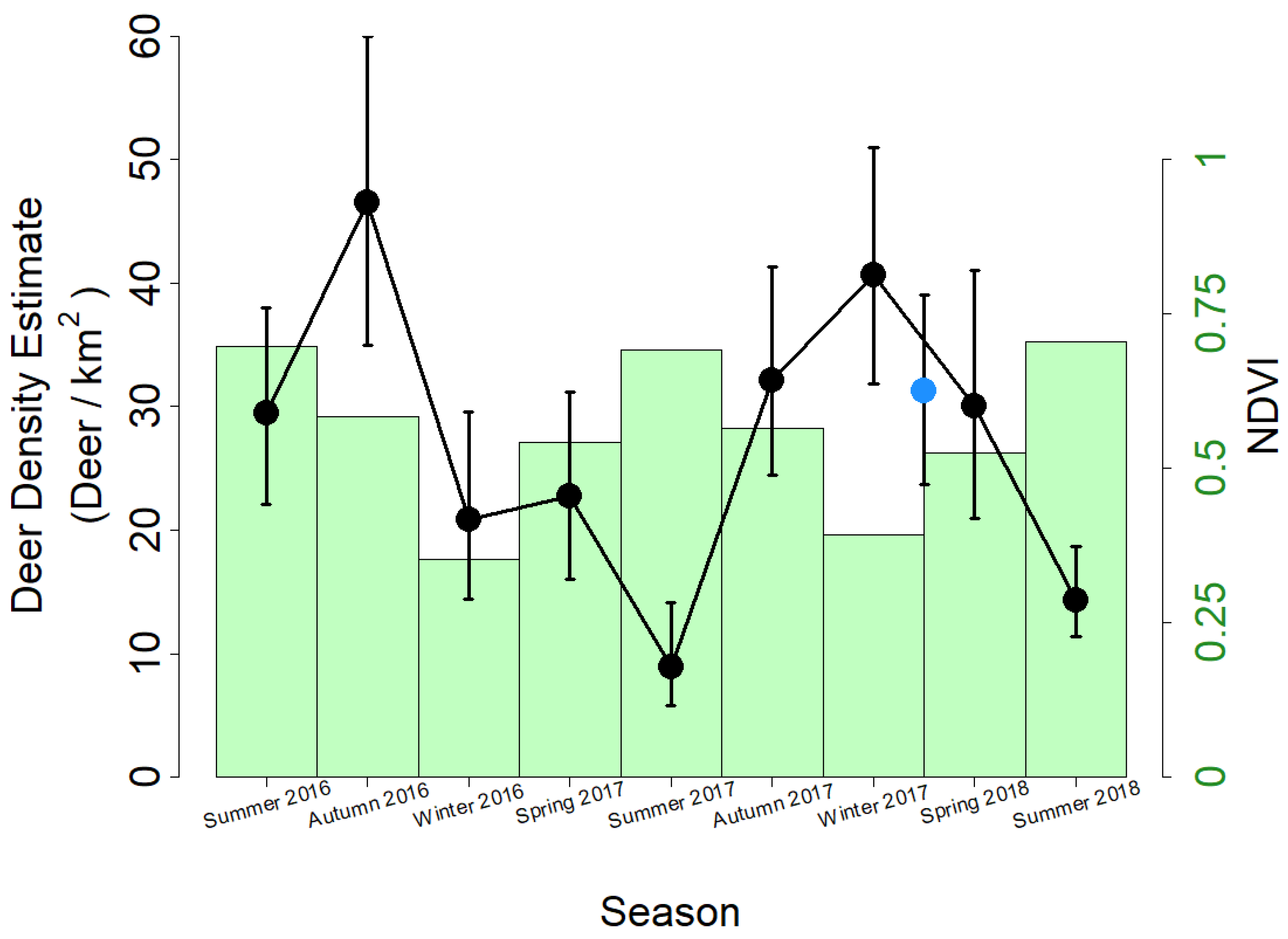
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Model Name | Detection | Abundance |
| m0 | ~1 | ~1 |
| m1 | ~ASPECT | ~SLOPE |
| m2 | ~ASPECT | ~ELE |
| m3 | ~ASPECT | ~EDGE |
| m4 | ~SLOPE | ~ASPECT |
| m5 | ~SLOPE | ~ELE |
| m6 | ~SLOPE | ~EDGE |
| m7 | ~ELE | ~ASPECT |
| m8 | ~ELE | ~SLOPE |
| m9 | ~ELE | ~EDGE |
| m10 | ~EDGE | ~ASPECT |
| m11 | ~EDGE | ~SLOPE |
| m12 | ~EDGE | ~ELE |
| m13 | ~ASPECT | ~SLOPE + ELE |
| m14 | ~ASPECT | ~SLOPE + EDGE |
| m15 | ~ASPECT | ~ELE + EDGE |
| m16 | ~ASPECT + SLOPE | ~ELE |
| m17 | ~ASPECT + SLOPE | ~EDGE |
| m18 | ~ASPECT + ELE | ~SLOPE |
| m19 | ~ASPECT + ELE | ~EDGE |
| m20 | ~ASPECT + EDGE | ~SLOPE |
| m21 | ~ASPECT + EDGE | ~ELE |
| m22 | ~SLOPE | ~ASPECT + ELE |
| m23 | ~SLOPE | ~ASPECT + EDGE |
| m24 | ~SLOPE | ~ELE + EDGE |
| m25 | ~SLOPE + ELE | ~ASPECT |
| m26 | ~SLOPE + ELE | ~EDGE |
| m27 | ~SLOPE + EDGE | ~ASPECT |
| m28 | ~SLOPE + EDGE | ~ELE |
| m29 | ~ELE | ~ASPECT + SLOPE |
| m30 | ~ELE | ~ASPECT + EDGE |
| m31 | ~ELE | ~SLOPE + EDGE |
| m32 | ~ELE + EDGE | ~ASPECT |
| m33 | ~ELE + EDGE | ~SLOPE |
| m34 | ~EDGE | ~ASPECT + SLOPE |
| m35 | ~EDGE | ~ASPECT + ELE |
| m36 | ~EDGE | ~SLOPE + ELE |
| m37 | ~ASPECT + SLOPE | ~ELE + EDGE |
| m38 | ~ASPECT + ELE | ~SLOPE + EDGE |
| m39 | ~ASPECT + EDGE | ~SLOPE + ELE |
| m40 | ~SLOPE + ELE | ~ASPECT + EDGE |
| m41 | ~SLOPE + EDGE | ~ASPECT + ELE |
| m42 | ~ELE + EDGE | ~ASPECT + SLOPE |
References
- Freckleton, R.P.; Gill, J.A.; Noble, D.; Watkinson, A.R. Large-Scale Population Dynamics, Abundance-Occupancy Relationships and the Scaling from Local to Regional Population Size. J. Anim. Ecol. 2005, 74, 353–364. [Google Scholar] [CrossRef]
- Vázquez, D.P.; Melián, C.J.; Williams, N.M.; Blüthgen, N.; Krasnov, B.R.; Poulin, R. Species Abundance and Asymmetric Interaction Strength in Ecological Networks. Oikos 2007, 116, 1120–1127. [Google Scholar] [CrossRef]
- Hochachka, W.M.; Dhondt, A.A. Density-Dependent Decline of Host Abundance Resulting from a New Infectious Disease. Proc. Natl. Acad. Sci. USA 2000, 97, 5303–5306. [Google Scholar] [CrossRef] [PubMed]
- Gotelli, N.J.; Colwell, R.K. Quantifying Biodiversity: Procedures and Pitfalls in the Measurement and Comparison of Species Richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Pierce, B.L.; Lopez, R.R.; Silvy, N.J. Estimating Animal Abundance. In The Wildlife Techniques Manual: Volume 1: Research. Volume 2: Management; JHU Press: Baltimore, MD, USA, 2020; pp. 297–325. ISBN 978-1-4214-3670-8. [Google Scholar]
- Lancia, R.A.; Kendall, W.L.; Pollock, K.H.; Nichols, J.D. Estimating the Number of Animals in Wildlife Populations. In Techniques for Wildlife Investigation and Management; Braun, C., Ed.; Wildlife Society: Bethesda, MD, USA, 2005; ISBN 978-0-933564-15-2. [Google Scholar]
- Witmer, G.W. Wildlife Population Monitoring: Some Practical Considerations. Wildl. Res. 2005, 32, 259–263. [Google Scholar] [CrossRef]
- Cutler, T.L.; Swann, D.E. Using Remote Photography in Wildlife Ecology: A Review. Wildl. Soc. Bull. 1999, 27, 571–581. [Google Scholar]
- Koerth, B.H.; Kroll, J.C. Bait Type and Timing for Deer Counts Using Cameras Triggered by Infrared Monitors. Wildl. Soc. Bull. 2000, 28, 630–635. [Google Scholar]
- Rowcliffe, J.M.; Field, J.; Turvey, S.T.; Carbone, C. Estimating Animal Density Using Camera Traps without the Need for Individual Recognition. J. Appl. Ecol. 2008, 45, 1228–1236. [Google Scholar] [CrossRef]
- Larrucea, E.S.; Brussard, P.F.; Jaeger, M.M.; Barrett, R.H. Cameras, Coyotes, and the Assumption of Equal Detectability. J. Wildl. Manag. 2007, 71, 1682–1689. [Google Scholar] [CrossRef]
- Heilbrun, R.D.; Silvy, N.J.; Peterson, M.J.; Tewes, M.E. Estimating Bobcat Abundance Using Automatically Triggered Cameras. Wildl. Soc. Bull. 2006, 34, 69–73. [Google Scholar] [CrossRef]
- Silveira, L.; Jácomo, A.T.A.; Diniz-Filho, J.A.F. Camera Trap, Line Transect Census and Track Surveys: A Comparative Evaluation. Biol. Conserv. 2003, 114, 351–355. [Google Scholar] [CrossRef]
- Meek, P.D.; Ballard, G.; Claridge, A.; Kays, R.; Moseby, K.; O’Brien, T.; O’Connell, A.; Sanderson, J.; Swann, D.E.; Tobler, M.; et al. Recommended Guiding Principles for Reporting on Camera Trapping Research. Biodivers. Conserv. 2014, 23, 2321–2343. [Google Scholar] [CrossRef]
- Burton, A.C.; Neilson, E.; Moreira, D.; Ladle, A.; Steenweg, R.; Fisher, J.T.; Bayne, E.; Boutin, S. REVIEW: Wildlife Camera Trapping: A Review and Recommendations for Linking Surveys to Ecological Processes. J. Appl. Ecol. 2015, 52, 675–685. [Google Scholar] [CrossRef]
- Kéry, M.; Royle, J.A. Chapter 6—Modeling Abundance with Counts of Unmarked Individuals in Closed Populations: Binomial N-Mixture Models. In Applied Hierarchical Modeling in Ecology; Kéry, M., Royle, J.A., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 219–312. ISBN 978-0-12-801378-6. [Google Scholar]
- Howe, E.J.; Buckland, S.T.; Després-Einspenner, M.-L.; Kühl, H.S. Distance Sampling with Camera Traps. Methods Ecol. Evol. 2017, 8, 1558–1565. [Google Scholar] [CrossRef]
- Pollock, K.H.; Nichols, J.D.; Brownie, C.; Hines, J.E. Statistical Inference for Capture-Recapture Experiments. In Wildlife Monographs; Wiley: Hoboken, NJ, USA, 1990; pp. 3–97. [Google Scholar]
- Karanth, K.U.; Nichols, J.D. Estimation of Tiger Densities in India Using Photographic Captures and Recaptures. Ecology 1998, 79, 2852–2862. [Google Scholar] [CrossRef]
- Jacobson, H.A.; Kroll, J.C.; Browning, R.W.; Koerth, B.H.; Conway, M.H. Infrared-Triggered Cameras for Censusing White-Tailed Deer. Wildl. Soc. Bull. 1997, 25, 547–556. [Google Scholar]
- Mccoy, J.C.; Ditchkoff, S.S.; Steury, T.D. Bias Associated with Baited Camera Sites for Assessing Population Characteristics of Deer. J. Wildl. Manag. 2011, 75, 472–477. [Google Scholar] [CrossRef]
- Royle, J.A.; Young, K.V. A Hierarchical Model for Spatial Capture-Recapture Data. Ecology 2008, 89, 2281–2289. [Google Scholar] [CrossRef]
- Keever, A.C.; McGowan, C.P.; Ditchkoff, S.S.; Acker, P.K.; Grand, J.B.; Newbolt, C.H. Efficacy of N-Mixture Models for Surveying and Monitoring White-Tailed Deer Populations. Mammal. Res. 2017, 62, 413–422. [Google Scholar] [CrossRef]
- Mackenzie, D.I.; Royle, J.A. Designing Occupancy Studies: General Advice and Allocating Survey Effort. J. Appl. Ecol. 2005, 42, 1105–1114. [Google Scholar] [CrossRef]
- Royle, J.A. N-Mixture Models for Estimating Population Size from Spatially Replicated Counts. Biometrics 2004, 60, 108–115. [Google Scholar] [CrossRef]
- Dénes, F.V.; Silveira, L.F.; Beissinger, S.R. Estimating Abundance of Unmarked Animal Populations: Accounting for Imperfect Detection and Other Sources of Zero Inflation. Methods Ecol. Evol. 2015, 6, 543–556. [Google Scholar] [CrossRef]
- Ahumada, J.A.; Hurtado, J.; Lizcano, D. Monitoring the Status and Trends of Tropical Forest Terrestrial Vertebrate Communities from Camera Trap Data: A Tool for Conservation. PLoS ONE 2013, 8, e73707. [Google Scholar] [CrossRef] [PubMed]
- Ficetola, G.F.; Barzaghi, B.; Melotto, A.; Muraro, M.; Lunghi, E.; Canedoli, C.; Lo Parrino, E.; Nanni, V.; Silva-Rocha, I.; Urso, A.; et al. N-Mixture Models Reliably Estimate the Abundance of Small Vertebrates. Sci. Rep. 2018, 8, 10357. [Google Scholar] [CrossRef] [PubMed]
- Kéry, M.; Royle, J.A.; Schmid, H. Modeling Avian Abundance from Replicated Counts Using Binomial Mixture Models. Ecol. Appl. 2005, 15, 1450–1461. [Google Scholar] [CrossRef]
- Barker, R.J.; Schofield, M.R.; Link, W.A.; Sauer, J.R. On the Reliability of N-Mixture Models for Count Data. Biometrics 2018, 74, 369–377. [Google Scholar] [CrossRef]
- Link, W.A.; Schofield, M.R.; Barker, R.J.; Sauer, J.R. On the Robustness of N-Mixture Models. Ecology 2018, 99, 1547–1551. [Google Scholar] [CrossRef]
- Duarte, A.; Adams, M.J.; Peterson, J.T. Fitting N-Mixture Models to Count Data with Unmodeled Heterogeneity: Bias, Diagnostics, and Alternative Approaches. Ecol. Model. 2018, 374, 51–59. [Google Scholar] [CrossRef]
- Knape, J.; Arlt, D.; Barraquand, F.; Berg, Å.; Chevalier, M.; Pärt, T.; Ruete, A.; Żmihorski, M. Sensitivity of Binomial N-Mixture Models to Overdispersion: The Importance of Assessing Model Fit. Methods Ecol. Evol. 2018, 9, 2102–2114. [Google Scholar] [CrossRef]
- Beaver, J.T.; Baldwin, R.W.; Messinger, M.; Newbolt, C.H.; Ditchkoff, S.S.; Silman, M.R. Evaluating the Use of Drones Equipped with Thermal Sensors as an Effective Method for Estimating Wildlife. Wildl. Soc. Bull. 2020, 44, 434–443. [Google Scholar] [CrossRef]
- Koger, B.; Deshpande, A.; Kerby, J.T.; Graving, J.M.; Costelloe, B.R.; Couzin, I.D. Quantifying the Movement, Behaviour and Environmental Context of Group-Living Animals Using Drones and Computer Vision. J. Anim. Ecol. 2023. early view. [Google Scholar] [CrossRef]
- Egan, C.C.; Blackwell, B.F.; Fernández-Juricic, E.; Klug, P.E. Testing a Key Assumption of Using Drones as Frightening Devices: Do Birds Perceive Drones as Risky? Condor 2020, 122, duaa014. [Google Scholar] [CrossRef]
- Waller, D.M.; Alverson, W.S. The White-Tailed Deer: A Keystone Herbivore. Wildl. Soc. Bull. 1997, 25, 217–226. [Google Scholar]
- Rossell, C.R.; Gorsira, B.; Patch, S. Effects of White-Tailed Deer on Vegetation Structure and Woody Seedling Composition in Three Forest Types on the Piedmont Plateau. For. Ecol. Manag. 2005, 210, 415–424. [Google Scholar] [CrossRef]
- Rossell, C.R.; Patch, S.; Salmons, S. Effects of Deer Browsing on Native and Non-Native Vegetation in a Mixed Oak-Beech Forest on the Atlantic Coastal Plain. Northeast. Nat. 2007, 14, 61–72. [Google Scholar] [CrossRef]
- Stewart, K.M.; Bowyer, R.T.; Weisberg, P.J. Spatial Use of Landscapes. In Biology and Management of White-Tailed Deer; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-0-429-07875-0. [Google Scholar]
- Johnson, J.T.; Chandler, R.B.; Conner, L.M.; Cherry, M.J.; Killmaster, C.H.; Johannsen, K.L.; Miller, K.V. Effects of Bait on Male White-Tailed Deer Resource Selection. Animals 2021, 11, 2334. [Google Scholar] [CrossRef]
- Darlington, S.; Ladle, A.; Burton, A.C.; Volpe, J.P.; Fisher, J.T. Cumulative Effects of Human Footprint, Natural Features and Predation Risk Best Predict Seasonal Resource Selection by White-Tailed Deer. Sci. Rep. 2022, 12, 1072. [Google Scholar] [CrossRef]
- Gardner, B.; Reppucci, J.; Lucherini, M.; Royle, J.A. Spatially Explicit Inference for Open Populations: Estimating Demographic Parameters from Camera-Trap Studies. Ecology 2010, 91, 3376–3383. [Google Scholar] [CrossRef]
- Randall, J.; Somers, A.; Lipscomb, M. Natural Areas Inventory for Surry County, North Carolina; N.C. Natural Heritage Program, Division of Parks and Recreation, Dept. of Environment, Health and Natural Resources: Surrey County, NC, USA, 1995. [Google Scholar]
- Hamrick, B.; Strickland, B.; Demarais, S.; McKinley, W.; Griffin, B. Conducting Camera Surveys to Estimate Population Characteristics of White-Tailed Deer; Mississippi State University: Starkville, MS, USA, 2013. [Google Scholar]
- Thomas, J.L. (Ed.) Deer Cameras the Science of Scouting; Quality Deer Management Association: Bogart, GA, USA, 2010; ISBN 978-0-9777104-5-4. [Google Scholar]
- Campbell, T.A.; Laseter, B.R.; Ford, W.M.; Miller, K.V. Feasibility of Localized Management to Control White-Tailed Deer in Forest Regeneration Areas. Wildl. Soc. Bull. 2004, 32, 1124–1131. [Google Scholar] [CrossRef]
- McKinley, W.T.; Demarais, S.; Gee, K.L.; Jacobson, H.A. Accuracy of the Camera Technique for Estimating White-Tailed Deer Population Characteristics. In Annual Conference of Southeastern Association of Fish and Wildlife Agencies; Southeastern Association of Fish and Wildlife Agencies: Norfolk, VA, USA, 2006; Volume 60, pp. 83–88. [Google Scholar]
- U.S. Geological Survey. USGS 1 Arc Second N37w081 20170414 2017. Available online: https://cmerwebmap.cr.usgs.gov/catalog/item/4f70aa71e4b058caae3f8de1 (accessed on 18 November 2019).
- Didan, K. MOD13Q1 MODIS/Terra Vegetation Indices 16-Day L3 Global 250m SIN Grid V006 2015. Available online: https://lpdaac.usgs.gov/products/mod13q1v006/ (accessed on 18 November 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Fiske, I.; Chandler, R. Unmarked: An R Package for Fitting Hierarchical Models of Wildlife Occurrence and Abundance. J. Stat. Softw. 2011, 43, 1–23. [Google Scholar] [CrossRef]
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c) 2023. Available online: https://cran.r-project.org/web/packages/AICcmodavg/index.html (accessed on 28 March 2023).
- Hijmans, R.J.; van Etten, J.; Sumner, M.; Cheng, J.; Baston, D.; Bevan, A.; Bivand, R.; Busetto, L.; Canty, M.; Fasoli, B.; et al. Raster: Geographic Data Analysis and Modeling 2023; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://cran.r-project.org/web/packages/raster/index.html (accessed on 28 March 2023).
- Detsch, F.; Mattiuzzi, M.; Forrest, M.; Mouselimis, L. MODIS-Package: MODIS Acquisition and Processing 2023; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://github.com/fdetsch/MODIS (accessed on 28 March 2023).
- Chandler, R.B.; Royle, J.A. Spatially Explicit Models for Inference About Density in Unmarked or Partially Marked Populations. Ann. Appl. Stat. 2013, 7, 936–954. [Google Scholar] [CrossRef]
- Newbolt, C.H.; Rankin, S.; Ditchkoff, S.S. Temporal and Sex-Related Differences in Use of Baited Sites by White-Tailed Deer. J. Southeast. Assoc. Fish Wildl. Agencies 2017, 4, 109–114. [Google Scholar]
- Foster, R.J.; Harmsen, B.J. A Critique of Density Estimation from Camera-Trap Data. J. Wildl. Manag. 2012, 76, 224–236. [Google Scholar] [CrossRef]
- Ketz, A.C.; Johnson, T.L.; Monello, R.J.; Mack, J.A.; George, J.L.; Kraft, B.R.; Wild, M.A.; Hooten, M.B.; Hobbs, N.T. Estimating Abundance of an Open Population with an N-Mixture Model Using Auxiliary Data on Animal Movements. Ecol. Appl. 2018, 28, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Yimer, F.; Ledin, S.; Abdelkadir, A. Soil Organic Carbon and Total Nitrogen Stocks as Affected by Topographic Aspect and Vegetation in the Bale Mountains, Ethiopia. Geoderma 2006, 135, 335–344. [Google Scholar] [CrossRef]
- Bennie, J.; Hill, M.O.; Baxter, R.; Huntley, B. Influence of Slope and Aspect on Long-Term Vegetation Change in British Chalk Grasslands. J. Ecol. 2006, 94, 355–368. [Google Scholar] [CrossRef]
- Pauley, G.R.; Peek, J.M.; Zager, P. Predicting White-Tailed Deer Habitat Use in Northern Idaho. J. Wildl. Manag. 1993, 57, 904–913. [Google Scholar] [CrossRef]
- Schmitz, O.J. Thermal Constraints and Optimization of Winter Feeding and Habitat Choice in White-Tailed Deer. Ecography 1991, 14, 104–111. [Google Scholar] [CrossRef]
- Beier, P.; McCullough, D.R. Factors Influencing White-Tailed Deer Activity Patterns and Habitat Use. Wildl. Monogr. 1990, 109, 3–51. [Google Scholar]
- Williams, R.M.; Oosting, H.J. The Vegetation of Pilot Mountain, North Carolina: A Community Analysis. Bull. Torrey Bot. Club 1944, 71, 23–45. [Google Scholar] [CrossRef]
- Igota, H.; Sakuragi, M.; Uno, H.; Kaji, K.; Kaneko, M.; Akamatsu, R.; Maekawa, K. Seasonal Migration Patterns of Female Sika Deer in Eastern Hokkaido, Japan. Ecol. Res. 2004, 19, 169–178. [Google Scholar] [CrossRef]
- Poole, K.G.; Mowat, G. Winter Habitat Relationships of Deer and Elk in the Temperate Interior Mountains of British Columbia. Wildl. Soc. Bull. 2005, 33, 1288–1302. [Google Scholar] [CrossRef]
- DeYoung, R.W.; Miller, K.V. White-Tailed Deer Behavior. In Biology and Management of White-Tailed Deer; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-0-429-07875-0. [Google Scholar]
- Grund, M.D.; McAninch, J.B.; Wiggers, E.P. Seasonal Movements and Habitat Use of Female White-Tailed Deer Associated with an Urban Park. J. Wildl. Manag. 2002, 66, 123–130. [Google Scholar] [CrossRef]
- Hebblewhite, M.; Merrill, E.H. Trade-Offs between Predation Risk and Forage Differ between Migrant Strategies in a Migratory Ungulate. Ecology 2009, 90, 3445–3454. [Google Scholar] [CrossRef]
- Hopcraft, J.G.C.; Olff, H.; Sinclair, A.R.E. Herbivores, Resources and Risks: Alternating Regulation along Primary Environmental Gradients in Savannas. Trends Ecol. Evol. 2010, 25, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Lone, K.; Loe, L.E.; Meisingset, E.L.; Stamnes, I.; Mysterud, A. An Adaptive Behavioural Response to Hunting: Surviving Male Red Deer Shift Habitat at the Onset of the Hunting Season. Anim. Behav. 2015, 102, 127–138. [Google Scholar] [CrossRef]
- Wiskirchen, K.H.; Jacobsen, T.C.; Ditchkoff, S.S.; Demarais, S.; Gitzen, R.A.; Wiskirchen, K.H.; Jacobsen, T.C.; Ditchkoff, S.S.; Demarais, S.; Gitzen, R.A. Behaviour of a Large Ungulate Reflects Temporal Patterns of Predation Risk. Wildl. Res. 2022, 49, 500–512. [Google Scholar] [CrossRef]
- DeVoe, J.D.; Proffitt, K.M.; Mitchell, M.S.; Jourdonnais, C.S.; Barker, K.J. Elk Forage and Risk Tradeoffs during the Fall Archery Season. J. Wildl. Manag. 2019, 83, 801–816. [Google Scholar] [CrossRef]
- Proffitt, K.M.; Gude, J.A.; Hamlin, K.L.; Messer, M.A. Effects of Hunter Access and Habitat Security on Elk Habitat Selection in Landscapes with a Public and Private Land Matrix. J. Wildl. Manag. 2013, 77, 514–524. [Google Scholar] [CrossRef]
- Shannon, G.; Lewis, J.S.; Gerber, B.D. Recommended Survey Designs for Occupancy Modelling Using Motion-Activated Cameras: Insights from Empirical Wildlife Data. PeerJ 2014, 2, e532. [Google Scholar] [CrossRef]
- Marchinton, R.; Hirth, D.; Halls, L. White-Tailed Deer: Ecology and Management; Stackpole: Washington, DC, USA, 1984. [Google Scholar]
- Nicholson, M.C.; Bowyer, R.T.; Kie, J.G. Habitat Selection and Survival of Mule Deer: Tradeoffs Associated with Migration. J. Mammal. 1997, 78, 483–504. [Google Scholar] [CrossRef]
- Latif, Q.; Ellis, M.; Amundson, C. A Broader Definition of Occupancy: Comment on Hayes and Monfils. J. Wildl. Manag. 2015, 80, 192–194. [Google Scholar] [CrossRef]
- Zabel, F.; Findlay, M.A.; White, P.J.C. Assessment of the Accuracy of Counting Large Ungulate Species (Red Deer Cervus Elaphus) with UAV-Mounted Thermal Infrared Cameras during Night Flights. Wildl. Biol. 2023, 2023, e01071. [Google Scholar] [CrossRef]
- de Kock, M.E.; O’Donovan, D.; Khafaga, T.; Hejcmanová, P. Zoometric Data Extraction from Drone Imagery: The Arabian Oryx (Oryx Leucoryx). Environ. Conserv. 2021, 48, 295–300. [Google Scholar] [CrossRef]
- Gordon, I.J.; Hester, A.J.; Festa-Bianchet, M. REVIEW: The Management of Wild Large Herbivores to Meet Economic, Conservation and Environmental Objectives. J. Appl. Ecol. 2004, 41, 1021–1031. [Google Scholar] [CrossRef]
- Engeman, R.M. Indexing Principles and a Widely Applicable Paradigm for Indexing Animal Populations. Wildl. Res. 2005, 32, 203–210. [Google Scholar] [CrossRef]
| Season | Parameter | Estimate | SE | Z-Value | p-Value |
|---|---|---|---|---|---|
| Summer 2016 | B-Abundance | 2.19 | 0.17 | 12.99 | <0.001 |
| Slope | −0.32 | 0.13 | −2.46 | 0.014 | |
| B-Detection | −3.12 | 0.17 | −18.68 | <0.001 | |
| Edge | 0.4 | 0.2 | 2.04 | 0.042 | |
| Elevation | −0.43 | 0.14 | −3.08 | 0.002 | |
| Autumn 2016 | B-Abundance | 2.68 | 0.16 | 16.74 | <0.001 |
| Elevation | −0.25 | 0.1 | −2.55 | 0.011 | |
| Slope | 0.19 | 0.1 | 1.99 | 0.046 | |
| B-Detection | −3.73 | 0.16 | −23.48 | <0.001 | |
| Aspect | 0.17 | 0.07 | 2.49 | 0.013 | |
| Winter 2016/17 | B-Abundance | 1.87 | 0.15 | 12.12 | <0.001 |
| Aspect | −0.2 | 0.1 | −2.03 | 0.042 | |
| B-Detection | −3.65 | 0.14 | −25.4 | <0.001 | |
| Elevation | 0.6 | 0.18 | 3.28 | 0.001 | |
| Slope | −0.47 | 0.16 | −2.91 | 0.004 | |
| Spring 2017 | B-Abundance | 1.94 | 0.16 | 11.78 | <0.001 |
| Edge | −0.29 | 0.12 | −2.33 | 0.02 | |
| B-Detection | −3.34 | 0.16 | −21.2 | <0.001 | |
| Elevation | 1.23 | 0.23 | 5.45 | <0.001 | |
| Slope | −0.99 | 0.19 | −5.3 | <0.001 | |
| Summer 2017 | B-Abundance | 0.99 | 0.21 | 4.75 | <0.001 |
| Aspect | 0.36 | 0.16 | 2.2 | 0.028 | |
| B-Detection | −3.39 | 0.19 | −18.31 | <0.001 | |
| Elevation | 1.08 | 0.21 | 5.06 | <0.001 | |
| Slope | −0.29 | 0.18 | −1.57 | 0.115 | |
| Autumn 2017 | B-Abundance | 2.22 | 0.16 | 13.82 | <0.001 |
| Edge | −0.22 | 0.12 | −1.89 | 0.059 | |
| Slope | −0.34 | 0.13 | −2.58 | 0.01 | |
| B-Detection | −3.21 | 0.15 | −20.7 | <0.001 | |
| Aspect | −0.2 | 0.08 | −2.56 | 0.01 | |
| Elevation | 0.3 | 0.14 | 2.05 | 0.04 | |
| Winter 2017/18 | B-Abundance | 2.51 | 0.14 | 18.54 | <0.001 |
| Aspect | −0.26 | 0.07 | −3.52 | <0.001 | |
| Elevation | 0.23 | 0.09 | 2.49 | 0.013 | |
| B-Detection | −3.19 | 0.13 | −24.53 | <0.001 | |
| Slope | −0.29 | 0.09 | −3.3 | <0.001 | |
| Spring 2018 | B-Abundance | 2.22 | 0.2 | 11.01 | <0.001 |
| Slope | −0.29 | 0.13 | −2.32 | 0.02 | |
| B-Detection | −3.76 | 0.2 | −18.84 | <0.001 | |
| Elevation | 0.4 | 0.13 | 2.98 | 0.003 | |
| Summer 2018 | B-Abundance | 1.56 | 0.15 | 10.21 | <0.001 |
| Elevation | 0.15 | 0.12 | 1.26 | 0.208 | |
| B-Detection | −2.71 | 0.13 | −20.76 | <0.001 | |
| Aspect | −0.3 | 0.11 | −2.83 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldwin, R.W.; Beaver, J.T.; Messinger, M.; Muday, J.; Windsor, M.; Larsen, G.D.; Silman, M.R.; Anderson, T.M. Camera Trap Methods and Drone Thermal Surveillance Provide Reliable, Comparable Density Estimates of Large, Free-Ranging Ungulates. Animals 2023, 13, 1884. https://doi.org/10.3390/ani13111884
Baldwin RW, Beaver JT, Messinger M, Muday J, Windsor M, Larsen GD, Silman MR, Anderson TM. Camera Trap Methods and Drone Thermal Surveillance Provide Reliable, Comparable Density Estimates of Large, Free-Ranging Ungulates. Animals. 2023; 13(11):1884. https://doi.org/10.3390/ani13111884
Chicago/Turabian StyleBaldwin, Robert W., Jared T. Beaver, Max Messinger, Jeffrey Muday, Matt Windsor, Gregory D. Larsen, Miles R. Silman, and T. Michael Anderson. 2023. "Camera Trap Methods and Drone Thermal Surveillance Provide Reliable, Comparable Density Estimates of Large, Free-Ranging Ungulates" Animals 13, no. 11: 1884. https://doi.org/10.3390/ani13111884
APA StyleBaldwin, R. W., Beaver, J. T., Messinger, M., Muday, J., Windsor, M., Larsen, G. D., Silman, M. R., & Anderson, T. M. (2023). Camera Trap Methods and Drone Thermal Surveillance Provide Reliable, Comparable Density Estimates of Large, Free-Ranging Ungulates. Animals, 13(11), 1884. https://doi.org/10.3390/ani13111884





