Camera Traps Uncover the Behavioral Ecology of an Endemic, Cryptic Monkey Species in the Congo Basin
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Camera Trap Surveys
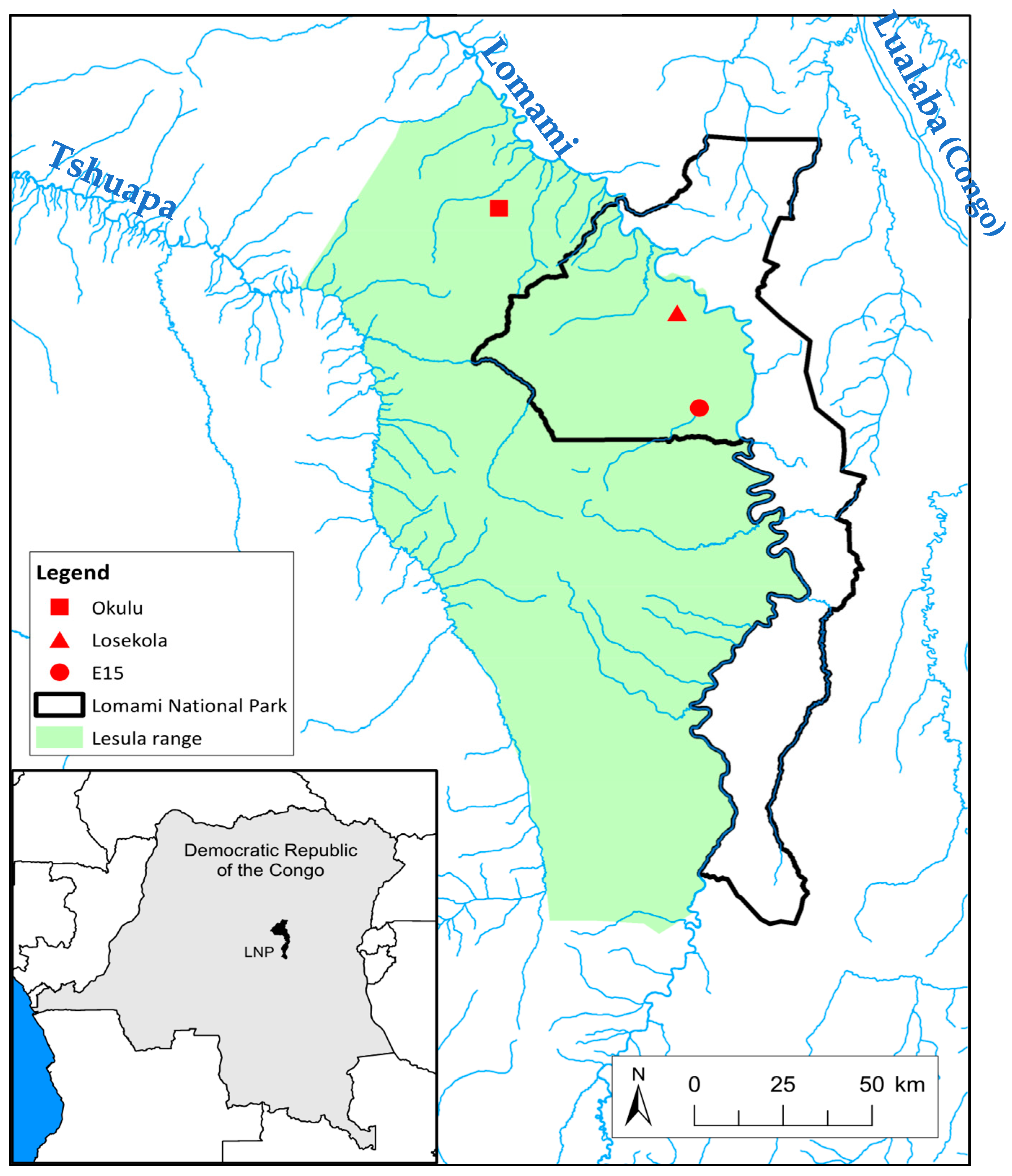
2.2.1. Study Area
2.2.2. Survey Sites
2.2.3. Survey Design
2.2.4. Data Analysis
2.2.5. Behavioral and Ecological Analyses
Degree of Terrestriality
Activity Pattern
Birth Synchronicity
Group Size and Composition
3. Results
3.1. Camera Trap Surveys
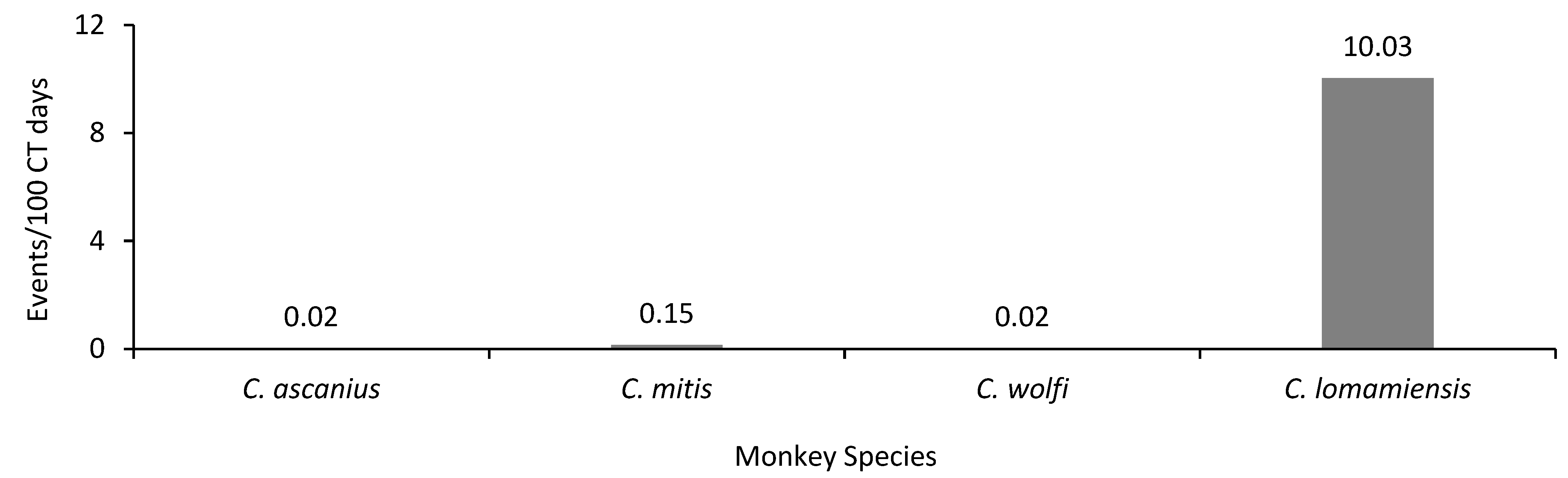
3.2. Terrestriality
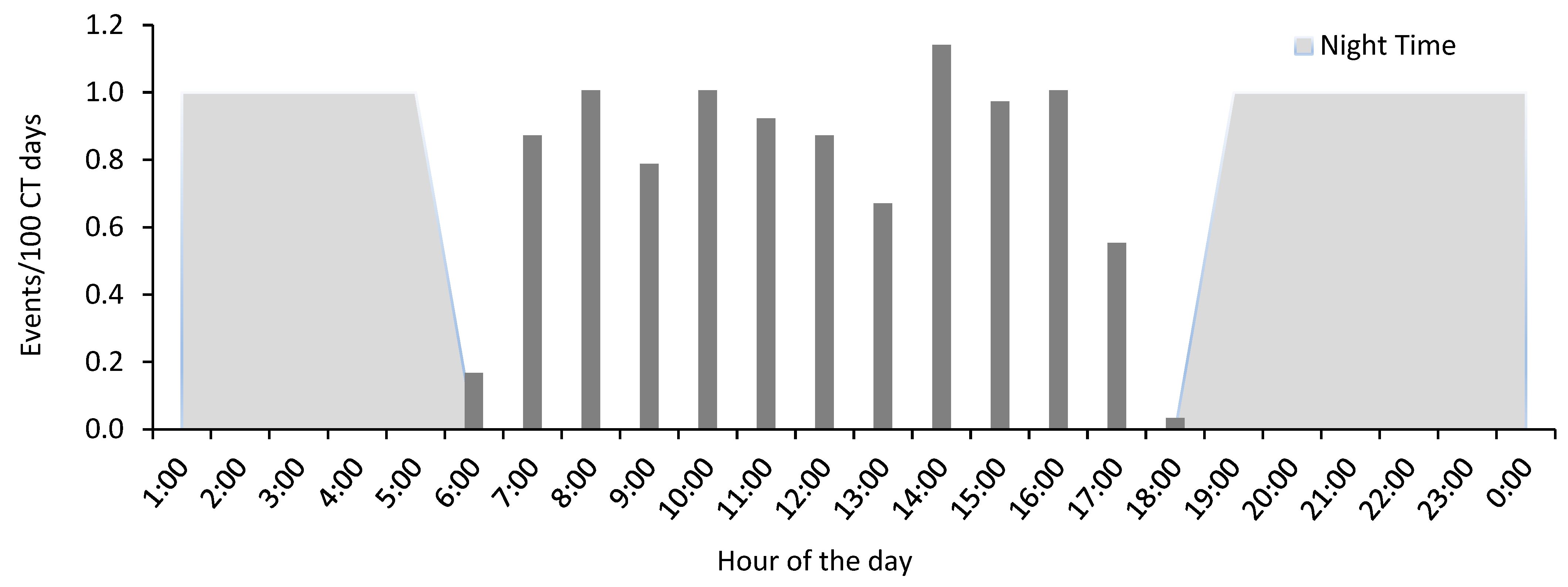
3.3. Activity Pattern
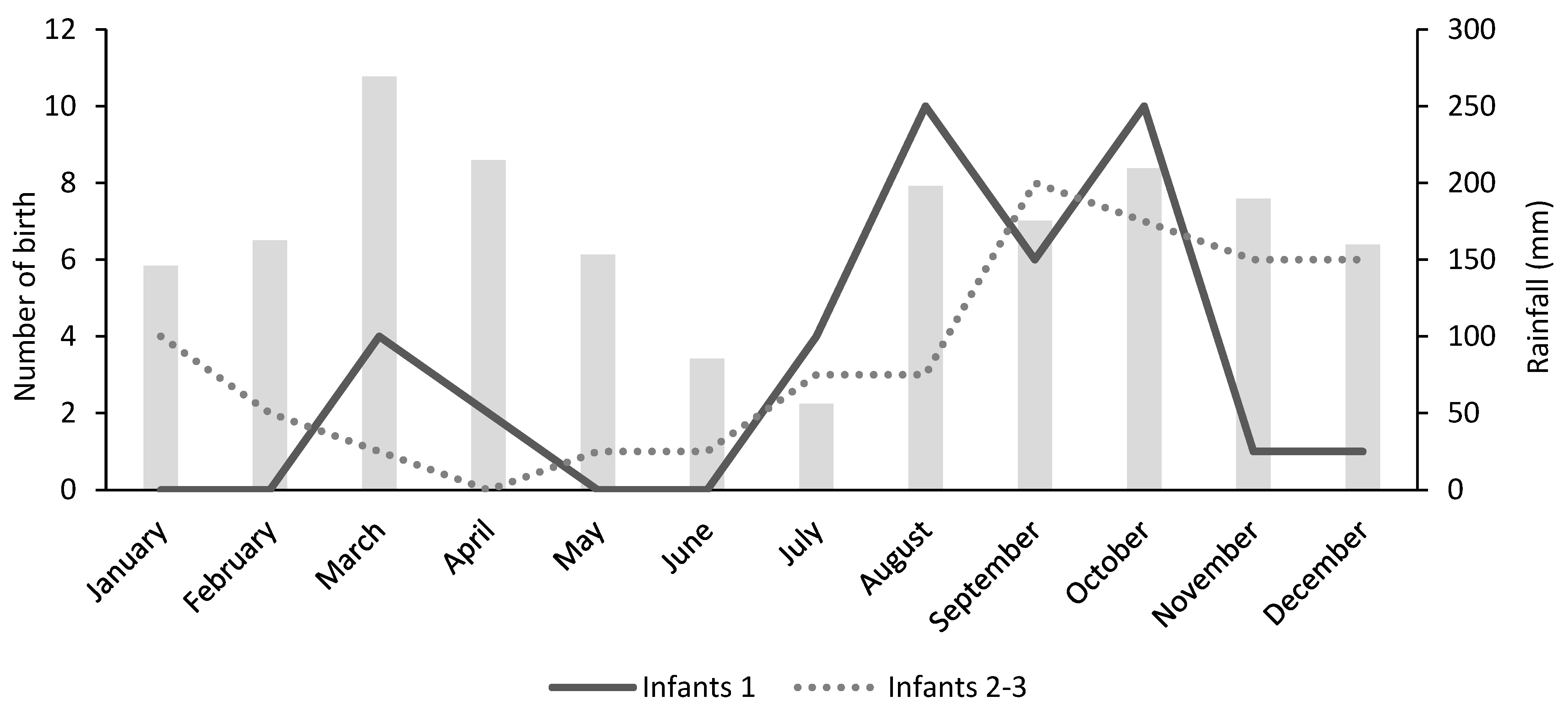
3.4. Birth Seasonality
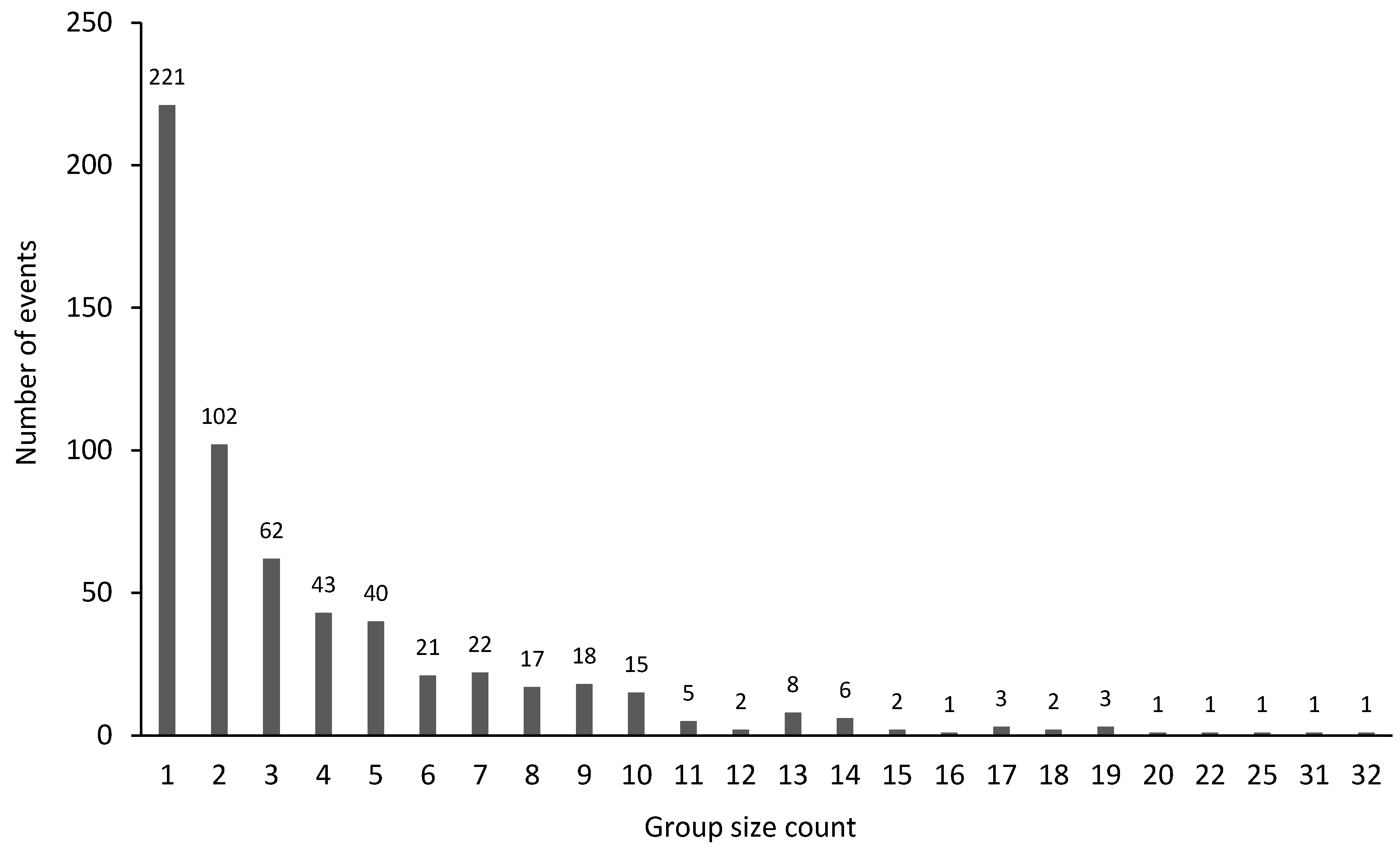
3.5. Group Size
3.6. Group Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hart, J.A.; Detwiler, K.M.; Gilbert, C.C.; Burrell, A.S.; Fuller, J.L.; Emetshu, M.; Hart, T.B.; Vosper, A.; Sargis, E.J.; Tosi, A.J. Lesula: A New Species of Cercopithecus Monkey Endemic to the Democratic Republic of Congo and Implications for Conservation of Congo’s Central Basin. PLoS ONE 2012, 7, e44271. [Google Scholar] [CrossRef]
- Guschanski, K.; Krause, J.; Sawyer, S.; Valente, L.M.; Bailey, S.; Finstermeier, K.; Sabin, R.; Gilissen, E.; Sonet, G.; Nagy, Z.T.; et al. Next-Generation Museomics Disentangles One of the Largest Primate Radiations. Syst. Biol. 2013, 62, 539–554. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Rylands, A.B.; Wilson, D.E. (Eds.) Handbook of the Mammals of the World; Primates; Lynx Edicions: Barcelona, Spain, 2013; Volume 3. [Google Scholar]
- Alempijevic, D.; Hart, J.A.; Hart, T.B.; Detwiler, K.M. Using Local Knowledge and Camera Traps to Investigate Occurrence and Habitat Preference of an Endangered Primate: The Endemic Dryas Monkey in the Democratic Republic of the Congo. Oryx 2021, 56, 260–267. [Google Scholar] [CrossRef]
- Detwiler, K.M.; Hart, J.A. Cercopithecus lomamiensis. The IUCN Red List of Threatened Species 2020: E.T92401376A92401776. Available online: https://www.iucnredlist.org/species/92401376/92401776 (accessed on 16 May 2023).
- Kamilar, J.M.; Martin, S.K.; Tosi, A.J. Combining Biogeographic and Phylogenetic Data to Examine Primate Speciation: An Example Using Cercopithecin Monkeys. Biotropica 2009, 41, 514–519. [Google Scholar] [CrossRef]
- Colyn, M.; Rahm, U. Cercopithecus hamlyni kahuziensis, a New Guenon Subspecies from the Bamboo Forest of the Kahuzi-Biega National Park (Zaire). Folia Primatol. 1987, 49, 203–208. [Google Scholar] [CrossRef]
- Easton, J.; Chao, N.; Mulindahabi, F.; Ntare, N.; Rugyerinyange, L.; Ndikubwimana, I. Status and Conservation of the Only Population of the Vulnerable Owl-Faced Monkey Cercopithecus hamlyni in Rwanda. ORYX 2011, 45, 435–438. [Google Scholar] [CrossRef]
- Hart, J.; Maisels, F. Cercopithecus hamlyni (Amended Version of 2019 Assessment). The IUCN Red List of Threatened Species 2020: E.T4219A166615690. Available online: https://doi.org/10.2305/IUCN.UK.2020-1.RLTS.T4219A166615690.en (accessed on 8 September 2020).
- Rowe, N.; Myers, M. All the World’s Primates; Pogonias Press: Charlestown, MA, USA, 2016. [Google Scholar]
- Thomas, S.C. Population Densities and Patterns of Habitat Use Among Anthropoid Primates of the Ituri Forest, Zaire. Biotropica 1991, 23, 68–83. [Google Scholar] [CrossRef]
- Arenson, J.L.; Sargis, E.J.; Hart, J.A.; Hart, T.B.; Detwiler, K.M.; Gilbert, C.C. Skeletal Morphology of the Lesula (Cercopithecus lomamiensis) and the Evolution of Guenon Locomotor Behavior. Am. J. Phys. Anthropol. 2020, 172, 3–24. [Google Scholar] [CrossRef]
- Kay, R.F.; Kirk, E.C. Osteological Evidence for the Evolution of Activity Pattern and Visual Acuity in Primates. Am. J. Phys. Anthropol. 2000, 113, 235–262. [Google Scholar] [CrossRef] [PubMed]
- Fournier, C.S.; Graefen, M.; McPhee, S.; Amboko, J.; Noonburg, E.G.; Ingram, V.; Hart, T.B.; Hart, J.A.; Detwiler, K.M. Impact of Hunting on the Lesula Monkey (Cercopithecus lomamiensis) in the Lomami River Basin, Democratic Republic of the Congo. Int. J. Primatol. 2022, 44, 282–306. [Google Scholar] [CrossRef]
- Ayers, A.M.; Allan, A.T.L.; Howlett, C.; Tordiffe, A.S.W.; Williams, K.S.; Williams, S.T.; Hill, R.A. Illuminating Movement? Nocturnal Activity Patterns in Chacma Baboons. J. Zool. 2020, 310, 287–297. [Google Scholar] [CrossRef]
- Hartel, E.F.; Noke Durden, W.; O’Corry-Crowe, G. Testing Satellite Telemetry within Narrow Ecosystems: Nocturnal Movements and Habitat Use of Bottlenose Dolphins within a Convoluted Estuarine System. Anim. Biotelemetry 2020, 8, 13. [Google Scholar] [CrossRef]
- LaFleur, M.; Sauther, M.; Cuozzo, F.; Yamashita, N.; Jacky Youssouf, I.A.; Bender, R. Cathemerality in Wild Ring-Tailed Lemurs (Lemur catta) in the Spiny Forest of Tsimanampetsotsa National Park: Camera Trap Data and Preliminary Behavioral Observations. Primates 2014, 55, 207–217. [Google Scholar] [CrossRef]
- Tan, C.L.; Yang, Y.; Niu, K. Into the Night: Camera Traps Reveal Nocturnal Activity in a Presumptive Diurnal Primate, Rhinopithecus brelichi. Primates 2013, 54, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Butynski, T.M. Guenon Birth Seasons and Correlates with Rainfall and Food. In A Primate Radiation: Evolutionary Biology of the African Guenons; Gauthier-Hion, A., Bourlière, F., Gautier, J.-P., Eds.; University Press: Cambridge, UK, 1988; pp. 284–322. [Google Scholar]
- Cords, M. Mating Systems of Forest Guenons: A Preliminary Review. In A Primate Radiation: Evolutionary Biology of the African Guenons; Gauthier-Hion, A., Bourlière, F., Gautier, J.-P., Eds.; University Press: Cambridge, UK, 1988; pp. 323–339. [Google Scholar]
- Most, C.A.; Strum, S.C. Bringing up Baby: Maternal Responsiveness, Secondary Attachments, and the Development of Infant Social Competence in Wild Olive Baboons (Papio anubis). Dev. Psychobiol. 2020, 62, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J. Baboon Mothers and Infants; Harvard University Press: Boston, MA, USA, 1980. [Google Scholar]
- Stone, A.I.; Ruivo, L.V.P. Synchronization of Weaning Time with Peak Fruit Availability in Squirrel Monkeys (Saimiri collinsi) Living in Amazonian Brazil. Am. J. Primatol. 2020, 82, e23139. [Google Scholar] [CrossRef]
- Cords, M. Forest Guenons and Patas Monkeys: Male-Male Competition in One-Male Groups. In Primates Societies; Smuts, B., Cheney, D., Seyfarth, R., Wrangham, R., Struhsaker, T., Eds.; The University of Chicago Press: Chicago, IL, USA; London, UK, 1987. [Google Scholar]
- Kingdon, J.; Groves, C.P. Tribe Cercopithecini. In Mammals of Africa: Volume II: Primates; Butynski, T.M., Kingdon, J., Kalina, J., Eds.; Bloomsbury Publishing: London, UK, 2013; pp. 245–387. [Google Scholar]
- Mwenja, I.; Maisels, F.; Hart, J.A. Cercopithecus neglectus. The IUCN Red List of Threatened Species 2019: E.T4223A17947167. Available online: https://doi.org/10.2305/IUCN.UK.2019-3.RLTS.T4223A17947167.en (accessed on 7 June 2020).
- Bennett, N.C. Towards a Better Understanding of Life History Strategies and the Implications of Habitat Destruction on Future Mammal Conservation. Front. Mammal Sci. 2023, 2. [Google Scholar] [CrossRef]
- Armsworth, P.R.; Benefield, A.E.; Dilkina, B.; Fovargue, R.; Jackson, H.B.; Le Bouille, D.; Nolte, C. Allocating Resources for Land Protection Using Continuous Optimization: Biodiversity Conservation in the United States. Ecol. Appl. 2020, 30, e02118. [Google Scholar] [CrossRef] [PubMed]
- Noss, A.J. The Impacts of Cable Snare Hunting on Wildlife Populations in the Forests of the Central African Republic. Conserv. Biol. 2008, 12, 390–398. [Google Scholar] [CrossRef]
- Boyer-Ontl, K.M.; Pruetz, J.D. Giving the Forest Eyes: The Benefits of Using Camera Traps to Study Unhabituated Chimpanzees (Pan troglodytes verus) in Southeastern Senegal. Int. J. Primatol. 2014, 35, 881–894. [Google Scholar] [CrossRef]
- Caravaggi, A.; Banks, P.B.; Burton, A.C.; Finlay, C.M.V.; Haswell, P.M.; Hayward, M.W.; Rowcliffe, M.J.; Wood, M.D. A Review of Camera Trapping for Conservation Behaviour Research. Remote Sens. Ecol. Conserv. 2017, 3, 109–122. [Google Scholar] [CrossRef]
- Pebsworth, P.A.; LaFleur, M. Advancing Primate Research and Conservation Through the Use of Camera Traps: Introduction to the Special Issue. Int. J. Primatol. 2014, 35, 825–840. [Google Scholar] [CrossRef]
- Marcus Rowcliffe, J. Key Frontiers in Camera Trapping Research. Remote Sens. Ecol. Conserv. 2017, 3, 107–108. [Google Scholar] [CrossRef]
- Hart, T.B. Lomami National Park: A New Protected Area in D. R. Congo. Searching for Bonobo in Congo. Available online: https://www.bonoboincongo.com/2016/07/13/lomami-national-park-a-new-protected-area-in-d-r-congo/ (accessed on 21 February 2023).
- Mcphee, S.G. A Camera Trap Study of the Cryptic, Terrestrial Guenon Cercopithecus lomamiensis in Central Democratic Republic of the Congo. Master’s Thesis, Florida Atlantic University, Boca Raton, FL, USA, 2015. [Google Scholar]
- Scotson, L.; Johnston, L.R.; Iannarilli, F.; Wearn, O.R.; Mohd-Azlan, J.; Wong, W.M.; Gray, T.N.E.; Dinata, Y.; Suzuki, A.; Willard, C.E.; et al. Best Practices and Software for the Management and Sharing of Camera Trap Data for Small and Large Scales Studies. Remote Sens. Ecol. Conserv. 2017, 3, 158–172. [Google Scholar] [CrossRef]
- Gregory, T.; Lunde, D.; Zamora-Meza, H.T.; Carrasco-Rueda, F. Records of Coendou ichillus (Rodentia, Erethizontidae) from the Lower Urubamba Region of Peru. Zookeys 2015, 2015, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Eppley, T.M.; Hoeks, S.; Chapman, C.A.; Ganzhorn, U.; Hall, K.; Owen, M.A.; Adams, D.B.; Estor Allgas, N.; Amato, K.R.; Andriamahaihavana, M.; et al. Factors Influencing Terrestriality in Primates of the Americas and Madagascar. Proc. Natl. Acad. Sci. USA 2022, 119, e2121105119. [Google Scholar] [CrossRef] [PubMed]
- Förster, S.; Cords, M. Development of Mother-Infant Relationships and Infant Behavior in Wild Blue Monkeys. In The Guenons: Diversity and Adaptation in African Monkeys; Glenn, M.E., Cords, M., Eds.; Kluwer Academic: New York, NY, USA, 2002; pp. 245–272. [Google Scholar]
- Krige, P.D.; Lucas, J.W. Behavioural Development of the Infant Vervet Monkey in a Free Ranging Troop. J. Behav. Sci. 1975, 2, 151–160. [Google Scholar]
- Lee, P.C. Early Infant Development and Maternal Care in Free-Ranging Vervet Monkeys. Primates 1984, 25, 36–47. [Google Scholar] [CrossRef]
- Glenn, M.E. Group Size and Group Composition of the Mona Monkey (Cercopithecus mona) on the Island of Grenada, West Indies. Am. J. Primatol. 1997, 43, 167–173. [Google Scholar] [CrossRef]
- Kautt, A.F.; Machado-Schiaffino, G.; Meyer, A. Lessons from a Natural Experiment: Allopatric Morphological Divergence and Sympatric Diversification in the Midas Cichlid Species Complex Are Largely Influenced by Ecology in a Deterministic Way. Evol. Lett. 2018, 2, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Stroud, J.T.; Losos, J.B. Ecological Opportunity and Adaptive Radiation. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 507–532. [Google Scholar] [CrossRef]
- Yoder, J.B.; Clancey, E.; des Roches, S.; Eastman, J.M.; Gentry, L.; Godsoe, W.; Hagey, T.J.; Jochimsen, D.; Oswald, B.P.; Robertson, J.; et al. Ecological Opportunity and the Origin of Adaptive Radiations. J. Evol. Biol. 2010, 23, 1581–1596. [Google Scholar] [CrossRef] [PubMed]
- Kingdon, J.; Happold, D.; Buttynski, T. Mammals of Africa; Volume II: Primates; Bloomsbury: London, UK; New Delhi, India; New York, NY, USA; Sydney, Australia, 2013. [Google Scholar]
- Henzi, S.P.; Lawes, M. Breeding Season Influxes and the Behaviour of Adult Male Samango Monkeys (Cercopithecus mitis albogularis). Folia Primatol. 1987, 48, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Nowell, A.A.; Fletcher, A.W. Development of Independence from the Mother in Gorilla gorilla gorilla. Int. J. Primatol. 2007, 28, 441–455. [Google Scholar] [CrossRef]
- Pazol, K. Mating in the Kakamega Forest Blue Monkeys (Cercopithecus mitis): Does Female Sexual Behavior Function to Manipulate Paternity Assessment? Behaviour 2003, 140, 473–499. [Google Scholar] [CrossRef]
- Bourlière, F.; Hunkeler, C.; Bertrand, M. Ecology and Behavior of Lowe’s Guenon (Cercopithecus campbell lowei) in the Ivory Coast. In Old World Monkeys: Evolution, Systematics, and Behavior; Napier, J.R., Napier, P.H., Eds.; Academic Press: New York, NY, USA, 1970; pp. 297–350. [Google Scholar]
- Galat-Luong, A. Notes Préliminaires sur l’écologie de Cercopithecus ascanius schmidti. Terre et Vie 1975, 122, 288–297. [Google Scholar]
- Loken, B.; Spehar, S.; Rayadin, Y. Terrestriality in the Bornean Orangutan (Pongo pygmaeus morio) and Implications for Their Ecology and Conservation. Am. J. Primatol. 2013, 75, 1129–1138. [Google Scholar] [CrossRef]
- Green, V.M.; Gabriel, K.I. Researchers’ Ethical Concerns Regarding Habituating Wild-Nonhuman Primates and Perceived Ethical Duties to Their Subjects: Results of an Online Survey. Am. J. Primatol. 2020, 82, e23178. [Google Scholar] [CrossRef] [PubMed]

| Category | Definition | Birth Month Estimate |
|---|---|---|
| Infant 1 | Newborn infant; Clinging to adult female; Skin can be seen through fine textured hair; Infant body size is relatively small compared to adult female body size (range from 1/4 to 1/3). | <1 month before event |
| Infant 2 | Hair fully grown; Can be clinging to adult female or walking by itself where female is in proximity (both individuals captured on the same screen); If clinging, infant covers the full ventral portion of the female (about 1/2). | 3 months before event |
| Infant 3 | Independent from adult female (>1 m away); Still possesses infant physical features, behaviors, and coloring; Transitioning from infant to juvenile. | 6 months before event |
| Site | Sampling Effort | Events | Capture Rate |
|---|---|---|---|
| Okulu | 1551 | 143 | 9.22 |
| Losekola | 2430 | 282 | 11.61 |
| E15 | 1979 | 173 | 8.74 |
| Total | 5960 | 598 | 10.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fournier, C.S.; McPhee, S.; Amboko, J.D.; Detwiler, K.M. Camera Traps Uncover the Behavioral Ecology of an Endemic, Cryptic Monkey Species in the Congo Basin. Animals 2023, 13, 1819. https://doi.org/10.3390/ani13111819
Fournier CS, McPhee S, Amboko JD, Detwiler KM. Camera Traps Uncover the Behavioral Ecology of an Endemic, Cryptic Monkey Species in the Congo Basin. Animals. 2023; 13(11):1819. https://doi.org/10.3390/ani13111819
Chicago/Turabian StyleFournier, Charlene S., Steven McPhee, Junior D. Amboko, and Kate M. Detwiler. 2023. "Camera Traps Uncover the Behavioral Ecology of an Endemic, Cryptic Monkey Species in the Congo Basin" Animals 13, no. 11: 1819. https://doi.org/10.3390/ani13111819
APA StyleFournier, C. S., McPhee, S., Amboko, J. D., & Detwiler, K. M. (2023). Camera Traps Uncover the Behavioral Ecology of an Endemic, Cryptic Monkey Species in the Congo Basin. Animals, 13(11), 1819. https://doi.org/10.3390/ani13111819






